Surface modifications of Pt-based atomically ordered nanoparticles to improve catalytic performances for oxygen reduction reaction
Libo Zhang,Xiaoran Wang,Hong Zhu
State Key Laboratory of Chemical Resource Engineering,Institute of Modern Catalysis,Department of Organic Chemistry,Beijing Engineering Centre for Hierarchical Catalysts,School of Science,Beijing University of Chemical Technology,Chaoyang,Beijing,100029,China
ABSTRACT Bimetallic platinum-cobalt(Pt-Co)nanostructure catalysts represent superior catalytic performances for oxygen reduction reaction (ORR).In a variety of Pt-Co catalyst structures,atomically ordered structure catalysts show excellent catalytic performances in the ORR.In this work,for promoting their catalytic performances,atomically ordered PtCo nanoparticles (PtCo/C) with carbon supported were successfully prepared by an improved impregnation method and annealing.Then,the ordered PtCo/C catalysts have been significantly improved by doped with ultralow amount of Au and Cr transition metal.The physical and electrochemical test results demonstrate the Cr-PtCo/C and Au-PtCo/C catalysts have superior catalytic performances including mass activity and stability compared to commercialized Johnson Matthey (JM) Pt/C,which was the result of the modified electronic properties of Pt surface and atomically ordered structure.The presence of Au and Cr enhances the stability of PtCo/C catalysts.This work represents a simple way to promote the catalytic performances of the atomically ordered catalysts.
Keywords:Low-pt Proton exchange membrane fuel cells Atomically ordered structure Oxygen reduction reaction Surface modification
1.Introduction
Proton exchange membrane fuel cells(PEMFC)are excellent energy conversion devices with superior fuel efficiency,high power density,and low maintenance cost.Platinum-based nanoparticles are generally used as efficient catalysts for the electrodes of PEMFC [1-5].However,the scarcity,high cost,and high loading of Pt hinder the extensive applications of PEMFCs.Many efforts have been devoted to improving the Pt utilization,catalytic performances and decreasing the Pt loading[6-8].As oxygen reduction reaction (ORR) occurs on Pt surface,increasing the turnover frequency and utilization of Pt atoms on the surface can enhance ORR activity and reduce Pt consumption.Forming alloy of Pt with transition metal,such as Cu,Ni,Co,and Fe has been used for enhancing the turnover frequency of Pt atoms on the surface and reducing the usage of Pt [9-12].However,in these alloy phases of the catalysts,the atomic arrangement is usually disordered;Pt and the transition metal atoms are randomly distributed.The alloy catalysts show superior catalyst activity,but they are expected higher catalytic activity and satisfactory stability under the harsh operation environment of fuel cell.The unsatisfactory stability is due to transition-metal dissolution,particle agglomeration,and corrosion of the carbon support.Carbon supported atomically ordered catalyst shows excellent catalytic performances in the ORR[13-17].
In recent times,atomically ordered structure catalysts have attracted much attention.The ternary and binary atomically ordered structure catalysts have been reported and were certified to be excellent catalysts with superior performances in the ORR [18-21].The superior ORR catalytic performances were the result of the ordered structure and change of electronic properties.In general,the formation of ordered structure needs the control of the synthesized temperature and catalyst composition.Annealing can turn the structure of the catalysts from disordered structure into ordered structure,the annealing temperature has been devoted to change the crystal structure of the catalysts and improve the degree of alloying [22-26].For preparing highly active atomically ordered catalysts,we can control the particle size(<10 nm)of prepared catalysts to increase the catalytic active sites,and some efforts dedicated to prepare binary and ternary atomically ordered catalysts such as ordered PtCo/C,PtFeCu/C,and PtCoNi NPs[27-30].Wang and co-workers systematically synthesized ternary alloy PtFeCu/C catalyst by impregnation reduction method [31].The ternary alloy PtFeCu/C catalyst is prepared with the introduction of Cu into PtFe lattices.In this structure,the presence of Cu enhances the catalytic performances of catalysts.
Another effective method is to modify the electronic property of Pt surface by doped transition metals.The surface electronic structure can significantly affect the ORR activity [32,33].So we used surface modification method with trace transition metals to improve the catalytic performances of the atomically ordered catalysts.Huang and co-workers prepared bimetallic Pt3Ni/C catalyst,and doped this catalyst with transition metals [34].The Mo-Pt3Ni/C demonstrated the better ORR catalytic performances than commercial Pt/C catalysts.
In this study,the surface electronic properties of ordered catalyst are tuned by doped special element (Au,Cr).We successfully prepared surface-doped ordered PtCo/C catalysts with chromium (Cr) and gold(Au),named as Cr-PtCo/C and Au-PtCo/C.Firstly,the ordered PtCo/C catalysts with small particle size were synthesized by an improved impregnation method and annealing under appropriate temperature.Then,the PtCo/C catalysts were doped by small amount of chromium(Cr) and gold (Au) on the surface.The structure characteristics and catalytic performances of the prepared catalysts were evaluated by physics characterizations and electrochemical tests.The test results demonstrated that surface modification can obviously enhance the catalytic performance of ordered PtCo/C catalyst;we have prepared ORR catalysts that exhibit high activity and stability.This work developed an effective method to prepare surface modified atomically ordered catalysts.A schematic of the synthesis of orderedM-PtCo/C catalyst is supplied in Scheme 1.
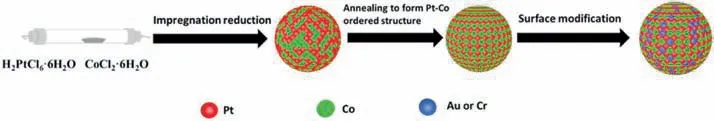
Scheme 1.Schematic of synthesis process of M-PtCo/C catalysts.
2.Experimental
2.1.Materials
Analytical grade chloroauric acid sodium(HAuCl44H2O),chromium chloride hexahydrate(CrCl3⋅6H2O),nitric acid(HNO3),ethylene glycol(EG),perchloric acid (HClO4),sodium borohydride (NaBH4),cobalt chloride hexahydrate (CoCl26H2O),and hexachloroplatinic acid(H2PtCl6⋅6H2O) were bought from J&K.Carbon black (EC-300J) was supplied by Chemical Shanghai Kajet Co.,Ltd.5 wt % Nafion was obtained from Dupont.
2.2.Preparation of atomically ordered PtCo/C catalysts
PtCo/C catalysts were synthesized by an improved impregnation method and annealing,and the Pt to Co molar ratio is 1:1.Impregnation method is a facile route,and we can obtain small particle size and high distribution NPs by this preparation method.40.7 mg of H2PtCl66H2O and 8.67 mg of CoCl26H2O were dissolved in 8 ml deionized water,and 80 mg of the carbon powder functionalized by nitric acid was added to the solution.The smooth thick slurry was obtained in ultrasonic sound bath for 30 min.After drying in a drying oven at 80°C for 10 h,the mixture was ground in an agate mortar.The powder was reduced in a tube furnace under flowing 5%H2/95%Ar at 200°C for 3 h.Then,the powder annealed under flowing 5%H2/95%Ar at 700°C for 2 h to form atomically ordered intermetallic(noted as PtCo/C).
2.3.Surface modification of ordered PtCo/C catalysts
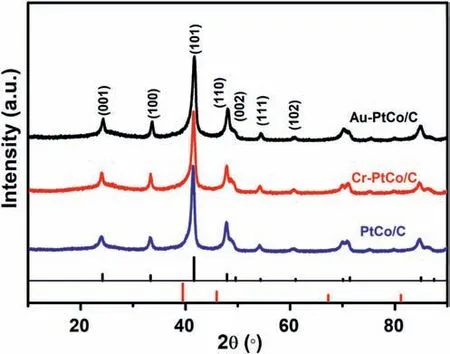
Fig.1.XRD patterns of Cr-PtCo/C,Au-PtCo/C,and PtCo/C catalysts.The red and black lines corresponding to the cards of pure Pt(PDFcard#87-0640)and intermetallic PtCo (PDFcard # 29-0498),respectively.
The surface modification of the ordered PtCo/C catalyst was synthesized by the addition of dopant Cr and Au precursors,the obtained catalysts were named as Cr-PtCo/C and Au-PtCo/C,respectively.The Cr-PtCo/C was prepared by the liquid phase reduction method with NaBH4.First,50 μl of CrCl36H2O(20 g/l)aqueous was added to 20 ml PtCo/C dispersion consist of 40 mg PtCo/C catalyst under magnetic stirring.Then,the NaBH4solution (1 M,10 ml) was added to the solution as reducing agent.After magnetic stirring under room temperature for 6 h,the sample was centrifuged and cleaned with deionized water and ethanol two times,and dried in a drying oven at 60°C for 8 h.The Au-PtCo/C was prepared by a superficial displacement reaction.The 40 mg PtCo/C was ultrasonically dispersed in 20 ml deionized water.Then 50 μl of HAuCl44H2O solution(20 g/l)was added to the above system with stirring.The solution was stirred for 4 h under room temperature,and the sample was centrifuged and cleaned with deionized water and ethanol two times,and dried in a drying oven at 60°C for 8 h.
2.4.Physics characterizations
Transmission electron microscopy (TEM,JEOL 2010 microscope) is used to characterize the morphology and distribution of obtained catalysts.X-ray diffraction (XRD,Shimadzu XD-3A diffractometer,Japan)was characterized the crystal structures of the obtained catalysts.Inductively coupled plasma (ICP,Agilent Technologies,USA) was used to measure the compositions of the catalysts.Thermo Fisher X-ray photoelectron spectrometer (XPS,USA,LAB250ESCA) was used to test the surface electronic structure of the catalysts.
2.5.Electrochemical tests
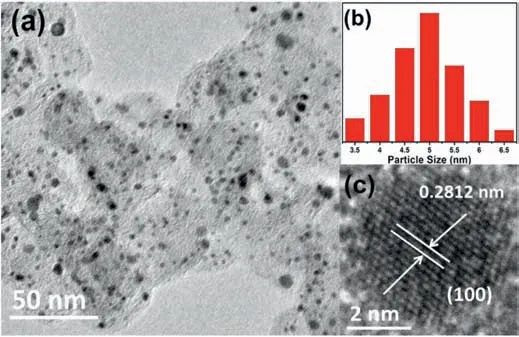
Fig.2.TEM image and particle size distribution histograms(a,b),and HRTEM image of PtCo/C catalyst (c).
The electrochemical tests were evaluated by a Germany Zennium electrochemical workstation (Zahner IM6e) with the three-electrode system.This system consists of a counter electrode (Pt wire),a reference electrode(silver-silver chloride electrode)and a working electrode.In this work,all the potentials were reported versus the reversible hydrogen electrode(RHE).The 20 μl of the catalysts was coated on the glassy carbon (GC) electrode to prepare the working electrode (area:0.196 cm2).The catalysts powder was dissolved in the mixing solution consisted of deionized water (750 μl),Nafion solution (5 μl),and isopropyl alcohol (250 μl).The cyclic voltammetry (CV) curves were obtained in HClO4(0.1 M,N2saturated)electrolyte at a scan rate of 50 mV s-1from 0.025 to 1.075 V.The linear sweep voltammetry(LSV)curves were collected in HClO4(0.1 M,O2saturated) electrolyte at a rotation rate of 1600 rpm and a scan rate of 10 mV s-1from 0.05 to 1.1 V.
3.Result and discussion
3.1.Structural and compositional results
The XRD patterns of ordered catalysts Cr-PtCo/C,Au-PtCo/C,and PtCo/C are supplied in Fig.1.The broad peaks corresponding to carbon support are at around 2θ=25°.The red and black lines are corresponding to the cards of pure Pt(PDFcard#87-0640)and intermetallic PtCo (PDFcard # 29-0498),respectively.The characteristic peaks at around 2θ=39.8°,46.9°,67.8°and 81.3°are attributed to the (111),(200),(220) and (311) of Pt crystal planes,respectively [35,36].However,four additional diffraction peaks can be observed at about 23°for(001),33°for (100),53°for (111),and 58°for (102) from the XRD patterns of PtCo/C,Cr-PtCo/C and Au-PtCo/C.The additional characteristic peaks indicate that ordered phase is formed[37].The diffraction peaks of the Cr-PtCo/C,Au-PtCo/C,and PtCo/C catalysts shift slightly in the positive direction compared to Pt/C.The shift of characteristic peaks is attributed to Co embedded into Pt lattice to cause lattice contraction to form alloy phase [38].The Cr-PtCo/C and Au-PtCo/C catalysts also possess similar characteristic peaks compared to PtCo/C.However,the intensity of the characteristic peaks was decreased after surface doping,which was attributed to the modification of surface crystal phase[39].
TEM and HRTEM images (as shown in Fig.2) are used to study the microstructure characteristics of PtCo/C catalysts.From Fig.2 (a)image,the ordered PtCo/C catalysts have high distribution on the carbon supports with little agglomeration.The average crystal size of PtCo/C was calculated to be 4.8 nm[40].The HRTEM image reveals that the PtCo/C NPs are spherical.From the HRTEM image,the PtCo/C catalysts have clear crystal lattice fringes.The lattice spacing (0.2812 nm) is attributed to (100) superlattice planes,demonstrating that the ordered structure is formed [41,42].As seen from Fig.S1,the Cr-PtCo/C and Au-PtCo/C catalysts showed similar microstructural characteristics compared to PtCo/C catalysts after surface modification.The average crystal size of Cr-PtCo/C and Au-PtCo/C was calculated to be 4.7 nm and 4.6 nm,respectively.The Cr-PtCo/C and Au-PtCo/C were also tested using HRTEM in Fig.S2.The existence of(100)superlattice plane was also clearly observed.This indicted atomically ordered structure was well-preserved after modification.
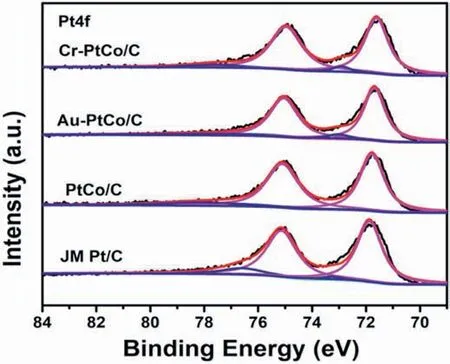
Fig.3.XPS spectra of PtCo/C,Au-PtCo/C,Cr-PtCo/C,and JM Pt/C in the Pt 4f region.
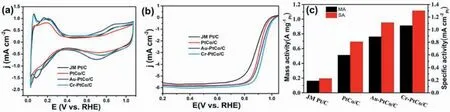
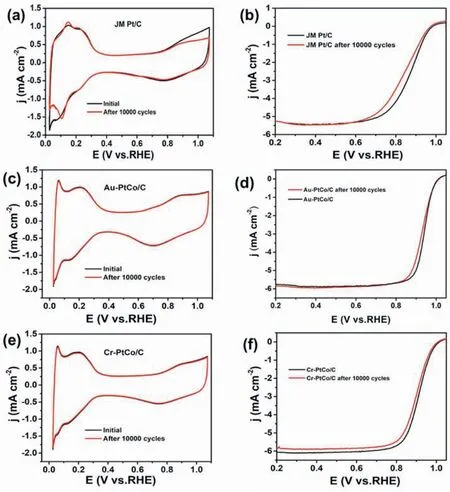
The composition of the as-synthesized catalysts was tested by the ICP test,and the surface electronic properties of Pt were determined by XPS,the components were provided in Table S1.The Pt 4f XP spectrum of PtCo/C,Au-PtCo/C,Cr-PtCo/C,and JM Pt/C was shown in Fig.3;the three peaks at about 71,72.4,and 74.8eV,which can be attributed to the Pt 4f7/2peaks of Pt0,PtII,and PtⅣ,respectively,demonstrate Pt is existed in the form of three oxidation states on the surface[43,44].The binding energy of Pt increased in the order of JM Pt/C The electrochemical performances of the as-obtained catalysts were tested for the ORR and the JM Pt/C catalysts were also tested for comparison.Fig.4(a)shows the CV curves of all the catalysts in N2-purged 0.1 M HClO4.As we can be seen from the curves,all the catalysts demonstrate the typical features of Pt.The peak near 0.1 V vs.RHE is attributed to the hydrogen adsorption peak,and the peak near 0.75 V vs.RHE is attributed to the oxygen reduction peak [47].After surface modification,the hydrogen adsorption area of Cr-PtCo/C and Au-PtCo/C catalysts display an increase.This demonstrated there is an increase in catalytic active sites,which is attributed to remove Co or Co oxides on the surface.Integrating the hydrogen adsorption range from 0.05 to 0.4 V can calculate the electrochemically active surface area(ECSA),which can evaluate the Pt utilization.The results are shown in Table S2.The Pt utilization was increased as a result of surface modification according to the ECSAs. Fig.4.Electrochemical testing results of JM Pt/C,PtCo/C,Au-PtCo/C,and Cr-PtCo/C catalysts,(a) CV curves,(b) LSV curves,(c) Mass and specific activities of the catalysts. Fig.5.Durability test CV results of (a) JM Pt/C,(c) Au-PtCo/C,and (e) Cr-PtCo/C catalysts;durability test LSV results of (b) JM Pt/C (d) Au-PtCo/C,and (f)Cr-PtCo/C catalysts. The LSV curves of the ORR obtained from the as-synthesized catalysts were supplied in Fig.4 (b).The curve of the JM Pt/C catalysts is also supplied in Fig.4 (b) for comparison.As can be seen from the images,all the atomically ordered catalysts have higher half-wave potentials(E1/2)than JM Pt/C catalysts.The E1/2of the catalysts increased in the order of JM Pt/C Fig.6.HRTEM images of Cr-PtCo/C (a) and Au-PtCo/C (b) catalysts after stability tests. A good stability of ORR catalyst is an important requirement for fuel cell applications.The catalysts durability is evaluated by using potential cycling stability test in the short time [49].The durability tests were employed by repeating 10,000 cycles at a scan rate of 500 mV s-1in 0.1 M N2-saturated HClO4electrolyte between 0.06 and 1.10 V.The stability of Cr-PtCo/C,Au-PtCo/C,and JM Pt/C were tested in the same conditions.Then the CV curves before and after 10,000 cycles were supplied in Fig.5(a),(c),and(e).The hydrogen adsorption area of JM Pt/C significantly reduced after 10,000 cycles,demonstrating poor structural stability.However,the CV tests of Cr-PtCo/C and Au-PtCo/C demonstrate the hydrogen adsorption peak of these catalysts have little difference,showing the catalytic sites are well preserved.The difference in hydrogen adsorption peak shows that the Cr-PtCo/C and Au-PtCo/C have higher structure stability than JM Pt/C,because of modification of trace transition metals Au,Cr and atomically ordered structure.To further evaluate the catalyst durability,Fig.5(b),(d),and(f)shows the LSV curves of the JM Pt/C,Cr-PtCo/C and Au-PtCo/C catalysts for initial curves and those after durability tests,respectively;obviously the large negative shifts of E1/2is for the JM Pt/C catalysts,which display poor structural stability.As we can see,the negative shifts in E1/2of Au-PtCo/C,Cr-PtCo/C,and JM Pt/C is 7 mV,12 mV,and 40 mV,respectively.The surface-doped catalysts show the enhanced catalytic stability for the ORR.The excellent stability is due to the surface modification of Cr and Au and the atomically ordered structure.The Au-PtCo/C possesses higher stability compared to Cr-PtCo/C,because Au has the better stabilizing effect on Pt. The TEM and HRTEM characterization results of Cr-PtCo/C and Au-PtCo/C after durability tests were also supplied in Fig.S4.The catalysts still have highly dispersed.The particle size of the Cr-PtCo/C and Au-PtCo/C catalysts has no obvious increase,which can also explain the excellent structural stability of the Cr-PtCo/C and Au-PtCo/C.The HRTEM images of Cr-PtCo/C and Au-PtCo/C catalysts are shown in Fig.6(a)and(b)after durability tests.The superlattice(100)plane of the NPs can be clearly seen,showing the well preservation of ordered structure.The durability tests show that the prepared Cr-PtCo/C and Au-PtCo/C catalysts possess higher structure stability compared to JM Pt/C catalysts. For studying the catalytic mechanism for ORR,the different rotation rates in the LSV test of the Cr-PtCo/C and Au-PtCo/C catalysts with ranging from 400 to 2500 rpm were recorded in HClO4electrolyte(0.1 M,O2saturated) [50];the test curves with Koutecky-Levich plots are supplied in Fig.S4.For Cr-PtCo/C and Au-PtCo/C catalysts,the electron transfer number was estimated to be 3.93 and 3.84 between 0.60 V and 0.80 V,respectively,demonstrating a nearly complete four-electron O2reduction process of these catalysts. In this work,an improved impregnation method and annealing were used to synthesize ordered PtCo/C catalyst with small particle size.After doped process,we synthesized modified Cr-PtCo/C and Au-PtCo/C catalysts,and the prepared catalysts were tested by XRD,TEM,HRTEM,ICP,XPS,CV,and LSV.These catalysts outperform JM Pt/C catalysts for the ORR.The catalytic performances of the ordered PtCo/C catalysts can be significantly enhanced by surface modification of Cr and Au,which obviously modified the surface electronic property of Pt.This improved ORR performances can be attributed to the ordered structure,increased catalytic active sites and tuned surface microstructure.This work presents a feasible and facile way to improve the catalytic performances of atomically ordered catalysts used in the oxygen reduction reaction. Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgements We gratefully appreciate the Joint Funds of the National Natural Science Foundation of China (No.U1705253 and 51961145107),the National Natural Science Foundation of China (No.21776014 and 21776012),the financial supports from the National Key Research and Development Program of China (No.2016YFB0101203),the International S&T Cooperation Program of China (No.2013DFA51860),and the Fundamental Research Funds for the Cornell University (No.12060093063). Appendix A.Supplementary data Supplementary data to this article can be found online at https://doi.org/10.1016/j.pnsc.2020.10.013.3.2.Electrochemical tests

4.Conclusion
 Progress in Natural Science:Materials International2020年6期
Progress in Natural Science:Materials International2020年6期
