Study of Density Functional Theory on Surface Enhanced Raman Spectroscopy of Fipronil
YI Zhen-fei, LIU Chun-yu, 2*, XIN Min-si, KUANG Shang-qi,ZHOU Cheng-cheng, YAO Zhi-hai, 2, CAI Hong-xing
1. Key Lab of Jilin Province for Spectral Detection Science and Technology, College of Science,Changchun University of Science and Technology, Changchun 130022, China 2. Jilin Qiushi Spectral Data Technology Co., Ltd., Changchun 130000, China
Abstract At the beginning of August 2017, Netherlands reported that a wide range of eggs were contaminated with the insecticide fipronil. In this study, the Raman spectroscopy was used to solve the problem of fipronil detection. The stable configuration and all vibration modes of the molecule were obtained after the geometrical structure optimization and frequency calculation, and the theoretical Raman Scattering spectroscopy of the stable configuration of fipronil was also calculated. Normal Raman spectroscopy and surface enhanced Raman spectroscopy of fipronil were collected by HORIBA’s T64000 grating confocal micro-Raman spectroscopy and Ag/Cu nano-substrate. The strong peaks appeared at 211, 308, 350, 867, 1 323, 1 432 cm-1, and the sub-strong peak appeared at 254, 407, 443, 463, 511, 607, 646, 712, 800, 1 065, 1 639 cm-1. The results show that the theoretically calculated vibration frequency agrees well with the experimental measurements at all strong peaks and most sub-strong peaks. The vibration modes corresponding to the frequencies of the fipronil molecule in the range of 200~2 000 cm-1 were assigned. The six strong peaks arranged from small to large were judged to be 21H-22H torsional vibration, 10F-11F deformation vibration, 21H-22H out-of-plane torsional vibration, 15N-22H twisting vibration, 6C stretching vibration and 21H in-plane torsional vibration, benzene ring breathing vibration and stretching vibration of 9C, 7H-8H in-plane torsional vibration. It was found that the surface-enhanced Raman spectrum has a slight frequency shift with respect to the Raman spectrum. The peaks at 211, 867, 1400, and 1 432 cm-1 in the surface-enhanced Raman spectroscopy were selectively enhanced. According to the selection rule of surface-enhanced Raman spectroscopy, it is interpreted as the atom corresponding to the relevant vibration peak and the surface of the silver substrate may be in a nearly vertical state and may be stick to the silver surface. In the next step, fipronil will be planned to be mixed into eggs, and the identification of fipronil in different concentrations in eggs will be carried out. The results of the study can provide a theoretical basis for the Raman spectroscopy of fipronil, which will promote the rapid detection and on-line detection of fipronil residues in food and agricultural products. Raman spectroscopy will be used as a supplement to conventional chemical detection methods.
Keywords Raman spectroscopy; Surface enhanced Raman spectroscopy; Density functional theory; Fipronil
Introduction
Fipronil has a molecular formula of C12H4Cl2F6N4OS and a molecular weight of 437.2. The molecular structure was shown in Figure 1. Fipronil is an insecticide belonging to the phenylpyrazole family. Fipronil destroys the insect central nervous system by blocking GABA-gated chloride channels and glutamate-gated chloride (GluCl) channels[1]. WHO has classed fipronil as a Class II moderately hazardous pesticide, and it is also a Group C (possibly human) carcinogen prescribed by the US Environmental Protection Agency (U.S. EPA). The 2017 fipronil-poisoned egg incident involved 16 EU countries and Hong Kong.
The EU stipulates that the residual amount of fipronil in egg products is no more than 0.02 mg·kg-1. The International Codex Alimentarius stipulates that the residual amount of fipronil in egg products is no more than 0.02 mg·kg-1, and the residual amount in poultry meat is no more than 0.01 mg·kg-1. GB2763—2016[2]stipulates that the residual amount of fipronil in grains and vegetables is no more than 0.02 mg·kg-1(no more than 0.1 mg·kg-1in corn), but its maximum residue limit in eggs and poultry has not been made by any regulation. On October 1, 2009, China specified that fipronil can only be used to treat household hygiene pests. The conventional fipronil detection methods are gas chromatography-electron capture detection (GC-ECD)[3], liquid chromatography (LC)[4], liquid chromatography tandem mass spectrometry (LC-MS/MS)[5-6], gas chromatography-mass spectrometry (GC-MS)[7]and high resolution chromatography-high resolution mass spectrometry (HRGCP LC-HRMS)[8]and so on. These chemical methods are complicated to operate, and the preparation is cumbersome and time consuming. At present, there are rare reports on the Raman spectroscopy of fipronil.
Raman spectroscopy is a scattering spectrum. It could reflect the structural characteristics of a molecule. However, in general, the Normal Raman spectroscopy(NRS) signal of the substance is very weak. In order to enhance this signal, the Surface Enhanced Raman Spectroscopy(SERS) is usually obtained by attaching the substance molecule on the surface of the roughened electrode. Raman spectroscopy does not need sample preparation, short measurement time, high sensitivity and accuracy, and is ideal for rapid detection and on-line detection.
Density Functional Theory (DFT) is a modeling method for physics, chemistry and materials science. It bases on ab initio method and quantum mechanics. It is commonly used to study the electronic structure of multi-body systems, especially atoms, molecules and condensed phases. Numerous studies conducted in Raman spectroscopy have shown its credibility[9-11]. In this paper, the theoretical calculation of the Raman spectra of fipronil molecules at the level of B3LYP/6-311G++(d, p) was carried out, and it was found to be in well agreement with the experimental measurements, and its vibration modes have been assigned.

Fig.1 Molecular structure of fipronil
1 Experimental Section
1.1 Materials and equipment
In the experiment, the spectrometer was a T64000 grating confocal micro-Raman spectrometer from HORIBA. Spectra-Physics’ Stabilite 2017 Ar ion gas laser was used as the excitation beam source and its excitation line is 514.5 nm. The laser power of the sample surface was approximately 9.64 mW. OLYMPUS’s BX41 (NA=0.25) 10× microscope. Fipronil (Purity≥98%) was purchased from Aladdin Reagent Company (China). Other reagents such as acetone, ethanol and silver nitrate were of analytical grade and were purchased from Beijing Chemical Company. Water used in this experiment was ultrapure water (18.2 MΩ).
1.2 Theoretical calculation
DFT can intuitively reflect molecular vibration information and was the most commonly used method in quantum chemical calculations. In this paper, the Raman spectrum of fipronil was calculated by G09W quantum chemical software package. The molecular configuration was calculated by Gauss View 5.0 at B3LYP/ 6-311G++(d, p) level. The keyword was opt freq=raman b3lyp/6-311++g(d, p).
1.3 Method
The Ag/Cu nano-substrate was prepared according to the method of Xiaohong Jiang[13]. The 2 cm×1 cm copper piece was ultrasonically cleaned with acetone, ethanol and ultrapure water for 10 min, then immersed in dilute nitric acid for 1 min to remove the surface oxide. Rinsed the treated copper foil with ultrapure water and dried it at room temperature. The dried copper sheet was immersed in the mixed solution containing 0.02 mol·L-1SnCl2and 0.02 mol·L-1HCl for 1 min, taken out, dried again at room temperature, and then immerse the dried copper sheet in the 0.01 mol·L-1AgNO3solution containing PVP [m(AgNO3)∶m(PVP)=2∶1] for 1 min. Finally, the copper piece was placed in the solution containing Sn2+and then immersed, taken out, rinsed with ultrapure water, and dried at room temperature. The 10-3mol·L-1fipronil solution was prepared with acetone as a solvent, and the Ag/Cu nano-substrate was immersed in the prepared sample solution for 30 min at room temperature, taken out, and dried at room temperature in a vacuum oven. Measured the spectrum directly after completion.
2 Results and Analysis
2.1 Optimization of Molecular Geometry
The calculation result had no imaginary frequency, and the stable structure of the fipronil molecule was obtained. As Fig.2 shows, the ring composed of 1C, 2C, 3C, 4C, 5C, 6C and the ring composed of 15N, 16C, 17C, 18C, 19N are not on a plane. The dihedral angle of the plane of the two rings is 86.432 71°. The triangular pyramid structure composed of 13Cl, 14Cl, 15N, 9C and 10-12F can be considered as a substitution of a benzene ring.

Fig.2 The optimized molecular structure of fipronil
2.2 Comparison of Raman Spectra and Calculation Results
Fig.3(a) shows the NRS of the fipronil, and Fig.3(b) shows the calculated Raman spectrum of fipronil. The calculation results were observed with Gauss View 5.0. The assignments of theoretical calculations and experimentally determined vibration peaks were finished with the help of Gauss View 5.0 and were listed in Table 1[13-15].
As the NRS shows, the Raman peaks of fipronil are in the range of 200~610, 640~900, 1 100~1 640 cm-1, and the higher intensity peaks are at 211, 308, 350, 867, 1 323, 1 432 cm-1. The peak at 211 cm-1is considered to be a strong tortuous vibration of 21H, 22H and 20N. The weaker deformation vibration of 10F-11F and the 21H-22H medium-intensity out-of-plane rocking vibration correspond to the peak at 308 cm-1. 350 cm-1is caused by the torsional vibration of the five-membered ring plane by 15N and 22H. The peak at 867 cm-1is in good agreement with the calculated value, which is considered to be 6C has a moderately severe stretching vibration in the 9C direction connected thereto and 21H doing strong bending vibration in the five-membered torus. The peak of 1 323 cm-1is due to the six-membered ring making moderate-intensity respiratory vibration and the 9C doing stretching vibration in the 6C direction. The peak at 1 432 cm-1is considered to be a strong in-plane bending vibration of 7H and 8H. The intensities of the peaks corresponding to the above vibrations are relatively high and appear in both the calculation spectrum and the experimental spectrum, and their matching degrees are also high, as shown in Table 1.
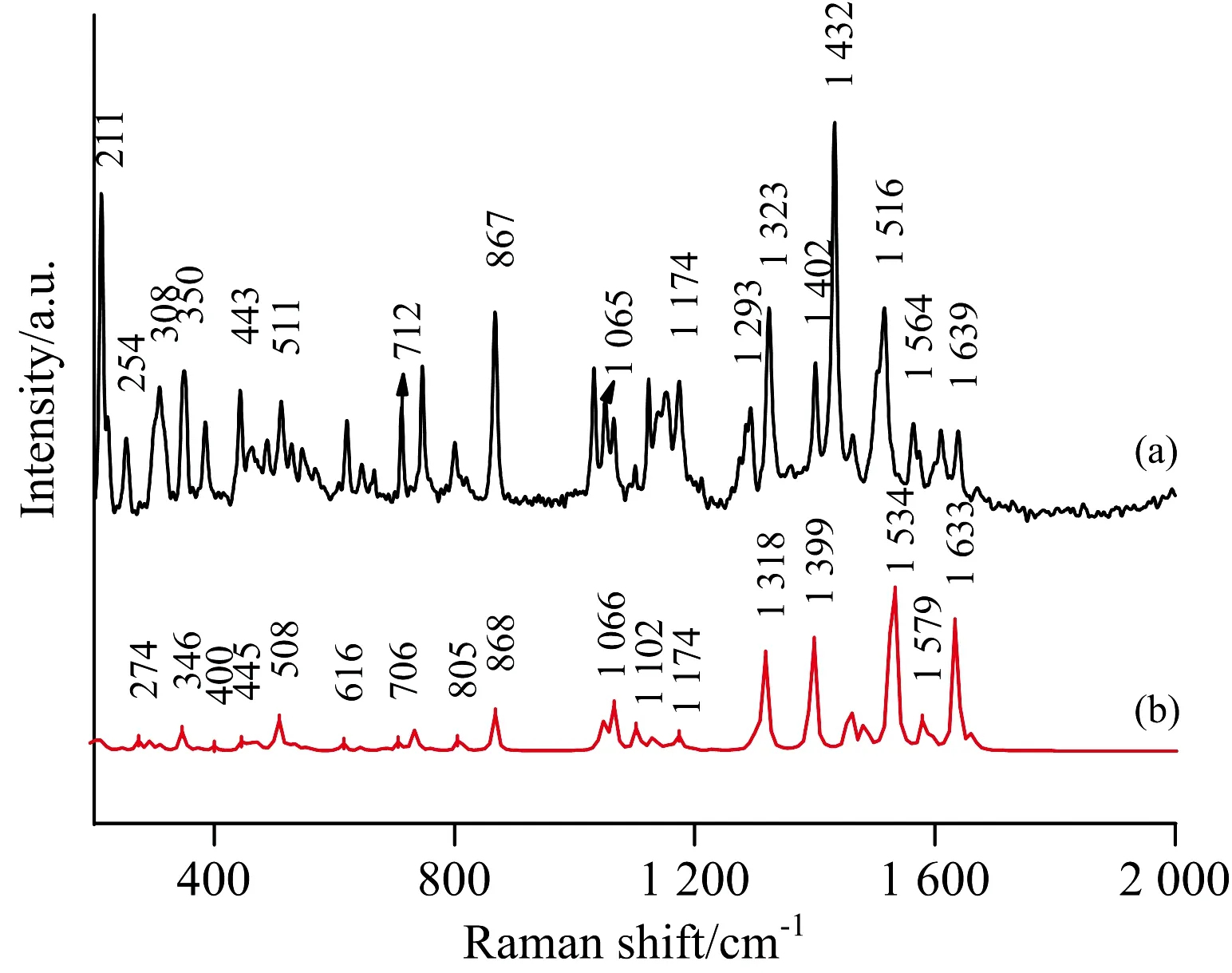
Fig.3ExperimentalnormalRamanspectrumoffipronil(a)andTheoreticalRamanspectrumoffipronil(b)
Comparing the calculated values of DFT with the measured values of NRS, it has been found that there are little differences between them, but most of them are the same[16]. Some peaks appear in the calculations but are not found in the measured spectrum, mainly because the theoretical calculation is simply simulation of pure theoretical vibrations of individual molecule. But in the experiment, what has been measured is the solid powder, and there are mutual influence factors such as the intermolecular force and the interaction between the groups; therefore, the theoretical calculation itself has a certain error anyway. In the comparison, we can also see the intensity of the experimental measured value of the solid powder is much higher than the theoretical calculation. But the theoretical calculation still provides a lot of important information for the basic information of the molecule.
The solubility of fipronil in acetone is high at normal temperature. Therefore, 10-3mol·L-1fipronil solution was prepared with acetone as solvent. The SERS was obtained with the help of Ag/Cu nano-substrate. Fig.4(a) is SERS, and Fig.4(b) is NRS. Peaks at 211, 867, 1 400, 1 432 cm-1are selectively enhanced. Overall, the SERS spectrum has some slight frequency shift relative to the NRS spectrum. For of 867 cm-1is 21H doing strong bending vibration in a five-membered torus, probably because the NH2connected to 21H is very close to the silver surface, and 21H itself may be stick to the silver. The peak of 1 400 cm-1is caused by the symmetric telescopic movement of 18C-15N, which has a frequency shift of 2 cm-1with respect to NRS, and is greatly enhanced. According to the selection rule of SERS, when the vibration damping mode of the adsorbed molecule involves the change of the vertical and surface components of the molecular polarizability, the vibration mode will be enhanced in the SERS, so the enhancement of the relevant vibration peak indicates that some atoms like 21N-H and the silver substrate may be in a nearly vertical state and sticks to the silver surface. 211, 867, 1 432 cm-1can be used as the Raman characteristic peak of fipronil, and 211, 867, 1 400, 1 432 cm-1can be meaningful for identification in SERS studies.
Table1Theassignmentsofthecalculatedvibrationalfrequenciesoffipronil(cm-1)atB3LYP/6-311G++(d,p)level

NRS/cm-1DFT/cm-1Assignment211218τ(21H-22H)254273ρ(18C-29C)τ(25C-26F)291ρ(9C-11F)308311δ(10F-11F)ω(21H-22H)350348τ(15N-22H)375ω(16C-17C)407401ω(7H-8H)443447τ(23S-24O)458δ(21H-22H)463460ρ(1C-5C-6C)511509ρ(ph) br(15N-16C-17C-18C-19N)529525ω(7H-8H)σ(10F-12F)557σ(26F-28F)607606τ(7H-8H)614ρ(16C-17C)646644τ(ph)ρ(18C-29C)712708δ(9C-10F-11F-12F)726724τ(16C-17C-18C)800806ρ(20N-21H)820815ν(2C-4C)867867ν(6C)τ(21H)889900ρ(7H-8H)1 062τ(17C-19N)1 0651 066δs(25C-26F-27F-28F)νs(23S-24O)1 1011 103ν(24O)1 1231 127τ(6C-9C)ρ(20N-21H-22H)1 1391 134ν(17C)1 1531 154τ(9C)1 1741 175ν(17C)τ(15N-19N)1 2931 294ν(9C)τ(7H-8H)1 3231 318br(pi)ν(9C)1 4021 396ν(18C-15N)1 4321 419τ(7H-8H)1 5161 530ν(3C-16C)1 5641 579ν(16C) τ(30N-29C)1 6391 635τ(1C-5C)1 662σ(21H-22H)
ν: stretching vibration;σ: scissor vibration;ρ: in-plane swing vibration;ω: out-of-plane swing vibration;τ: torsional vibration;δ: deformation vibration; br: breath vibration; s: symmetry; ph: benzene ring
example, the peak of 1 402 cm-1in NRS corresponds to 1 400 cm-1in SERS. The peak at 211 cm-1is considered to be a strong tortuous vibration of 21H, 22H and 20N, and the peak

Fig.4 SERS of fipronil (a) and NRS of fipronil (b)
3 Conclusions
The theoretical calculation of Raman spectra of fipronil was studied and it was in good agreement with NRS. The G09W software package was used as the calculation tool to optimize the spatial structure of fipronil and finally we obtained the stable structure. The theoretical calculation Raman spectrum was given at B3LYP/ 6-311G++(d, p) level. Combined with the vibration mode displayed by the visualization software Gauss View 5.0, the Raman vibration modes of fipronil were assigned. The results of quantitative calculations provide an effective theoretical basis for spectral analysis. Based on Ag/Cu, the SERS of fipronil-acetone solution was studied, and the adsorption mode was inferred. It provided a theoretical basis for the rapid analysis and detection of fipronil.

