UL56基因下游3513bp对鸭肠炎病毒生物特性的影响
毛娅卿,张兵,王团结,侯力丹,黄小洁,刘丹,赵俊杰,李启红,王乐元,李俊平,杨承槐
UL56基因下游3513bp对鸭肠炎病毒生物特性的影响
毛娅卿,张兵,王团结,侯力丹,黄小洁,刘丹,赵俊杰,李启红,王乐元,李俊平,杨承槐
(中国兽医药品监察所,北京 100081)
【】鸭肠炎病毒(duck enteritis virus, DEV)属于疱疹病毒,能感染鸭、鹅等雁形目禽类,可引起产蛋下降及高死亡率,已报道了多株DEV的全基因组序列,DEV基因组大,长度约158—162 kb。与疫苗株相比,DEV疫苗检验用参考强毒株基因组UL区5′端连续插入3 513 bp(2 716—6 228位核苷酸)导致UL56基因移框突变。目前关于DEV基因功能、致病机理等报道较少。本研究通过构建了重组病毒,以研究连续3 513 bp插入对DEV生物特性的影响;以提取的DEV基因组DNA为模板,分别扩增UL56基因上下游两端同源臂UL56-u、UL56-d。将两同源臂片段克隆到pMD18T-Simple载体,获得重组质粒pT-UL56ud。将重组质粒pT-UL56ud用I酶切,电泳回收去磷酸化后,将红色荧光蛋白(RFP)表达盒插入到pT-UL56ud中,获得重组质粒pT-UL56-RFP。DEV接种鸭胚成纤维细胞(DEF)(MOI=0.1),吸附1—2 h后,按Lipofectamine 2000说明书转染高纯质粒pT-UL56-RFP,通过同源重组的方法,以红色荧光蛋白(RFP)基因为报告基因,构建了缺失DEV UL56下游3 513 bp的重组病毒DEV△3513及其回复株DEVΔ3513(R)。将重组病毒及其亲本病毒分别接种25 cm2的细胞瓶中(MOI=0.01),测量其病毒含量,绘制一步生长曲线;将重组病毒及其亲本病毒分别作适当稀释,接种已长成单层DEF,加入M199营养琼脂糖覆盖,培养5—7 d,随机选取20个蚀斑,测量蚀斑面积;将重组病毒、回复株及其亲本病毒分别以105.0TCID50肌肉注射6周龄易感麻鸭进行致病性试验,观察10 d,每天记录发病死亡情况。试验结束后剖检所有存活鸭。对全部动物取肝脏组织,固定于4%多聚甲醛溶液,按常规程序制备病理切片并进行H.E染色;将重组病毒及鸭瘟商品化活疫苗株(CVCC AV1222)分别以103.0TCID50肌肉注射6周龄易感麻鸭进行免疫原性试验,在免疫后14 d,腿部肌肉注射接种DEV强毒(CVCC AV1221),每只103.0MLD。观察10 d,每天记录发病死亡情况。通过同源重组的方法,获得携带RFP的重组病毒DEV△3513-RFP、缺失DEV UL56下游3 513 bp的重组病毒DEV△3513及其回复株DEVΔ3513(R)。重组病毒DEVΔ3513、DEVΔ3513(R)及其亲本毒均在接种DEF后72 h,病毒含量达到峰值(106.2-6.5TCID50/0.1 mL),病毒含量无明显差异;均能在DEF细胞中形成清晰蚀斑,大小无明显差异;DEVΔ3513对6周龄易感麻鸭毒力明显减弱,未出现任何临床症状和死亡;DEVΔ3513以103.0TCID50/只免疫麻鸭,能抵抗DEV强毒株的攻击;成功构建了缺失UL56基因下游3 513 bp的重组病毒,首次证实UL56基因下游3 513 bp对DEV在细胞中的复制是非必需的;缺失UL56基因下游3 513 bp后病毒仍保留良好的免疫原性;UL56基因下游3 513 bp是DEV的一个毒力决定因子。
鸭肠炎病毒;UL56基因下游3513 bp;重组病毒;生物特性
0 引言
【研究意义】鸭肠炎病毒(duck enteritis virus, DEV),又称鸭瘟病毒,属于疱疹病毒科,能引起鸭、鹅等雁形目禽类发生急性、热性、败血性的传染病。该病也称为鸭瘟,发病率和死亡率很高,对水禽养殖业危害很大[1-2]。本研究根据DEV强弱株基因组差异,拟分析DEV毒力相关基因,解析DEV致病机制,探索外源基因插入位点、活载体疫苗开发的可行性。【前人研究进展】目前已完成多株DEV全基因组测序[3-7]。DEV疫苗检验用参考强毒株(CVCC AV1221)于1962年从南京一鸭场分离,是我国较早分离的一个强毒,毒力稳定,是我国官方评价鸭瘟疫苗免疫效果的参考强毒,其基因组全长为162 131 bp[8]。与参考强毒株基因组相比,DEV疫苗株UL区5′端连续缺失了3 513 bp(2 716—6 228位核苷酸)导致UL56基因移框突变;德国分离DEV 2085株基因组在2 561位核苷酸后连续缺失1 170 bp[3-4,8-9],该区域是否与DEV毒力相关,尚未见报道。【本研究切入点】本研究以红色荧光蛋白(RFP)为报告基因,构建缺失UL56基因下游3 513 bp的重组病毒,比较缺失前后病毒在细胞中的生长特性以及对易感鸭的致病性、免疫原性。【拟解决的关键问题】本研究旨在分析UL56基因下游3 513 bp对DEV生物特性的影响,为揭示DEV UL56基因下游3 513 bp功能、DEV活载体疫苗研究奠定基础。
1 材料与方法
1.1 试验时间、地点
试验于2015年6月至2017年7月在中国兽医药品监察所完成。
1.2 试验材料
1.2.1 毒株和细胞 DEV参考强毒株(CVCCAV1221)由中国兽医微生物菌种保藏管理中心保存;SPF鸭胚购自中国农业科学院哈尔滨兽医研究所。
1.2.2 试验动物 6周龄易感麻鸭(中和抗体<1﹕4)购自北京昌平某鸭场。
1.2.3 质粒和菌株 含有红色荧光蛋白RFP基因的载体pT-RFP由中国兽医药品监察所病毒制品检测室构建保存;pMD18T-Simple载体购自大连TaKaRa公司,DH5α受体菌购自天根生化科技(北京)有限公司。
1.2.4 主要试剂 Ex Taq DNA 聚合酶、限制性内切酶、T4 DNA 连接酶、T4 DNA 聚合酶购自大连TaKaRa公司;胶回收试剂盒等均购自天根生化科技(北京)有限公司;胎牛血清、M199培养液购自Hyclone公司;OPTI-MEM培养液购自Gibco公司;lipofectamine2000购自Invitrogen公司;无内毒素高纯质粒提取试剂盒Endo-free Plasmid Mini Kit II购自Omega公司。
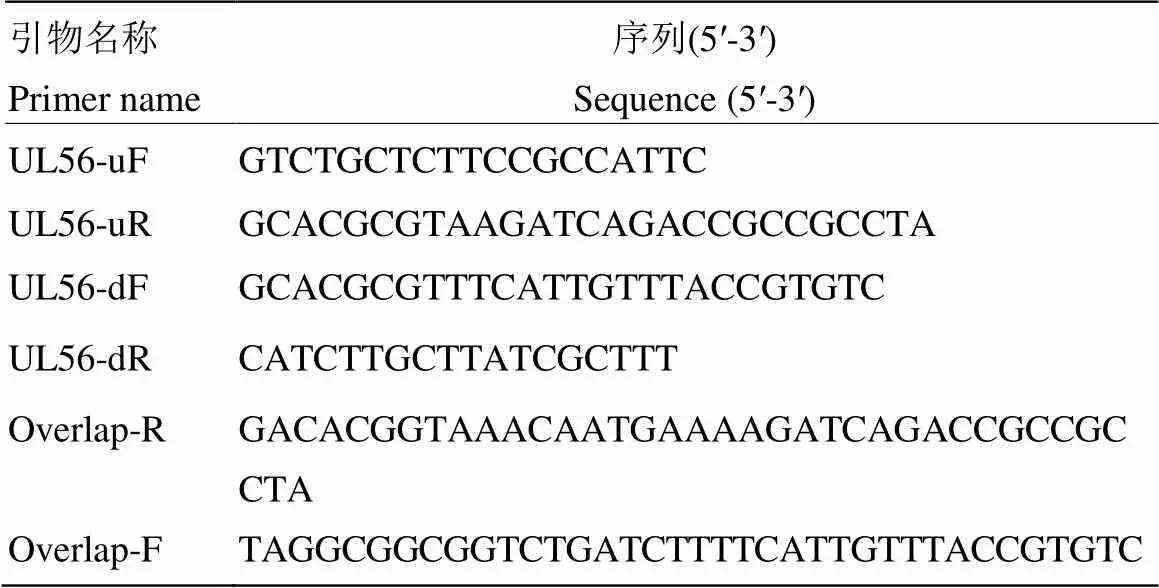
表1 目的基因的扩增引物及鉴定引物
1.2.5 引物试验所用PCR引物见表1,由上海Invitrogen生物公司合成。
1.3 试验方法
1.3.1 基因组DNA的提取 参照文献[10-12]进行。
1.3.2 重组质粒的构建 重组质粒pT-UL56-RFP构建策略见图1。以提取的DEV基因组DNA为模板,分别扩增UL56基因上下游两端同源臂UL56-u、UL56-d。将两同源臂片段克隆到pMD18T-Simple载体,获得重组质粒pT-UL56ud。将重组质粒pT-UL56ud用I酶切,电泳回收去磷酸化后,与用I酶切的pT-RFP连接,将RFP表达盒插入到pT-UL56ud中,获得重组质粒pT-UL56-RFP。按Endo-free plasmid mini kit II说明书提取高纯度质粒备用。
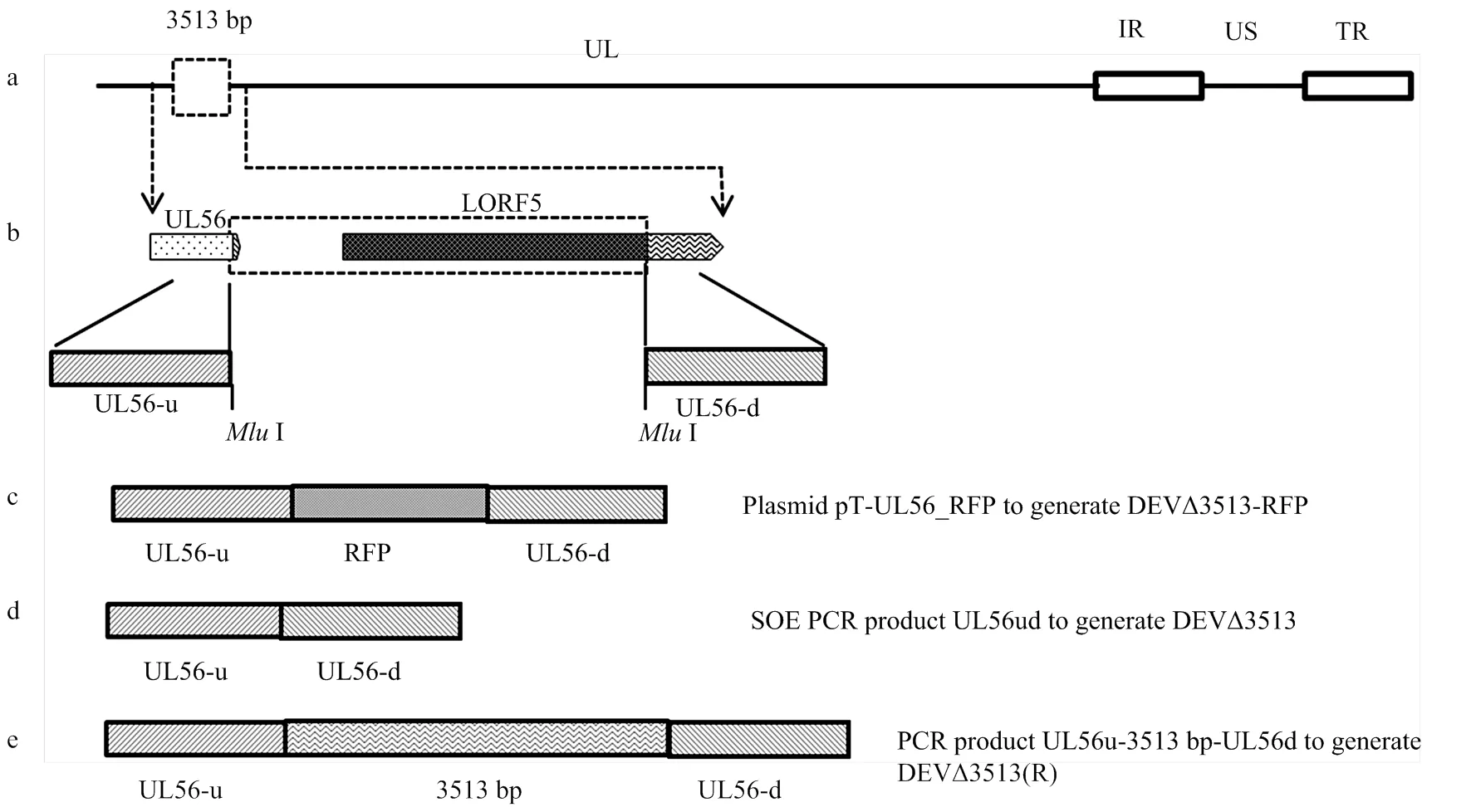
a:DEV基因组示意图,包括UL, IRS, US和TRS区域;b:PCR扩增UL56基因的上下游片段UL56-u,UL56-d;c:DEVΔ3513-RFP的构建,将RFP基因表达盒插入到DEV UL56基因组;d: 应用融合PCR从DEV亲本毒中扩增UL56-ud。PCR产物与DEVΔ3513-RFP进行同源重组,获得重组病毒DEVΔ3513;e: 应用PCR从DEV亲本毒中扩增UL56u-3 513 bp-UL56d。PCR产物与DEVΔ3513-RFP进行同源重组,获得重组病毒DEVΔ3513(R)
1.3.3 重组病毒DEVΔ3513-RFP的构建 DEV接种DEF(MOI=0.1),吸附1—2 h后,按Lipofectamine 2000说明书转染高纯质粒pT-UL56-RFP。按文献[10, 13]进行蚀斑纯化。
1.3.4 重组病毒DEVΔ3513的构建 以DEV基因组DNA为模板,用引物UL56-uF、Overlap-R以及Overlap-F、UL56-dR进行融合PCR。纯化的PCR产物与重组病毒DEVΔ3513-RFP共转染。
1.3.5 重组病毒DEVΔ3513(R)的构建 以DEV基因组DNA为模板,用引物UL56-uF、UL56-dR进行PCR扩增。纯化的PCR产物与重组病毒DEVΔ3513-RFP共转染。
1.3.6一步生长曲线 将重组病毒及其亲本病毒分别接种25cm2的细胞瓶中(moi=0.01),接种后每隔12h取出1瓶接毒细胞,测量其病毒含量,绘制一步生长曲线。
1.3.7 蚀斑大小分析 将重组病毒及其亲本病毒分别作适当稀释,接种已长成单层DEF,加入M199营养琼脂糖覆盖,培养5—7 d。随机选取20个蚀斑,应用CellSens v1.6软件(Olympus Company,Japan)对蚀斑面积进行测量[13]。
1.3.8 致病性试验 将20只6周龄易感麻鸭,随机分成4组,每组5只。第1组肌肉注射DEVΔ3513,每只105.0TCID50;第2组肌肉注射DEVΔ3513(R),每只105.0TCID50;第3组肌肉注射DEV亲本毒,每只105.0TCID50;第4组注射生理盐水,作对照。每组单独隔离饲养,观察10 d,每天记录发病死亡情况。试验结束后剖检所有存活鸭。对全部动物取肝脏组织,固定于4%多聚甲醛溶液,按常规程序制备病理切片并进行H.E.染色。
1.3.9 免疫原性测定 将15只6周龄易感麻鸭,随机分成3组,每组5只。第1组肌肉注射DEVΔ3513,每只103.0TCID50;第2组肌肉注射鸭瘟活疫苗种毒(CVCC AV1222),每只103.0TCID50;第3组注射生理盐水,作对照。每组单独隔离饲养。在免疫后14天,腿部肌肉注射接种DEV强毒(CVCC AV1221),每只103.0MLD。观察10 d,每天记录发病死亡情况。
2 结果
2.1 重组病毒的构建及鉴定
DEV感染DEF细胞2 h后,用Lipofectamine 2000转染高纯质粒pT-UL56-RFP。转染后约10 h,即可见转染细胞中有带有红色荧光的梭形细胞。72 h左右80%细胞出现病变,收获病毒,冻融3次,用DEF细胞分离、纯化红色蚀斑,经过5代筛选,获得纯化的重组病毒DEVΔ3513-RFP。
按照同样的方法,去除RFP获得了缺失UL56基因下游3 513 bp的重组病毒DEVΔ3513;用UL56基因下游3 513 bp替换病毒DEVΔ3513-RFP中的RFP,获得了回复突变株DEVΔ3513(R)。经测序鉴定,所构建的3个重组病毒均正确。
2.2 一步生长曲线
一步生长曲线结果表明,重组病毒DEVΔ3513、DEVΔ3513(R)及其亲本毒均在接种后72 h,病毒含量达到峰值(106.2-6.5TCID50/0.1mL),随后病毒含量下降(图5)。表明UL56下游3 513 bp的缺失对DEV的复制没有明显影响,缺失后病毒滴度无显著变化。
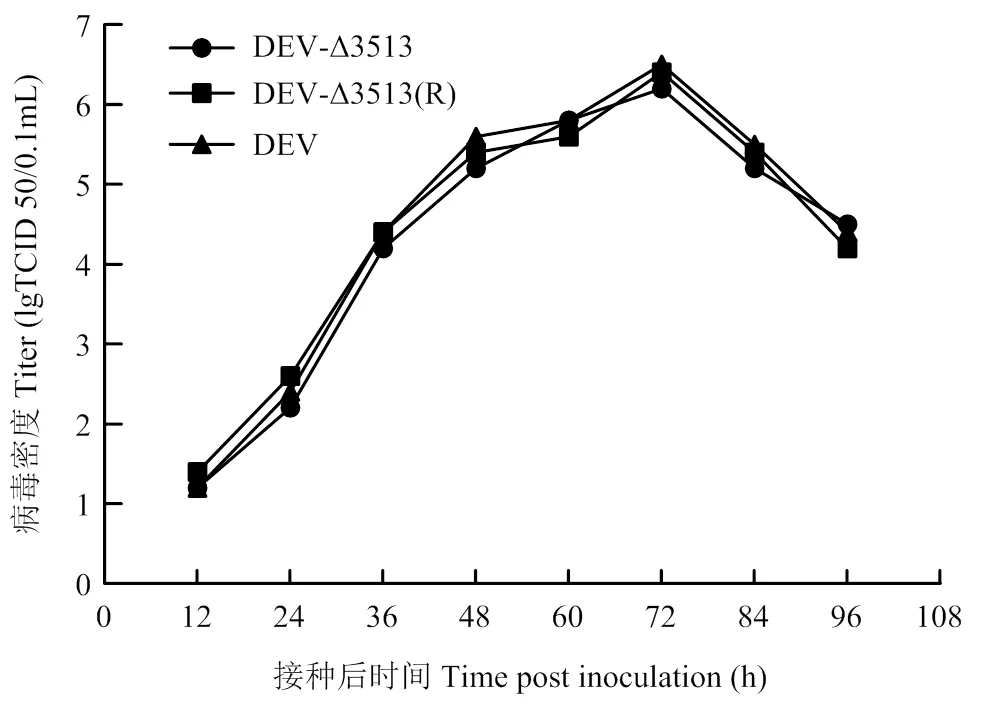
图2 重组病毒的一步生长曲线
2.3 蚀斑大小分析
重组病毒及其亲本毒均能在DEF细胞中形成清晰蚀斑;亲本毒、DEV-Δ3513、DEV-Δ3513(R)蚀斑面积分别为(1.91±0.28)mm2、(1.82±0.31)mm2、(1.98± 0.25)mm2,无显著差异(>0.05)(图5-9),说明UL56基因下游3 513 bp缺失不影响DEV在细胞间的传播能力。

图3 重组毒及其亲本毒蚀斑面积
2.4 致病性试验
DEV亲本毒、重组病毒DEV-Δ3513(R)在接种后3日麻鸭开始出现精神沉郁状;7日全部死亡,剖检肝脏表面有大量散在的白色坏死点。病理组织学观察可见:静脉和肝血窦淤血,充满大量红细胞;肝细胞内有大量脂滴呈空泡状,发生脂肪变性;部分区域出现局灶性肝细胞坏死,在坏死区域伴有炎性细胞,胞核溶解,与其他研究结果一致[14-16]。而DEV-Δ3513接种鸭和对照鸭在14 d观察期内,未出现任何临床症状和死亡。病理组织学诊断可见肝脏组织结构清晰,肝细胞形态正常,未见明显病理变化。说明UL56基因下游3 513 bp缺失后毒力明显减弱。
2.5 免疫原性测定
重组病毒DEVΔ3513以103.0TCID50/只、104.0TCID50/只剂量,免疫麻鸭后14 d,均能100%抵抗DEV强毒攻击(表2),表明UL56基因下游3 513 bp缺失后仍具有良好免疫原性。
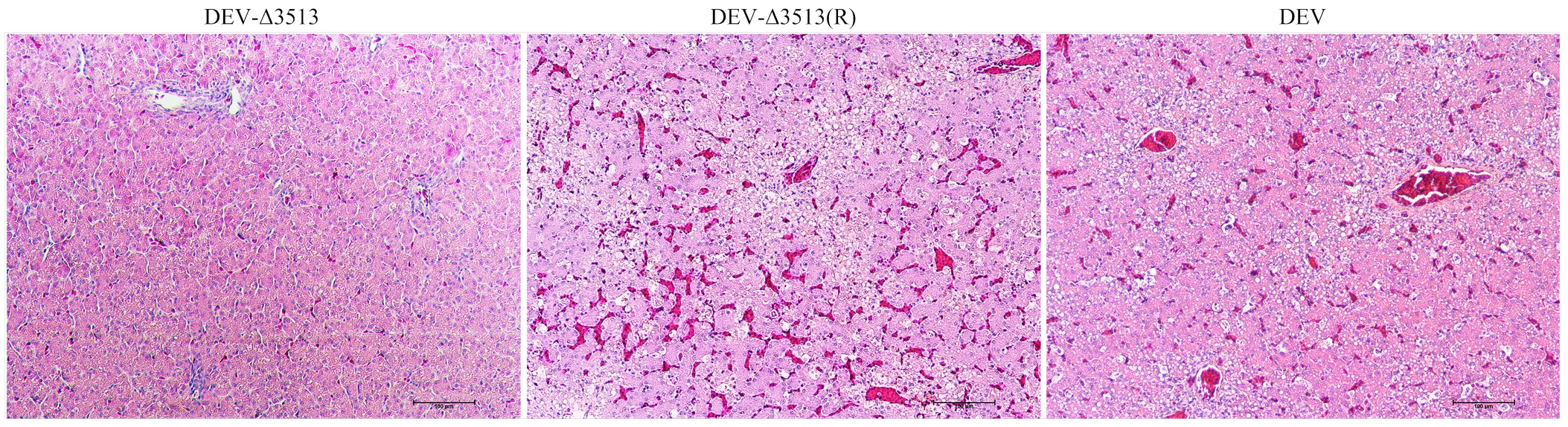
图4 肝脏病理切片(H.E.染色)

表2 免疫后的攻毒保护效力
3 讨论
本研究以红色荧光蛋白RFP为报告基因,以DEV参考强毒株为亲本毒,通过同源重组,构建了缺失UL56基因下游3 513 bp的重组病毒DEVΔ3513及其回复突变株DEVΔ3513(R)。并对其细胞生长特性、致病性和免疫原性进行研究。体外试验表明,重组病毒在细胞中的一步生长曲线、蚀斑大小与亲本毒相似,说明UL56基因下游3 513 bp缺失不影响DEV在细胞上的生长,是病毒复制非必需基因。动物试验结果表明,UL56基因下游3 513 bp缺失能显著降低DEV的毒力,是DEV的一个毒力决定因子;缺失后仍保留良好的免疫原性,能抵抗致死性强毒攻击,与现有商品化鸭瘟活疫苗免疫原性相当。

表3 免疫后的攻毒保护效力
我国鸭瘟活疫苗毒株,其亲本毒于1957年从我国广东分离,然后经鸡胚连续传代60多代致弱[17]。全基因组序列比较发现,与DEV参考强毒株相比,疫苗株基因组UL区5′端连续缺失了3 513 bp(2 716— 6 228位核苷酸)导致UL56蛋白C端第215位氨基酸后移框变和UL55缺失[1]。在α疱疹病毒中,除了牛疱疹病毒1型和5型外,均含有UL56同源物[18-26]。UL56蛋白存在病毒粒子中[27-28]。疱疹病毒UL56蛋白C端有一个疏水区,含跨膜区和多个PPxY 基序等,为典型的跨膜锚定功能域[29-30]。该功能域对维持单纯疱疹病毒1型(herpes simplex virus 1,HSV-1)毒力表型具有重要作用。致病性HSV-1的UL56被LacZ替代后,腹膜内注射不能引起树熊发病[29, 31-33];弱毒HSV-1 HFEM株UL56基因被强毒HSV-1 17株的UL56替换后,能恢复其毒力表型,进一步研究表明,缺失UL56蛋白C末端疏水区(217—234位氨基酸)导致HSV-1毒力丧失[31]。
本研究从DEV参考强毒与疫苗株基因组差异入手,分析了UL56基因下游3 513 bp对DEV参考强毒生物特性的影响,揭示了该区域对维持DEV毒力发挥了重要作用。下一步将缩小缺失范围,重点研究UL56蛋白C末端疏水区对DEV毒力的影响。
4 结论
4.1 重组病毒生长特性与亲本毒相似,首次证明UL56基因下游3 513 bp对DEV在细胞中的复制是非必需的。
4.2 缺失UL56基因下游3 513 bp能显著降低DEV的毒力,首次证明是UL56基因下游3 513 bp是DEV的一个毒力决定因子。
4.3 缺失UL56基因下游3 513 bp后仍保留良好的免疫原性。
[1] SANDHU T S, METWALLY S A. Duck Virus Enteritis(Duck Plague) //SAIF Y M.. Singapore; Blackwell Publishing. 2008: 384-393.
[2] BAUDET A E R F. Mortality in ducks in the Netherlands caused by a filterable virus. Fowl Plague., 1923, 50: 455-459.
[3] WU Y, CHENG A, WANG M, YANG Q, ZHU D, JIA R, CHEN S, ZHOU Y, WANG X, CHEN X. Complete genomic sequence of Chinese virulent duck enteritis virus., 2012, 86 (10): 5965. doi:10.1128/JVI.00529-12.
[4] WU Y, CHENG A, WANG M, ZHU D, JIA R, CHEN S, ZHOU Y, CHEN X. Comparative genomic analysis of duck enteritis virus strains.2012, 86 (24): 13841-13842. doi:10.1128/ JVI.01517-12.
[5] YANG C, ZHANG B, LI J, HUANG X, LIU D, HOU L, LI Q, LI H. Complete genomic sequence of a duck enteritis virus attenuated via serial passage in chick embryos., 2017, 162 (11): 3549-3550. doi:10.1007/s00705-017-3491-1.
[6] YANG C, LI J, LI Q, LI H, XIA Y, GUO X, YU K, YANG H. Complete genome sequence of an attenuated duck enteritis virus obtained by in vitro serial passage.2013, 1(5): 10.1128/genomeA.00685-13.
[7] LI Y, HUANG B, MA X, WU J, LI F, AI W, SONG M, YANG H. Molecular characterization of the genome of duck enteritis virus., 2009, 391(2): 151-161. doi:10.1016/j.virol.2009. 06.018.
[8] YANG C, LI Q, LI J, ZHANG G, LI H, XIA Y, YANG H, YU K. Comparative genomic sequence analysis between a standard challenge strain and a vaccine strain of duck enteritis virus in China.2014, 48 (2): 296-303. doi:10.1007/s11262-013-1009-9.
[9] WANG J, HOPER D, BEER M, OSTERRIEDER N. Complete genome sequence of virulent duck enteritis virus (DEV) strain 2085 and comparison with genome sequences of virulent and attenuated DEV strains.2011, 160 (1/2): 316-325. doi:10.1016/ j.virusres.2011.07.004.
[10] 孙莹, 李俊平, 黄小洁, 李岭, 曹明慧, 李启红, 李慧姣, 杨承槐. 表达绿色荧光蛋白重组鸭肠炎病毒构建. 中国农业科学, 2016, 49 (14): 2805-2812.
SUN Y, LI J P, HUANG X J, LI L, CAO M H, LI Q H, LI H J, YANG C H. Construction and characterization of recombinant duck enteritis virus expressing the green fluorescent protein.2016,49(14):2805-2812. (in Chinese)
[11] SINZGER C, KNAPP J, SCHMIDT K, KAHL M, JAHN G. A simple and rapid method for preparation of viral DNA from cell associated cytomegalovirus.1999, 81(1/2): 115-122. doi:10.1016/S0166-0934(99)00058-0.
[12] SUN Y, YANG C, LI J, LI L, CAO M, LI Q, LI H. Construction of a recombinant duck enteritis virus vaccine expressing hemagglutinin of H9N2 avian influenza virus and evaluation of its efficacy in ducks.2017, 162(1): 171-179. doi:10.1007/s00705- 016-3077-3.
[13] YANG C, LI J, LI Q, LI L, SUN M, LI H, XIA Y, YANG H, YU K. Biological properties of a duck enteritis virus attenuated via serial passaging in chick embryo fibroblasts.2015, 160 (1): 267-274. doi:10.1007/s00705-014-2275-0.
[14] SHAWKY S. Target cells for duck enteritis virus in lymphoid organs.2000, 29(6): 609-616. doi:10.1080/ 03079450020016869.
[15] SHAWKY S, SANDHU T, SHIVAPRASAD H L. Pathogenicity of a low-virulence duck virus enteritis isolate with apparent immunosuppressive ability., 2000, 44 (3): 590-599.
[16] QI X, YANG X, CHENG A, WANG M, ZHU D, JIA R. Quantitative analysis of virulent duck enteritis virus loads in experimentally infected ducklings.2008, 52(2): 338-344. doi:10. 1637/8120-100207-ResNote.1.
[17] HUANG Y X. Study on duck plague-like disease., 1959, 1: 1-12.
[18] BAUMEISTER J, KLUPP B G, METTENLEITER T C. Pseudorabies virus and equine herpesvirus 1 share a nonessential gene which is absent in other herpesviruses and located adjacent to a highly conserved gene cluster., 1995, 69(9): 5560-5567.
[19] DAVISON A J, SCOTT J E. The complete DNA sequence of varicella-zoster virus.1986, 67(Pt 9): 1759-1816. doi:10.1099/0022-1317-67-9-1759.
[20] TELFORD E A, WATSON M S, MCBRIDE K, DAVISON A J. The DNA sequence of equine herpesvirus-1.1992, 189 (1): 304-316.
[21] CULLINANE A A, RIXON F J, DAVISON A J. Characterization of the genome of equine herpesvirus 1 subtype 2.1988, 69(Pt 7): 1575-1590. doi:10.1099/0022-1317-69-7- 1575.
[22] SHAKYA A K, O'CALLAGHAN D J, KIM S K. Comparative genomic sequencing and pathogenic properties of equine herpesvirus 1 KyA and RacL11.2017, 4: 211. doi:10.3389/fvets.2017. 00211.
[23] FULTON R W, D'OFFAY J M, EBERLE R. Bovine herpesvirus-1: Comparison and differentiation of vaccine and field strains based on genomic sequence variation.2013, 31(11): 1471-1479. doi:10. 1016/j.vaccine.2013.01.013.
[24] MACDONALD S J, MOSTAFA H H, MORRISON L A, DAVIDO D J. Genome sequence of herpes simplex virus 1 strain KOS.2012, 86 (11): 6371-6372. doi:10.1128/JVI.00646-12.
[25] LIU C, GUO W, LU G, XIANG W, WANG X. Complete genomic sequence of an equine herpesvirus type 8 Wh strain isolated from China.2012, 86 (9): 5407. doi:10.1128/JVI. 00445-12.
[26] LEE S W, MARKHAM P F, MARKHAM J F, PETERMANN I, NOORMOHAMMADI A H, BROWNING G F, FICORILLI N P, HARTLEY C A, DEVLIN J M. First complete genome sequence of infectious laryngotracheitis virus.2011, 12: 197. doi:10.1186/1471-2164-12-197.
[27] KEHM R, LORENTZEN E, ROSEN-WOLFF A, DARAI G. In vitro expression of UL56 gene of herpes simplex virus type 1; detection of UL56 gene product in infected cells and in virions.1994, 33 (1): 55-66. doi:10.1016/0168-1702(94)90017-5.
[28] KEHM R, GELDERBLOM H R, DARAI G. Identification of the UL56 protein of herpes simplex virus type 1 within the virion by immuno electron microscopy., 1998, 17 (1): 49-53.
[29] DAVISON A J. Herpesvirus systematics.2010, 143 (1): 52-69. doi:10.1016/j.vetmic.2010.02.014.
[30] KOSHIZUKA T, GOSHIMA F, TAKAKUWA H, NOZAWA N, DAIKOKU T, KOIWAI O, NISHIYAMA Y. Identification and characterization of the UL56 gene product of herpes simplex virus type 2.2002, 76(13): 6718-6728. doi:10.1128/ jvi.76. 13.6718-6728.2002.
[31] KEHM R, ROSEN-WOLFF A, DARAI G. Restitution of the UL56 gene expression of HSV-1 HFEM led to restoration of virulent phenotype; deletion of the amino acids 217 to 234 of the UL56 protein abrogates the virulent phenotype.1996, 40 (1): 17-31. doi:10.1016/0168-1702(96)80248-6.
[32] MORI I, LIU B, GOSHIMA F, ITO H, KOIDE N, YOSHIDA T, YOKOCHI T, KIMURA Y, NISHIYAMA Y. HF10, an attenuated herpes simplex virus (HSV) type 1 clone, lacks neuroinvasiveness and protects mice against lethal challenge with HSV types 1 and 2.2005, 7 (15): 1492-1500. doi:10.1016/j.micinf. 2005.05.007.
[33] ROSEN-WOLFF A, LAMADE W, BERKOWITZ C, BECKER Y, DARAI G. Elimination of UL56 gene by insertion of LacZ cassette between nucleotide position 116030 to 121753 of the herpes simplex virus type 1 genome abrogates intraperitoneal pathogenicity in tree shrews and mice., 1991, 20 (3): 205-221. doi:10.1016/ 0168-1702(91)90076-8.
The Effects of Downstream 3513bp of UL56 on Characterization of Duck Enteritis Virus
Mao YaQing, Zhang Bing, Wang TuanJie, Hou LiDan, Huang XiaoJie, Liu Dan, Zhao JunJie, Li QiHong, Wang LeYuan, Li JunPing, Yang ChengHuai
(China Institute of Veterinary Drug Control, Beijing 100081)
【】Duck enteritis virus (DEV) taxonomically belongs to familyand infects ducks, geese, and swans, which results in high mortality and decreased egg production in domestic and wild waterfowl. Several DEV whole genomic sequences were published, which contained 158-162 kb. Compared with DEV vaccine strain, the virulent DEV strain for vaccine evaluation had a 3513-bp insertion, resulting to UL56 frameshift mutation. At present, there are few papers about DEV gene function and pathogenic mechanism. To study the effect of the 3513-bp insertion on DEV characterization, a recombinant DEV with the 3513-bp deletion was constructed.【】The extracted DEV genomic DNA was used as template to amplify the UL56-u and UL56-d of the upstream and downstream ends of UL56 gene, respectively. The two homologous arm fragments were cloned into pMD18T-Simple vector, and the recombinant plasmid pT-UL56ud was obtained. The recombinant plasmid pT-UL56ud was cut byI enzyme, and after electrophoretic recovery and dephosphorylation, the recombinant plasmid pT-UL56-RFP was obtained by inserting RFP expression box into pT-UL56ud. After DEV was inoculated with duck embryo fibroblast (DEF) (MOI=0.1) for 1 to 2 h, the purified plasmid pT-UL56-RFP was transfected according to Lipofectamine 2000 instructions, and then purified by plaque. A 3513-bp deletion mutant, DEVΔ3513-RFP, was generated by targeted homologous recombination, in which red fluorescent protein (RFP) as a reporter replaced the 3 513 bp. The RFP was then removed to generate DEV△3513. A rescue mutant, DEVΔ3513(R), was constructed by reinserting the 3 513 bp into the genome of DEVΔ3513-RFP. The recombinant virus and its parent virus were inoculated in DEF (MOI =0.01), the virus titer was measured and the growth curve was plotted. The recombinant virus and its parent virus were diluted properly and inoculated into monolayers DEF, covered with M199 agarose and cultured 5-7 days. Twenty plaques were selected randomly and the area of plaques was assayed. Six-week old susceptible ducks were inoculated with 105.0TCID50of the 3513-bp deletion mutant, rescue mutant and its parental virus, respectively. After 10 days, all the surviving ducks were euthanized. The liver tissues were taken from all the animals and fixed to 4% poly Formaldehyde Solution; the pathological sections were prepared by routine procedures and stained with HE. The recombinant virus and the Duck Plague Live Vaccine (CVCC AV1222) were injected with 103.0TCID50in muscles for 6 weeks of age susceptible ducks to be tested for immunogenicity. After 14 days, all vaccinated ducks were challenged with 103.0MLD of lethal DEV (CVCC AV1221).【】The recombinant viruses, DEVΔ3513 and DEVΔ3513(R), and their parental virus possessed similar growth kinetics, and their titer peaked at 72 hours with the peak titer 106.2-6.5TCID50/0.1mL. Their average plaques sizes were not significantly different; DEVΔ3513 was avirulent in 6-week ducks; the ducks vaccinated with 103TCID50were protected against subsequent challenge with lethal DEV.【】We successfully constructed a DEV mutant with 3 513 bp deletion, and firstly confirmed that the deletion of the 3 513 bp had no effect on virus replication in cells and immunogenicity in ducks. Moreover, the 3 513 bp was associated with DEV virulence.
duck enteritis virus; downstream 3513 bp of UL56; recombinant virus; property

10.3864/j.issn.0578-1752.2019.23.019
2019-04-22;
2019-07-22
国家重点研发计划(2017YFD0500800)、北京市自然科学基金(6162025)
毛娅卿,Tel:010-62103641;E-mail:wcpmyq @163.com。
李俊平,Tel:010-62103637;E-mail:lijunping03@163.com。通信作者杨承槐,Tel:010-61255386;E-mail:ychenghuai@163.com
(责任编辑 林鉴非)

