Large specific surface area S-doped Fe-N-C electrocatalysts derived from Metal-Organic frameworks for oxygen reduction reaction
Xiohui Yn ,Xiolin Li ,Cehung Fu ,Chen Lin ,Hunming Hu ,Shuiyun Shen ,Gunghu Wei,Junling Zhng,d,*
a Institute of Fuel Cells,School of Mechanical Engineering,Shanghai Jiao Tong University,Dongchuan Rd.800,Shanghai,China
b Central Research Institute,Shanghai Electric Group,Shanghai,200070,China
c SJTU-Paris Tech Elite Institute of Technology,Shanghai Jiao Tong University,Dongchuan Rd.800,Shanghai,China
d MOE Key Laboratory of Power Machinery and Engineering,Shanghai Jiao Tong University,Dongchuan Rd.800,Shanghai,China
ABSTRACT It is highly desired but challenging to develop platinum group metal-free electrocatalysts for oxygen reduction reaction (ORR),which can promote the commercialization of fuel cell technology.To achieve this target,we report a one-step doping method to prepare S-doped Fe-N-C catalysts using zeolite imidazole framework(ZIF-8)and iron (III) thiocyanate (Fe(SCN)3) as precursor.Different from conventional doping approach, i.e. physical mixing,Fe(SCN)3 is in-situ added during ZIF-8 formation which would encapsulate Fe(SCN)3 molecules inside ZIF-8 to avoid structure destruction and create potential replacement of Zn ions by Fe ions to form uniform Fe-N4 complexes.As a result,the prepared S-doped Fe-N-C catalysts own large specific surface areas with a maximum value of 1326 m2 g-1 and a dual-scale porous structure that benefits mass transport.Significantly,the composition-optimized catalyst exhibits superior ORR activity in both 0.1 M HClO4 electrolyte and 0.1 M KOH electrolyte,in which the half-wave potential reaches 0.81 V and 0.92 V (vs.RHE),respectively.Remarkable stability is also attained,which loses 2 mV only after 10000 potential cycles in O2-saturated 0.1 M HClO4 and remains almost constant in O2-saturated 0.1 M KOH,surpassing commercial Pt/C catalyst in both acidic and alkaline medium.
Keywords:Fuel cells Oxygen reduction reaction Non-precious metal catalyst Co-doping Acidic and alkaline medium
1.Introduction
Proton exchange membrane fuel cell(PEMFC),an energy conversion device with high-efficiency and zero-pollution[1-3],holds the potential to replace internal combustion engine in vehicle industry due to the increasingly stringent emission requirements [4-7].However,its widespread application is still hindered by cost issue which mainly arises from the use of platinum group metals (PGM) to catalyze the sluggish oxygen reduction reaction (ORR) at the fuel-cell cathode.To address this issue,great attentions have been paid on developing low-cost and high-performance ORR catalysts.For instance,three strategies are generally utilized for Pt based catalysts to boost the mass activity,i.e.(i) alloying Pt with other transition metals (Pt-M;M=Fe,Co,Ni …) [8,9],(ii) synthesizing shape-controlled Pt or Pt-M catalysts[10,11],and (iii) preparing core-shell structure with few atomic layers of Pt as shell and non-Pt materials as core [12,13],all of which can reduce Pt dosage in fuel cells without compromising performance.
Another and a more radical solution is the development of PGM-free ORR catalysts[14-17].Up to now,various types of materials,including heteroatom doped carbon,transition metal chalcogenides,metal-nitrogen-carbon materials,metal chelate [18,19],metal oxides,metal nitrides,metal oxynitrides[20-23],and etc have been synthesized and demonstrated to be active towards ORR[22,24-30],among which metal-nitrogen-carbon (particularly Fe-N-C) is considered as the most promising substitute of PGM due to its superior activity.
Previous theoretical calculations have suggested that the possible formation of Fe-N4clusters in Fe-N-C materials would promote oxygen adsorption and subsequentbond breaking which even own similar activity as Pt[31].Therefore,maximizing the exposure of Fe-N4sites at catalyst surface,i.e.increasing the active site density,is a direct way to improve the catalyst performance,which requires Fe-N-C catalysts to own large specific surface area.Meanwhile,a porous catalyst structure is also highly desired,not simply as it can enlarge the surface area,but also because such structure can facilitate the mass transport from bulk to surface,thus ensuring the active sites could be fully utilized.Considering the structural property,metal-organic frameworks (MOFs),a class of organic-inorganic hybrid crystalline materials with porous structure,are thus selected as ideal catalyst precursor[32].The MOF-derived Fe-N-C catalysts are commonly prepared by physically mixing MOF with iron salts,followed by pyrolysis at high temperatures.However,both these two steps would damage the pore structure,and thus resulting in a loss of surface area.In addition,S-doping was found capable of tuning electron spin density and electronegativity of carbon which benefits ORR process[33,34].But one more step to introduce sulfur into carbon skeleton would further aggravate the structure destruction,although the intrinsic activity is improved after the co-doping with sulfur.Therefore,there exists a trade-off between active sites density and intrinsic activity during Fe-N-C catalysts preparation that limits the catalyst performance improvement.
Herein,we propose to prepare MOF-derived catalysts via one-step doping to address above-mentioned issue using zinc-based zeolite imidazole framework(ZIF-8).After screening,Fe(SCN)3was selected as iron salt since it can simultaneously introduce Fe and S to achieve the one-step co-doping.Besides,Fe(SCN)3was in-situ added during ZIF-8 formation rather than physically mixing with formed ZIF-8 particles,which would encapsulate Fe(SCN)3molecules inside ZIF-8 without the porous-structure destruction and lead to potential replacement of Zn ions by Fe ions to form uniform Fe-N4complexes.The specific surface area of pristine ZIF-8 was found to be 1183.78 m2g-1,in contrast,this value for S-doped Fe-N-C catalysts after the pyrolysis of ZIF-8 based precursor can achieve 1000-1300 m2g-1,demonstrating that such onestep doping method is able to preserve the desired MOF-like structure.The electrochemical tests confirmed the consequent superior catalyst performance,which shows a half-wave potential of 0.81 V and 0.92 V in acidic and alkaline medium,respectively.
2.Experimental
2.1.Chemicals and reagents
Zinc nitrate hexahydrate(Zn(NO3)2⋅6H2O,99%),2-methylimidazole(99%),iron(III) chloride (FeCl3⋅6H2O,99.99%),potassium thiocyanate(KSCN,99%) were obtained from Sigma-Aldrich.Perchloric acid(HClO4,70% solution),potassium hydroxide (KOH,85%),methanol(CH3OH,AR) and ethanol (C2H5OH,AR) were purchased from Sinopharm Chemical Reagent Co.,Ltd.Commercial Nafion solution (5%,Dupont)and Pt/C(Pt-46.7%)were utilized for electrochemical tests.All solutions were prepared with the ultrapure water(18 MΩ)generated by Millipore Milli-Q system.
2.2.Synthesis of ZIF-8 derived S-doped Fe-N-C
Fe(SCN)3was firstly prepared by mixing 1.0 M KSCN and 1.0 M FeCl3⋅6H2O solution with a volume ratio of 7:2,which would simultaneously act as the source of iron,sulfur and part of nitrogen.ZIF-8 colloidal particles were prepared based on the method reported before[35,36]:1.695 g Zn(NO3)2⋅6H2O and 1.97 g 2-methylimidazole were dissolved into 200 mL and 100 mL methanol respectively,which were further mixed together rapidly and kept at 60°C for MOF formation.After 6 h,a certain amount(XmL,X=0.35,0.45,0.55 and 0.65)of 0.22 M Fe(SCN)3solution was added dropwise during the MOF incubation process,and the corresponding prepared S-doped Fe-N-C catalyst was denoted as Fe-SNC-X.Afterward,the solution was kept at 60°C for another 24 h for complete MOF formation,followed by centrifugation and ethanol washing which was further dried in vacuum overnight.At last,the obtained powders were pyrolyzed at 1000°C in Ar atmosphere for 1 h,followed by thermal treatment at 900°C in a mixture of NH3and Ar for another 15 min and then cooling to ambient temperature under Ar stream to obtain the final catalysts.
2.3.Catalysts characterization
Scanning electron microscopy (SEM,NOVA NanoSEM 230),transmission electron microscopy (TEM,JEOL 2100F) and high-angle annular dark-field scanning transmission electron microscopy(HAADF-STEM,JEOL JEM-ARM200F S/TEM) were utilized to perform the morphology characterization.X-ray photoelectron spectroscopy(XPS)measurement was performed to analyze the doping results based on an AXIS UltraDLD electron spectrometer.The surface area and pore distributions were tested by the Horvaih-Kawazoe (HK) method and Barrett-Joyner-Halenda(BJH)method based on a 3He2000PS1 analyzer(BeiShiDe Instruments S&T).
2.4.Electrochemical measurements
Activity and stability of the prepared Fe-SNC catalysts (the final prepared S-doped Fe-N-C catalysts) were tested in both acidic and alkaline medium.The catalyst ink was prepared as follows:5 mg Fe-SNC catalysts were added in a mixture solution containing 990 μL ethanol and 40 μL Nafion solution (5 wt%),which was dropped onto a glassy carbon electrode (GCE) with a catalyst loading of 700 μg cm-2after ultrasonic dispersion.Additionally,GCE coated with commercial Pt/C catalyst was also prepared with a Pt loading of 20 μg cm-2as the control group.Cyclic voltammetry (CV),linear sweep voltammetry (LSV) and chronoamperometric measurements were conducted with an electrochemical workstation (CHI 760e,CH Instruments) in a three-electrode cell at room temperature.Within the three-electrode cell,GCE coated with catalysts acts as the working electrode,platinum foil acts as the counter electrode,Hg/Hg2Cl2and Hg/HgO work as the reference electrode in acidic and alkaline medium,respectively.
Catalytic activity was evaluated by LSV which was obtained in O2-saturated 0.1 M HClO4or O2-saturated 0.1 M KOH with a scan rate of 5 mV s-1.Meanwhile,the contrastive CV curves in N2-saturated or O2-saturated electrolyte were obtained with a scan rate of 20 mV s-1.Catalyst stability was evaluated by accelerated degradation test (ADT)and chronoamperometric technique.ADT test was performed by applying potential cycles at the voltage range of 0.6-1.0 V with a scan rate of 100 mV s-1,followed by recording ORR polarization curve after 10000 potential cycles.The chronoamperometrici-tcurves were recorded at the potential of 0.65 V (vs.RHE) in the O2-saturated electrolyte with a rotation rate of 1600 rpm.
2.5.Computational details
Theoretical calculation was performed to investigate the effect of codoping.All calculation was carried out by density functional theory with DMol3 on Material Studio 8.0[37].The generalized gradient-corrected Perdew-Burke-Ernzerhof functional(GGA-PBE)was used to describe the exchange-correlation energy in Kohn-Sham function [38].The core treatment was DFT semi-core pseudopotentials.Tkatchenko-Scheffler(TS) method was applied to correct the van der Waals interaction and the atomic orbitals were described by double numerical plus polarization (DNP)basis set [39].
The Fe-N4C model is designed by monolayer graphite and sampled with 3 × 3 × 1 Monkhorst-Pack grid.20 Å vacuum slab and dipole correction are used to eliminate the interaction between slabs.The convergence tolerances were 0.00001 Ha,0.002 Ha/Å,0.005 Å for energy,max force and max displacement.
The ΔG of elementary reaction are calculated as follow:

whereGstate2andGstate1are the free energies of product and reactant in ORR elementary reaction.The energy of(H++e-)is defined as half of H2on the standard condition that U=0 and pH=0.The free energy is given as:

whereEis the structure energy andZPEis the zero-point energy which is equal to ∑(hvi/2) (his the Planck constant andviis the vibrational frequency).Tis the temperature (298.15 K),Sis the entropy of the structure,eis the charge constant andUis the potential[40,41].
The energy of H2O was calculated by:

whereRis gas constant,P0=1 bar andP=0.035 bar.The free energy of O2was calculated by the reaction of 2H2+O2→2H2O of which ΔG is 4.92 eV [40,41].
3.Results and discussion
3.1.Structural and compositional features of Fe-SNC catalysts
ZIF-8 is an ideal precursor for M-C-N catalysts,since it can simultaneously provide N and C sources and offer a three-dimensional porous structure that ensures high specific surface area.In order to minimize the structure damage,iron salt,Fe(SCN)3,was in-situ added to one-step introduce S and Fe along with the ZIF-8 incubation process.Herein,XmL(0.35,0.45,0.55 and 0.65 mL)Fe(SCN)3solution was added and the corresponding final catalyst was denoted as Fe-SNC-X.After that,hydrocarbon networks of prepared precursor was carbonized at 1000°C,followed by thermal treatment at 900°C in NH3-Ar mixture to increase the N-doping level and enlarge the surface area.The morphology of prepared S-doped Fe-N-C catalysts was characterized by SEM,which are depicted in Fig.1a and b that shows a ZIF-8-like structure,demonstrating the Fe(SCN)3based in-situ doping can help to preserve the desired structure of ZIF-8.The nanostructure of prepared catalysts was further characterized by TEM and HAADF-STEM.As shown in Fig.1c,there exist numerous mesopores and micropores within Fe-SNC catalysts that can increase the active site density at surface and promote the oxygen transport towards active sites,which are able to synergistically boost the catalytic activity.In HAADF-STEM images of Fig.1d and e,the porous structure can be further observed,besides,bright spots,i.e.Fe atoms,are also observed,indicating the successful embedment of Fe in carbon skeleton.The elemental mapping of Fig.1e was performed and depicted in Fig.1f-i,in which the existence of S,Fe and N elements over the whole carbon supports can be found,demonstrating the uniform doping of N and S.Moreover,the high intensity of N signal in Fig.1i suggests that the N content in Fe-SNC catalysts is high,although such measurement is a semi-quantitative test.
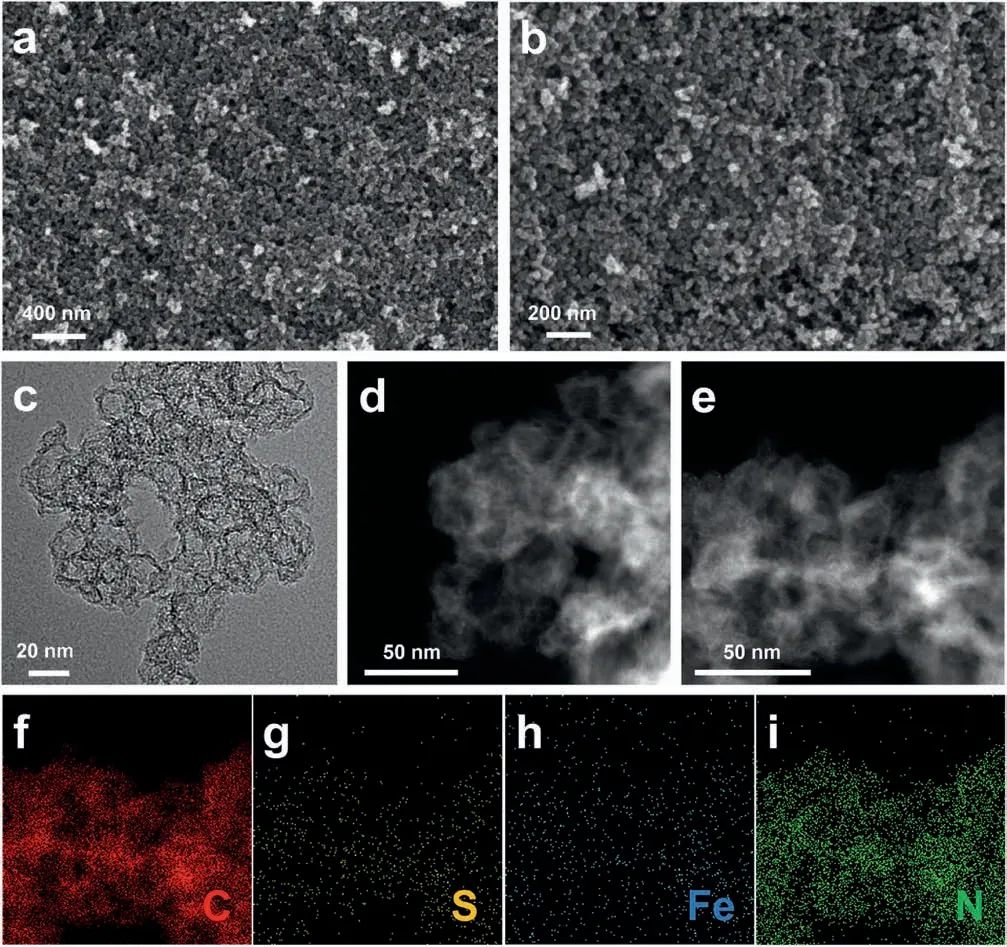
Fig.1.(a,b) SEM;(c) TEM;(d,e) HAADF-STEM images of Fe-SNC catalysts and (f-i) corresponding elements mapping.
According to N2adsorption/desorption isotherm of ZIF-8,its specific surface area without carbonization is 1183.78 m2g-1.Large surface area is a basis of high-performance metal-free ORR catalysts,therefore,insitu doping with Fe(SCN)3and thermal treatment with NH3were conducted to fully take the advantage of large-surface-area of ZIF-8.The N2adsorption/desorption isotherms and corresponding BET surface area of prepared Fe-SNC catalysts are given in Fig.2a,in which it can be found that the surface area would decrease with more Fe(SCN)3addition.For instance,the specific surface area of Fe-SNC-0.35 is 1326.04 m2g-1,in contrast,the value decreases to 1173.51 m2g-1for Fe-SNC-0.45.Nevertheless,the surface area is still as high as 1062.67 m2g-1even after adding 0.55 mL Fe(SCN)3,i.e.Fe-SNC-0.55,only slightly smaller than pristine ZIF-8 which can be attributed to the one-step doping strategy.This is because iron salt was in-situ introduced that could preserve the desired MOF structure,meanwhile,micro-pores would be partially formed during NH3treatment.Therefore,large specific surface area could be maintained after high-temperature pyrolysis.For clear presentation,N2adsorption/desorption isotherm of Fe-SNC-0.45 is shown alone in Fig.2b,and relevant pore size analysis is performed via Horvath-Kawazoe (HK) method and Barrett-Joyner-Halenda (BJH)method respectively which are shown in Fig.2c and d.According to HK analysis,there exist abundant micropores with average pore size of 0.6 nm that can also be observed in TEM image of Fig.1c.Such micropores are originated from the evaporation of Zn in ZIF-8 and decomposition of organic fragments during pyrolysis [42,43].HAADF-STEM images(Fig.1d and e) show the existence of mesopores,and the detailed structure is revealed by BJH analysis that pore-size of mesopores ranges from 10 nm to 30 nm with an average value of 22.5 nm.Such hierarchically porous structure is highly desired,since it can simultaneously enlarge the active site density to promote intrinsic activity and build continuous oxygen transport pathway to ensure sufficient oxygen supply.
The chemical composition of prepared Fe-SNC was further characterized by XPS.As shown in Fig.3a,elements of S,N and Fe can be clearly identified in corresponding XPS spectrum.For example,mass fraction of S,Fe and N in Fe-SNC-0.45 is 0.21%,0.39% and 5.94%respectively,indicating that N doping amount is high.In C 1s spectrum of Fig.3b,four types of carbon appear in which the peaks locate around 287.8 eV and 284.1 eV belong to C-N and C-S,demonstrating the successful doping of N and S into carbon lattice.For sulfur,besides S atoms connected with carbon whose XPS peak locates at 163.8 eV in Fig.3d,there also exists oxidized S species as depicted by the peaks at 169.5 eV and 167.9 eV,suggesting that S is partially doped in carbon lattice but this type of S accounts for the main part.Among different N types,i.e.pyridinic N (Py-N),iron-coordinated N (Fe-N),pyrrolic N (Pyr-N) and graphite-like N(G-N),Fe-N accounts for a major part which is expected as the highly active sites towards ORR [44,45].The detailed element composition,content and chemical state of N in Fe-SNC catalysts prepared by different Fe(SCN)3amount is summarized in Table 1.Clearly,when more Fe(SCN)3was added during catalyst preparation process,Fe-N sites would decrease while pyridinic N sites would increase,which would in turn tune the activity.
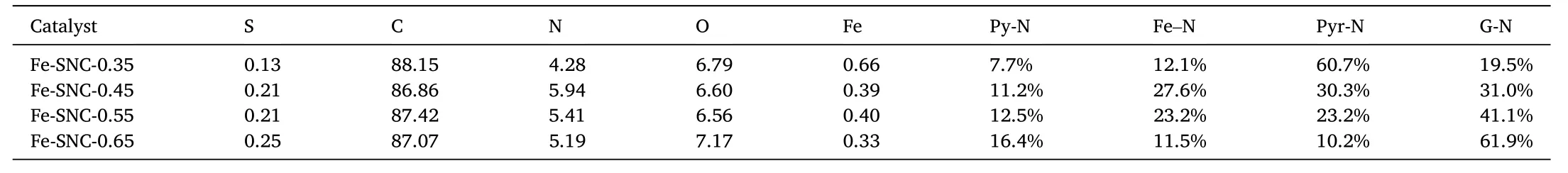
Table 1 Elemental composition and contents of different N for Fe-SNC-X catalysts.
3.2.Electrochemical performance in acidic medium
The ORR activity of prepared Fe-SNC catalysts in acidic medium was evaluated in 0.1 M HClO4.As shown in Fig.4a,all these Fe-SNC catalysts present good catalytic activity which can be attributed to the combined effect from porous catalyst structure and highly active Fe-Nxsites.Among these catalysts,Fe-SNC-0.45 shows the best performance with an onset potential(Eonset)of 0.95 V and a half-wave potential(E1/2)of 0.81 V.It should be noted that although Fe-SNC-0.45 is still less active than commercial Pt/C (Eonset=1.01 V;E1/2=0.90 V),it is among the best non-precious metal catalysts for ORR in acidic medium compared with reported results [42,46-49].Considering Fe-N sites content in Fe-SNC-0.45 is the most relative to other samples,it can be speculated that the high catalytic activity of Fe-SNC in acidic medium mainly arises from Fe-Nxsites which is consistent with previous report.
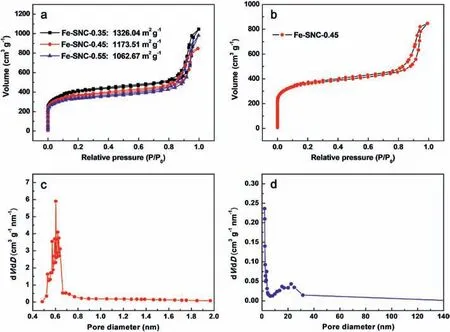
Fig.2.(a)N2 adsorption/desorption isotherms of Fe-SNC catalysts with different addition amount of Fe(SCN)3;(b)the detailed isotherm of Fe-SNC-0.45 for a clear view;(c) micropore analysis of Fe-SNC-0.45 based on HK method;(d) mesopore analysis of Fe-SNC-0.45 based on BJH method.

Fig.3.(a) XPS spectra;(b) C 1s spectra;(c) N 1s spectra and (d) S 2p spectra of Fe-SNC-0.45.
Performance of Fe-SNC-0.45 is further demonstrated by CV.As shown in Fig.4b,the cyclic voltammetry curves of both Fe-SNC-0.45 and Pt/C display a typical capacitor behavior in O2-free condition and the double-layer capacitance of Fe-SNC-0.45 is extremely large.Such large double-layer capacitance not only confirms the high-surface-area of Fe-SNC-0.45,but also demonstrates that the large surface area derived from numerous micro-and meso-pores can be easily infiltrated by liquid electrolyte,implying such porous structure can be fully used in catalysis process with no mass transport concern.Fig.4c shows the LSV curves in O2saturated 0.1 M HClO4solution with rotation speed varying from 100 rpm to 2500 rpm,all of which reach well-defined diffusion limited current density.The related Koutecky-Levich (K-L) plot is also illustrated in Fig.4c,and the electron-transfer number(n)of Fe-SNC-0.45 is calculated to be 3.70 based on this plot,indicating that four-electron pathway is dominant for ORR in acidic medium over Fe-SNC-0.45 surface.
Besides activity,durability is also critical for practical application.Therefore,ADT and chronoamperometric methods were adopted to evaluate the catalyst durability.As shown ini-tcurves of Fig.4d,the current of Fe-SNC-0.45 remains 90%of the initial value after 10 h test at a constant potential of 0.65 V(vs.RHE)in the O2-saturated electrolyte,in contrast,the current retention of Pt/C is 8%only after 10 h test under same condition.This phenomenon clearly demonstrates that the durability of Fe-SNC catalysts in acidic medium is better than that of Pt/C.Fig.4e and f display the ADT results,in which theE1/2decrease 2 mV only for Fe-SNC-0.45 after 10000 potential cycles while the corresponding value for Pt/C is 15 mV,further confirming the satisfactory electrochemical durability of Fe-SNC in acidic medium.
3.3.Electrochemical performance in alkaline medium
Recently,ORR catalysts in alkaline medium have received increasing attentions for its wide-use in electrochemical energy system such as alkaline fuel cell and metal-air batteries,therefore,the electrochemical performance of Fe-SNC catalysts in alkaline medium is also investigated using 0.1 M KOH as the electrolyte.As shown in LSV curves of Fig.5a,all these Fe-SNC catalysts perform much better than commercial Pt/C,among which Fe-SNC-0.55 is the best one that displays anEonsetof 1.05 V and anE1/2of 0.92 V.Considering the contents of Py-N and Fe-N are relatively high in Fe-SNC-0.55 compared with other samples(Table 1),it implies that both Py-N and Fe-N sites play a vital role in catalyzing ORR in alkaline medium which is consistent with previous reports [50,51].CV curves in Fig.5b confirm the large double-layer capacitance of Fe-SNC-0.55,that in turn demonstrates the high surface area derived from micro-and meso-pores is also accessible for ORR relevant active species in alkaline medium.Moreover,K-L plot in Fig.5c indicates the electron-transfer number of Fe-SNC-0.55 in alkaline medium is 3.98 which is superior to that in acidic medium,suggesting why Fe-SNC performs better in alkaline medium.
Excellent durability of Fe-SNC catalysts is also found.Durability test results are illustrated in Fig.5d-f,where the current retention of Fe-SNC-0.55 is as high as 94.3% after 10 h chronoamperometric test,in contrast,the current retention of Pt/C is 69%.ADT tests reveal thatE1/2of Fe-SNC-0.55 remains almost constant after 10000 potential cycles,which obviously surpasses Pt/C withE1/2decrease of 16 mV after same test.The simultaneous excellent activity and durability enable Fe-SNC catalysts to own great application potentials in alkaline medium.

Fig.4.(a) LSV curves of various catalysts tested in 0.1 M HClO4;(b) CV curves of Fe-SNC-0.45 and Pt/C in N2-saturated and O2-saturated 0.1 M HClO4;(c) LSV curves of Fe-SNC-0.45 at different rotation rates and corresponding K-L plots;(d)normalized i-t curves of Fe-SNC-0.45 and Pt/C tested at 0.65 V(vs.RHE);(e)ADT test result of Fe-SNC-0.45 after 10 000 potential cycles;(f) ADT test result of Pt/C after 10 000 potential cycles.
3.4.Effect of S-doping on catalytic activity
The role of S-doping in the high activity of Fe-SNC catalysts was investigated by density functional theory (DFT).4 models of S-doped Fe-N-C are considered in our DFT calculation as shown in Fig.6.Fig.6b and c show the structures which contain N-S bond and other model are used to describe the situation when N-S bond is nonexistent.According to the comparison of thermodynamic energy among 4 models(Fig.6b-e),the energy of non-NS-bond models are higher than others,which indicates that N-S bond is useful to stabilize the doping of sulfur.The calculation of ORR elementary step is performed on the models with N-S bond,although the experimental detection of N-S bond is hard due to the ultralow content of S.It is worth mentioning that the oxygen atom prefer to bond with S and it is hard to form OH.Thus,it could be deduced that the S site would be poisoned by O during ORR process as described by Fig.7a and b.The energy profile is calculated by DFT and the process could be described as follows:
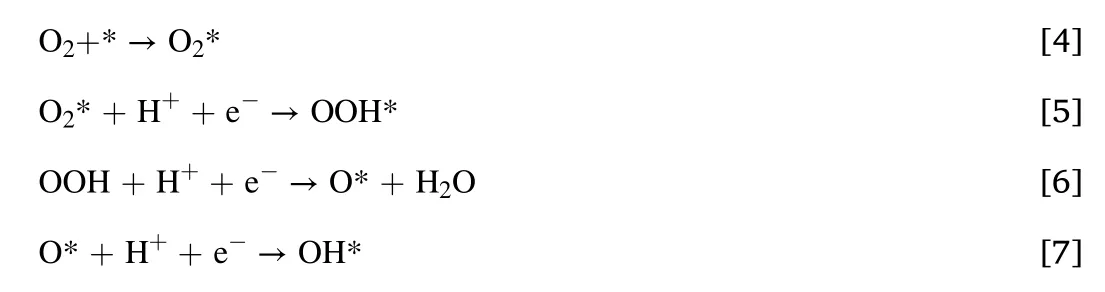

As shown in Fig.7c,compared with that on Fe-N4C surface without S doping,the ΔG of the elementary reaction is greater on S-doped FeN4C,which means a lower energy barrier of this reaction [40].Thus,the breaking of O-O bond,which is a key process in ORR and described as the energy barrier of the elementary reaction,becomes easier to occur on S-doped Fe-N4C sites,meaning that S-doping is able to promote the intrinsic activity.
4.Conclusion
In summary,an in-situ co-doping method is proposed to prepare Sdoped Fe-N-C catalysts in order to avoid the destruction of 3D porous structure derived from ZIF-8.This method brings three benefits,i.e.(i)creating a large specific area to increase the active site density;(ii) offering abundant meso-and micro-pores to ensure the sufficient oxygen transport towards active sites;(iii) boosting the intrinsic activity by Sdoping without compromising the structure property.As a results,Fe-SNC-0.45 exhibits a large specific surface area of 1173.51 m2g-1and consequent superior ORR activity in 0.1 M HClO4withEonsetof 0.95 V andE1/2of 0.81 V,which is among the best non-precious metal catalysts in acidic medium.Meanwhile,the composition-optimized catalyst in alkaline medium,Fe-SNC-0.55,exhibits a much better ORR activity than Pt/C does (E1/2=0.92 V for Fe-SNC-0.55vs.E1/2=0.84 V for Pt/C in 0.1 M KOH).Remarkable stability is also attained for Fe-SNC catalysts,demonstrated by ADT test and chronoamperometric test,which surpass commercial Pt/C catalyst in both acidic and alkaline medium.This work demonstrates that the prepared S-doped Fe-N-C catalysts do hold the potential to act as a substitute of PGM catalysts in fuel cell applications.

Fig.5.(a)LSV curves of various catalysts tested in 0.1 M KOH;(b)CV curves of Fe-SNC-0.55 and Pt/C in N2-saturated and O2-saturated 0.1 M KOH;(c)LSV curves of Fe-SNC-0.55 at different rotation rates and corresponding K-L plots;(d)normalized i-t curves of Fe-SNC-0.55 and Pt/C tested at 0.65 V(vs.RHE);(e)ADT test result of Fe-SNC-0.55 after 10000 potential cycles;(f) ADT test result of Pt/C after 10000 potential cycles.

Fig.6.Four kinds of catalyst structures marked as (a) Fe-N4C;(b) S-doping-1;(c) S-doping-2;(d) S-doping-3 and (e) S-doping-4.

Fig.7.(a) S-doping-1 and (b) S-doping-2 sites poisoned by O;(c) the energy profile of ORR elementary reaction.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the National Key Research and Development Program of China [grant number 2016YFB0101201];the National Natural Science Foundation of China [grant numbers 21706158,21533005].
 Progress in Natural Science:Materials International2020年6期
Progress in Natural Science:Materials International2020年6期
- Progress in Natural Science:Materials International的其它文章
- Key materials and technologies for fuel cells
- Experimental measurement of proton conductivity and electronic conductivity of membrane electrode assembly for proton exchange membrane fuel cells
- Nickel-introduced structurally ordered PtCuNi/C as high performance electrocatalyst for oxygen reduction reaction
- Surface modifications of Pt-based atomically ordered nanoparticles to improve catalytic performances for oxygen reduction reaction
- Accelerated Test of Silicone Rubbers Exposing to PEMFC environment
- A novel graphite/phenolic resin bipolar plate modified by doping carbon fibers for the application of proton exchange membrane fuel cells
