Description of a New Species of Amolops(Anura:Ranidae)from Tibet,China
Shuo QI ,Zhengyan ZHOU ,Zhitong LYU,Yuyan LU,Han WAN,Mian HOU,Keji GUO and Pipeng LI
1 Institute of herpetology, Shenyang Normal University, Shenyang 110034, Liaoning, China
2 The Museum of Biology, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, Guangdong, China
3 College of Continuing (Online) Education, Sichuan Normal University, Chengdu 610068,Sichuan, China
4 Central South Inventory and Planning Institute of National Forestry and Grassland Administration, Changsha 410014,Hunan, China
Abstract A new species,Amolops pallasitatus sp.nov.is described based on specimens collected from Chentang Town,Dinggyê County,southern Tibet,China.The new species can be distinguished from other known congeners by mitochondrial divergence and morphological characteristics including:(1)medium body size,SVL 70.6-72.3 mm in adult females;(2)skin smooth over the entire body;(3)absence of dorsolateral fold;(4)tympanum small,edge indistinct,less than half of eye diameter;(5)vomerine teeth in two short oblique;(6)circummarginal and transverse grooves absent on disk of the first finger;(7)presence of inner metacarpal tubercle;(8)toes fully webbed,webbing formula I 0-0- II 0-½ III0-1+ IV 1+-0 V;(9)absence of outer metatarsal tubercle and tarsal glands;(10)tibiotarsal articulation of the hind limb reaches posterior corners of the eye;(11)dorsum yellow-green,with irregular dark brown blotches without margins;(12)blotches concentrated on the dorsum,less on the flanks.In morphology,Amolops pallasitatus sp.nov.is similar to A.himalayanus and A.formosus,the difference between them is length of hind limbs,web of toe and dorsal colour pattern.The systematic placement of the new species within the genus is unresolved and it is not assigned to any recognized species group,for the lack of convictive evidences.
Keywords Amolops pallasitatus sp.nov.,mitochondrial,morphology,unknown species group,torrent frog
1.Introduction
The ranid genusAmolopsCope,1865,as a typical representative for Oriental fauna,is widespread from Nepal and northern India,eastward to southeastern mainland China,and southward to Malaya Peninsula(Frost,2019).These frogs inhabit swift,rocky streams,including torrents,rapids and waterfalls,enabled by abdominal suckers in larvae and enlarged digital pads in adults,which lead to their common name“torrent frogs”or“sucker frogs”.
Tibet is an important element across two biodiversity hotspots of the world,the Himalaya Hotspot and Indo-Burma Hotspot,which is well-known as the hottest of the hotspots(Tordoffet al.,2012).The mountains rise abruptly,resulting in a diversity of ecosystems that range from alluvial grassland and subtropical broadleaf forests to alpine meadows above the tree line.This area also holds remarkable endemism and is the home to the important populations of numerous amphibians and reptiles.
Previous studies have reported eight species of genusAmolopsin this region:A.aniqiaoensisDong,Rao,and Lü,2005,A.chayuensisSun,Luo,Sun,and Zhang,2013,A.formosus(Günther,1876),A.gerbillus(Annandale,1912),A.marmoratus(Blyth,1855),A.medogensisLi and Rao,2005,A.monticola(Anderson,1871)andA.nyingchiensisJiang,Wang,Xie,Jiang,and Che,2016.During our herpetological surveys in Chentang Town,Dinggyê County,southern Tibet,China(Figure 1),two specimens ofAmolopswere collected in June 2015.The morphological examinations and further molecular analysis supported the two specimens represented an independent evolutionary lineage of genusAmolopsand can be consistently distinguished from other known molecular data congeners.Therefore in this study we describe them as a new species,whose phylogenetic placement is also discussed.
2.Materials and Methods
2.1.SamplingTwo individuals of the new species were collected from Chentang Town,Dinggyê County,southern Tibet,China.Following euthanasia,all specimens were fixed in 10% buffered formalin solution for preservation after sampling of liver tissues from the adults in 95%ethanol,and subsequently transferred to 75% ethanol.All specimens were deposited in The Institute of herpetology,SYNU.We obtained diagnostic characters ofA.formosus(Günther,1876),A.himalayanus(Boulenger,1900),A.medogensisandA.viridimaculatus(Jiang,1983)from literature(Feiet al.,2012;Liet al.,2010;Nidupet al.,2016;Schleich and Kästle,2002;Subbaet al.,2016).In addition,a total of 25 museum specimens of eight species ofAmolopswere examined for comparison,which are listed in Appendix I.
2.2.Taxon samplingA total of 29 samples of genusAmolopswere used for molecular analysis in this study.All samples were attained from euthanasia specimens and then preserved in 95% ethanol and stored at-40°C.In addition,43 sequences were obtained from GenBank and incorporated into our dataset for phylogenetic analysis.Detail information of these materials is shown in Table 1.
2.3.Extraction,PCR amplification,and sequencingGenomic DNA was extracted from muscular sample by using a DNA extraction kit from Tiangen Biotech(Beijing)Co.,Ltd.Partial 16S ribosomal RNA gene(16S)and partial cytochrome C oxidase 1 gene(CO1)were amplified.Primers used for 16S were L3975(5'-CGCCTGTTTACCAAAAACAT-3')and H4551(5'-CCGGTCTGAACTCAGATCACGT-3'),and L2A(5'-CCAAACGAGCCTAGTGATAGCTGGTT-3')and H10(5'-TGATTACGCTACCTTTGCACGGT-3'),and for CO1 were Chmf4(5'-TYTCWACWAAYCAYAAAGAYA TCGG-3')and Chmr4(5'-ACYTCRGGRTGRCCRAAR AATCA-3'),and dgLCO(5'-GGTCAACAAATCATAAA GAYATYGG-3')and dgHCO(5'-AAACTTCAGGGTGA CCAAARAAYCA-3')following Lyuet al.,(2019).PCR amplifications were processed in a 20 reaction volume with the cycling conditions with an initial denaturing step at 95°C for 4 min,35 cycles of denaturing at 94°C for 40 s,annealing at 53°C(for 16S)/48°C(for CO1)for 40 s and extending at 72°C for 1 min,and final extending step of 72°C for 10 min.PCR products were purified with spin columns.The purified products were sequenced with both forward and reverse primers using a BigDye Terminator Cycle Sequencing Kit per the guidelines,on an ABI Prism 3730 automated DNA sequencer by Shanghai Majorbio Bio-pharm Technology Co.,Ltd and Beijing Genomics Institute.All sequences were deposited in GenBank(Table 1).

Figure 1 Map showing the collecting location of Amolops pallasitatus sp.nov.indicated by red star.
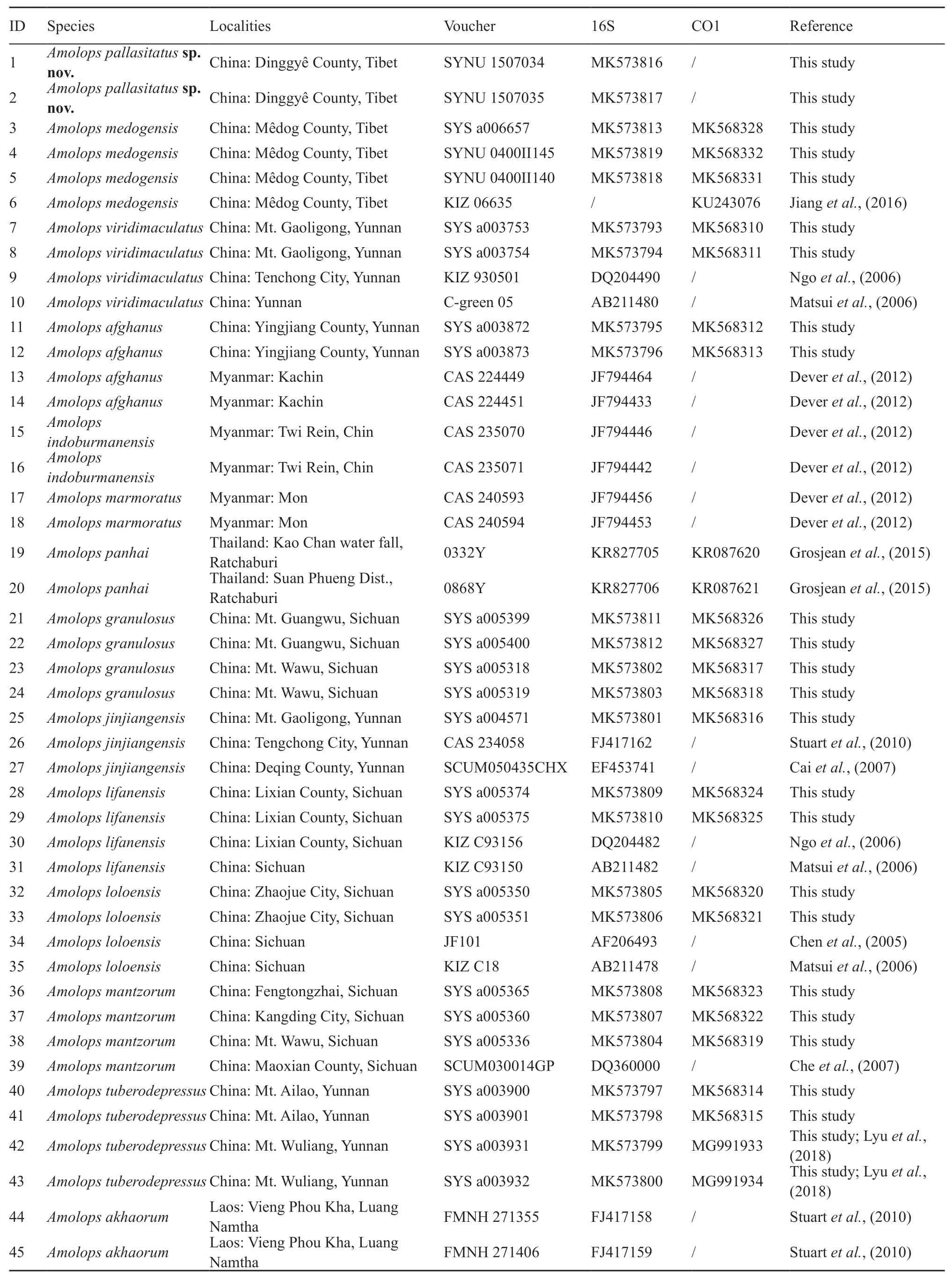
Table 1 Localities,voucher information and GenBank numbers for all samples used in this study.

Continued Table 1
2.4.Phylogenetic analysisDNA sequences were aligned by the Clustal W algorithm with default parameters(Thompsonet al.,1997)and trimmed with the gaps partially deleted in MEGA 6(Tamuraet al.,2013),while within highly variable regions,all gaps were removed.Two genes,1080 base pairs(bp)of 16S and 639 bp of CO1,were concatenated seriatim into a 1719-bp sequence,and further divided into two partitions by gene.Partitions were tested respectively in jmodeltest v2.1.2 with Akaike and Bayesian information criteria,all resulting in the best-fitting nucleotide substitution models of GTR+I+G.Sequenced data were analyzed using maximum likelihood(ML)implemented in RaxmlGUI 1.3(Silvestro and Michalak,2012)with the sequences ofA.rickettias out-group.The bootstrap consensus tree inferred from 1000 replicates was used to represent the evolutionary history of the taxa analyzed.The phylogenetic tree was displayed and annotated using iTOL v3(Letunic and Bork,2016).Because most of the GenBank sequences lacked CO1 gene data,pairwise distances(p-distance)were calculated only for 16S in MEGA 6,using the uncorrectedp-distance model.
2.5.Morphology and morphometricsMeasurements follow Feiet al.,(2009)and Lyuet al.,(2019),webbing formula followed Savage and Heyer(1997)and were taken with digital calipers(Mitutoyo 500-752-20 Stainless Steel Digital Caliper,Japan)to the nearest 0.1 mm,including snout-vent length(SVL)from tip of snout to posterior margin of vent,head length(HDL)from tip of snout to the articulation of the jaw,head width(HDW)at the commissure of the jaws,snout length(SNT)from tip of snout to the anterior corner of the eye,internasal distance(IND),interorbital distance(IOD),eye diameter(ED)from the anterior corner of the eye to posterior corner of the eye,tympanum horizontal diameter(TD),tympanum-eye distance(TED)from anterior edge of tympanum to posterior corner of the eye,hand length(HND)from distal end of radioulna to tip of distal phalanx III,radioulna length(RAD)from the flexed elbow to the base of the outer palmar tubercle,foot length(FTL)from distal end of tibia to tip of distal phalanx IV,tibial length(TIB)from the outer surface of the flexed knee to the heel,hindlimb length(HLL)from the vent to tip of distal phalanx IV,width of finger III digital disc(F3W)and width of toe IV digital disc(T4W).We determined sex by observation of secondary sexual characters,i.e.the presence of nuptial pads in males.In the webbing formula,Roman numbers refer to the fingers and toes,and the Arabic number refer to the number of subarticular tubercles.
3.Results
3.1.Morphological comparisonThe unnamedAmolopsspecimens from Dinggyê County,southern Tibet were compared with all recognized species of the genus,and they can significantly differ from the congeners by the following characteristics:(1)absence of dorsolateral fold[vs.present inA.akhaorum,A.aniqiaoensis,A.archotaphus,A.bellulus,A.chakrataensisRay,1992,A.chayuensis,A.chunganensis,A.compotrix,A.cremnobatusInger and Kottelat,1998,A.cucae,A.daorum,A.gerbillus,A.granulosus(Liu and Hu,1961),A.iriodes,A.jaunsariRay,1992,A.jinjiangensisSu,Yang,and Li,1986,A.kohimaensisBiju,Mahony,and Kamei,2010,A.longimanus(Andersson,1939),A.mengyangensisWu and Tian,1995,A.minutusOrlov and Ho,2007,A.monticola,A.nyingchiensis,A.vitreaandA.wenshanensis];(2)presence of vomerine teeth[vs.absent inA.daiyunensis(Liu and Hu,1975),A.hainanensis(Boulenger,1900),A.hongkongensis(Pope and Romer,1951),A.torrentis(Smith,1923)andA.wuyiensis(Liu and Hu,1975)];(3)dorsal and lateral skin absolutely smooth in adult females[vs.dorsal and lateral rough inA.albispinusSung,Hu,Wang,Liu,and Wang,2016,A.australisChan,Abraham,Grismer,and Grismer,2018,A.gerutuChan,Abraham,Grismer,and Grismer,2018,A.larutensis(Boulenger,1899),A.ricketti,A.sinensisLyu,Wang,and Wang,2019,A.yatseniLyu,Wang,and Wang,2019 andA.yunkaiensisLyu,Wang,Liu,Zeng,and Wang,2018,or dorsal and lateral granular inA.afghanus,A.marmoratus,A.panhaiandA.spinapectoralisInger,Orlov,and Darevsky,1999,or smooth middorsally but tuberculate laterally inA.assamensisSengupta,Hussain,Choudhury,Gogoi,Ahmed,and Choudhury,2008,A.indoburmanensis,A.kaulbacki(Smith,1940),A.lifanensis,A.loloensis,A.mantzorum,A.tuberodepressusandA.xinduqiaoFei,Ye,Wang,and Jiang,2017)];(4)Tibio-tarsal articulation of the hind limb reaches posterior corners of the eye[vs.reaches the nostril or the tip of the snout inA.formosus];(5)tibia significantly shorter than the trunk[vs.tibia as long as the trunk inA.himalayanus];(6)dorsum with irregular dark brown blotches without margins[vs.dorsum with irregular dark brown blotches with black margins or entirely black inA.formosus,or dorsum with numerous small round lightyellow spots inA.caelumnoctisRao and Wilkinson,2007 andA.splendissimusOrlov and Ho,2007,or dorsum with small irregularly arranged cobalt green spots inA.nidorbellusBiju,Mahony,and Kamei,2010];(7)blotches concentrated on the dorsum,less on the flanks[vs.blotches covered the dorsum and flanks inA.medogensisandA.viridimaculatus];(8)webbing formula I 0-0-II 0-½ III0-1+IV 1+-0 V[vs.I 0-1½ II 0-2+III 0-2-IV 2--0 V in front inA.formosus].
In morphology,these unnamed specimens are similar toA.himalayanusandA.formosus,more principal morphological differences between the three taxa are given in Table 2.The detailed morphological comparisons indicated that the unnamed specimens are significantly different fromA.formosusby its shorter hindlimb,tibiotarsal articulation reaches posterior corners of the eye(vs.reaches the nostril or the tip of the snout),webbingformula I 0-0-II 0-½ III0-1+IV 1+-0 V(vs.I 0-1½ II 0-2+III 0-2-IV 2--0 V),skin without spinules(vs.spinules limited to side of the head and base of the forelimb),dorsum yellow-green above with irregular dark brown blotches without margins on the head and body;blotches concentrated on the dorsum,less on the flanks(vs.dorsum olive or greyish above with indistinct darker spots on the body),lower parts light yellow and whitish(vs.brownish or pale olive).
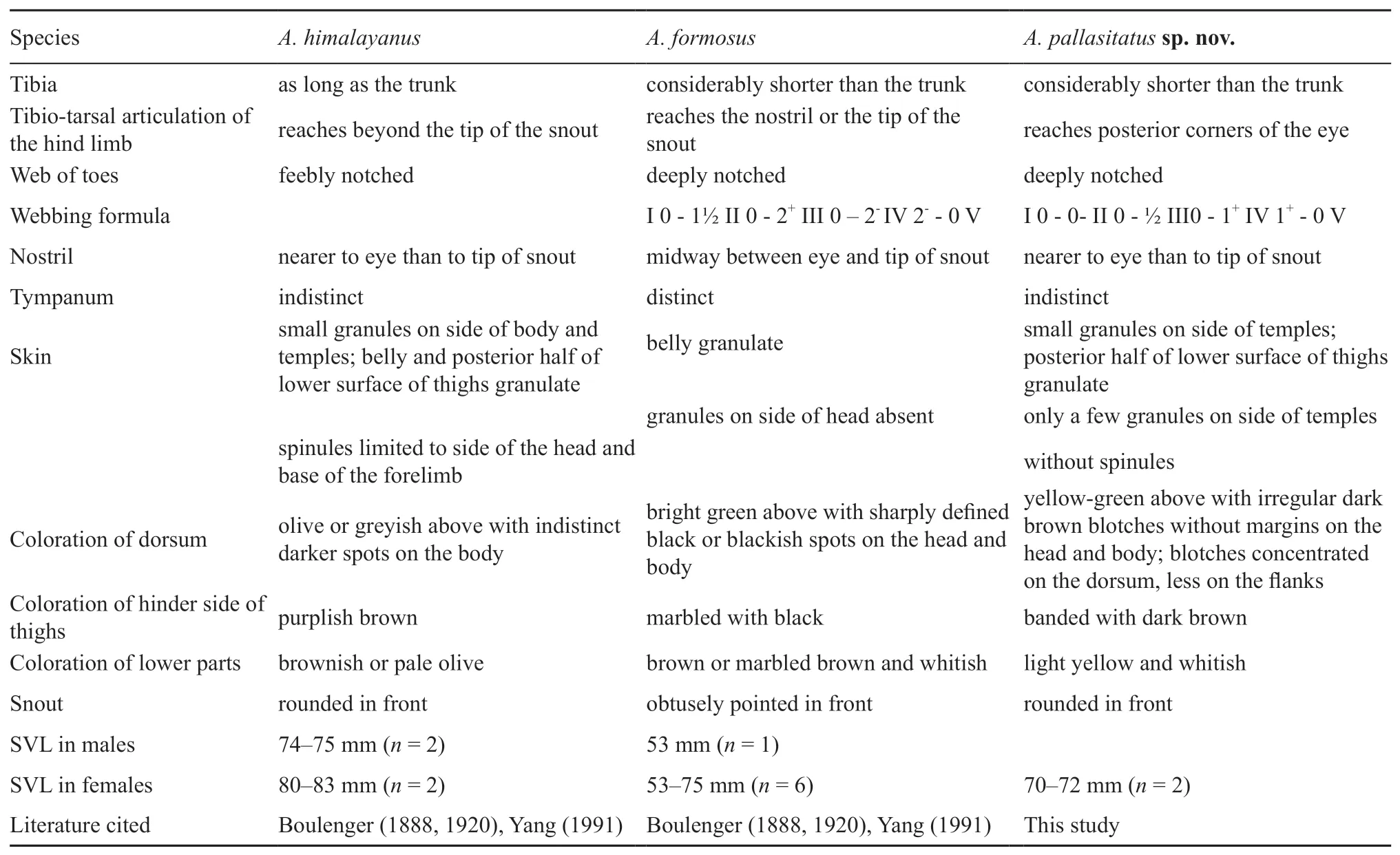
Table 2 The principal morphological differences between A.himalayanus (Boulenger,1888),A.formosus (Günther,1875)and A.pallasitatus sp.nov.,reference from Nidup et al.,(2016).
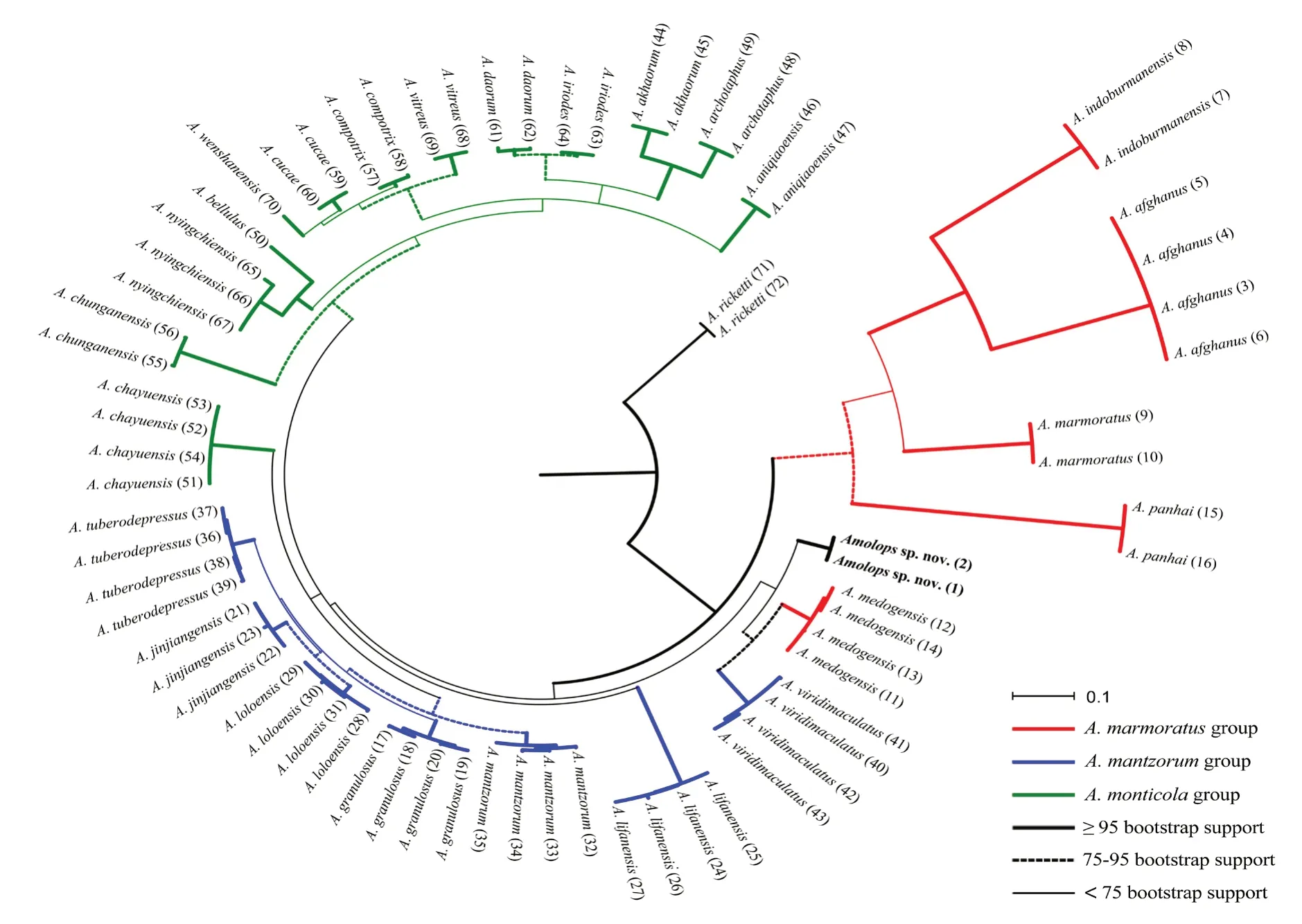
Figure 2 Maximum-likelihood phylogenic tree.Numbers in parentheses coresponding to the ID in Table 1.

Figure 3 Holotype(SYNU 1507035,female)of Amolops pallasitatus sp.nov.in life.Photo by Zhengyan ZHOU.
3.2.Phylogenetic analysesThe ML phylogenetic tree was shown in Figure 2.According to the phylogenetic results,theAmolopsspecimens from Dinggyê County,Tibet,gathered together in a lineage with strong support value and without genetic distance(bootstrap support=100,p-distance=0).Despite of the lineage being a sister taxon to(A.medogensis+A.viridimaculatus),the taxonomic placement of this lineage was unresolved for the relatively insignificant bootstrap supports of<75.The lowestp-distances between this lineage and a known congener(A.akhaorum)was 2.4%-2.4%,which was significant when compared to the values among described species,for example,betweenA.tuberodepressusandA.jinjiangensis(p-distances of 1.7%-2.0%).These results indicate that there are substantial genetic divergences between the recognizedAmolopsspecies and the lineage from Dinggyê County,Tibet,indicating that these two specimens represented a separately evolving lineage within the genus.
Based on the molecular and morphological evidences,we hereby describe the specimens from Dinggyê County,Tibet as a new species,Amolops pallasitatussp.nov.
3.3.Taxonomic account
Amolops pallasitatus Qi,Zhou,Lyu,Lu &Li,sp.nov.Holotype:SYNU 1507035(Figures 3,4,5),adult female,collected by Zhengyan ZHOU on June 2015 from a stream close to the China-Nepal border(27.92° N,87.47°E;3270 m a.s.l.),Chentang Town,Dinggyê County,Tibet Autonomous Region,China.
Paratype:SYNU 1507034(Figure 6),adult female,the same collection information as the holotype.

Figure 4 Holotype of Amolops pallasitatus sp.nov.in preservative.A and B:Dorsal and ventral view of the holotype;C:Ventral view of the left hand;D:Ventral view of the left foot;E:Lateral view of the head.Photos by Shuo QI.

Figure 5 Shows the tibio-tarsal articulation of the hind limb reaches posterior corners of the eye(SYNU 1507035).Photo by Zhengyan ZHOU.
Diagnosis.Amolops pallasitatussp.nov.is distingusished from its congeners by a combination of the following morphological characteristic:(1)medium body size,SVL 70.6-72.3 mm in adult females;(2)skin smooth over the entire body;(3)absence of dorsolateral fold;(4)tympanum small,edge indistinct,less than half of eye diameter;(5)vomerine teeth in two short oblique;(6)circummarginal and transverse grooves absent on disk of the first finger;(7)presence of inner metacarpal tubercle;(8)toes fully webbed,webbing formula I 0-0-II 0-½ III0-1+IV 1+-0 V;(9)absence of outer metatarsal tubercle and tarsal glands;(10)tibio-tarsal articulation of the hind limb reaches posterior corners of the eye;(11)dorsum yellow-green,with irregular dark brown blotches without margins;(12)blotches concentrated on the dorsum,less on the flanks.
Description of holotypeAdult female,body stout,SVL 72.3 mm,medium size in the genusAmolops.Head flattened,width slightly smaller than length(HDW/HDL=0.91);snout short(SNT/HDL=0.40)and rounded in profile,projecting beyond lower jaw;nostril closer to eye than tip of snout;eye large(ED/HDL=0.29)and convex;canthus rostralis rounded;pineal body invisible;tympanum small,edge indistinct,less than half of eye diameter(TD/ED=0.42);tympanum-eye distance larger than tympanum,TED/TD 1.10;supratympanic fold distinct;vomerine teeth distinct,on two short oblique between choanae,converging posteriorly,teeth ridges longer than space between them;tongue cordiform,deeply notched posteriorly.
Forelimbs moderately long;hands moderately long(HND/SVL=0.36);relative finger lengths I<II=IV<III;tip of the first finger slightly dilated,large disks with circummarginal grooves on tips of three outer fingers,relative width of finger disks I<II<III=IV;subarticular tubercles prominent,rounded;supernumerary tubercles indistinct below the base of fingers II,III and IV,butabsent below the base of fingers I;inner metacarpal tubercles oval,outer metacarpal tubercle separated with the outer part flat and barely visible;absence of webbing and lateral fringes on fingers.
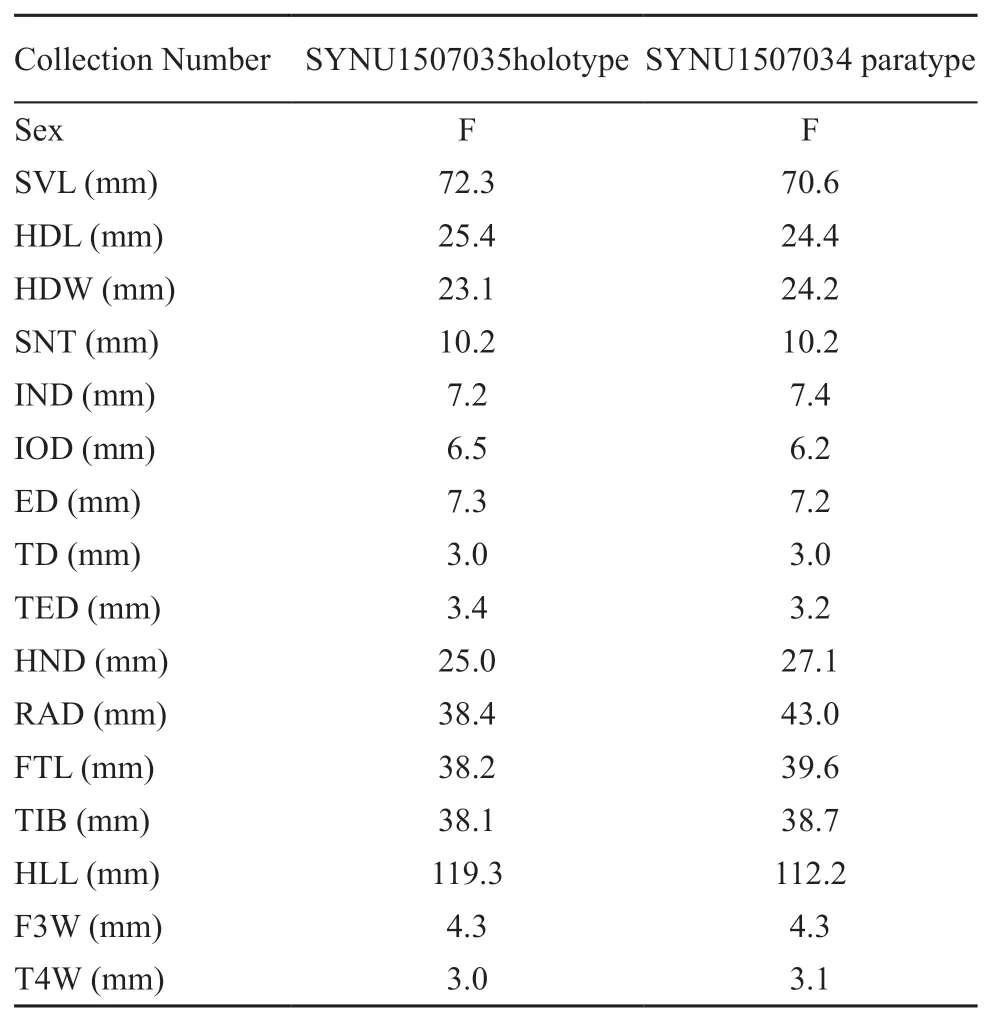
Table 3 Measurements(in mm)of the type series of Amolops pallasitatus sp.nov.

Figure 7 Habitat of Amolops pallasitatus sp.nov.in the type locality.Photo by Zhengyan ZHOU.
Hindlimbs long and robust(TIB/SVL=0.53,TIB/HLL=0.32);tibio-tarsal articulation reaching posterior corners of the eye when hindlimb stretched alongside of body;heels overlapping when hindlimbs flexed at right angles to axis of body;relative toe lengths I<II<III<V<IV;tips of all toes expanded to well-developed oval discs with circummarginal grooves;subarticular tubercles oval and distinct;inner metatarsal tubercle prominent,elongated;outer metatarsal tubercle and tarsal glands absent;toes fully webbed,webbing formula is I 0-0-II 0-½ III 0-1+IV 1+-0 V;lateral fringes of toes I and V developed.
Skin dorsally and ventrally smooth,including throat,chest,abdomen,ventral surface of limbs,and flanks;dorsolateral fold absent;only a few raised warts on both sides of temporal region;rictal gland prominent and ellipsoidal,posterior to corner of mouth;a few warts surrounding cloaca.
Measurements of holotype(in mm)SVL 72.3;HDL 25.4,HDW 23.1;SNT 10.2;IND 7.2;IOD 6.5;ED 7.3;TD 3.1;TED 3.4;HND 25.0;RAD 38.4;FTL 36.9;TIB 38.1;F3W 4.1;T4W 3.0.
Color of holotype in lifeDorsum yellow-green,with irregular dark brown blotches without margins;blotches concentrated on the dorsum,less on the flanks;irregular dark brown patches on dorsal surface of upper arms,distinct dark brown transverse bars on dorsal surface of lower arms and hindlimbs;a dark brown stripe along lower edge of the canthus rostralis extending from the snout tip across the eyes,to the anterior edge of supratympanic fold;ventral surfaces of the forelimbs light yellow;hinder side of thighs banded with dark brown.
Color of holotype in preservativeAfter preservation,dorsal surface fades to dark blue gray with irregular dark taupe blotches;ventral surface of body and limbs beige;transverse bars on limbs blur;webs on toes grayish white.
VariationsMeasurements of type series specimens are given in Table 3.Two specimens are very similar in morphology,but paratype shows more irregularly blotches on the dorsum than holotype.
EtymologyThe specific namepallasitatusmeans“pallasite like”,which derived from meteoritics term pallasite(a class of stony-iron meteorite).The name refers to the numerous irregular dark brown blotches on the dorsal background resembling olivine crystals in an iron-nickel matrix.According to the type locality,we suggest the English common name as“Chentang cascade frog”,and the Chinese common name as“chén táng tuān wā”(陈塘湍蛙).
DistributionCurrently,this species is only known from Chentang Town,Dinggyê County,Tibet,China,presumably also distributed in Nepal.
Ecology and habitatAll the specimens were caught at night,living in high-altitude(3270 m a.s.l.)streams within moist forest and grassland habitats(Figure 7).The frog is hidden underwater during the day.When disturbed,it jumped into the water immediately.
4.Discussion
Currently,Amolopscontains 58 recognized species,making it one of the largest Asian frog genera.Because of the large number of these frogs,six species groups based on morphological comparisons were proposed for the Chinese congeners(Pang 1992;Feiet al.,;2005;Feiet al.,2009),namelyA.marmoratusgroup,A.monticolagroup,A.mantzorumgroup,A.rickettigroup,A.daiyunensisgroup andA.hainanensisgroup.This species group division were followed by subsequent investigations and further applied to the species out of China(Matsuiet al.,2006;Ngoet al.,2006;Caiet al.,2007;Stuartet al.,2010;Deveret al.,2012;Lyuet al.,2018;Lyuet al.,2019).The respective monophylies ofA.rickettigroup,A.daiyunensisgroup andA.hainanensisgroup were well supported by mitochondrial data(Lyuet al.,2019),while the monophylies ofA.marmoratusgroup,A.monticolagroup andA.mantzorumgroup have not been tested,despite that the studies on each species group were conducted(Stuartet al.,2010;Deveret al.,2012;Luet al.,2014).
Morphologically,Amolops pallasitatussp.nov.can be steadily distinguished from all congeners but more similar toA.himalayanusandA.formosuswhose molecular data are unavailable.They differ in length of hind limbs,web of toe and dorsal colour pattern.In the molecular analysis of this study,we used as many samples fromA.marmoratusgroup,A.monticolagroup andA.mantzorumgroup as we can,to reconstruct the phylogenetic relationship of these three species group from southwestern China.The phylogenetic result disagreed with the monophylies of these three morphological species groups.The placements ofA.chayuensisfromA.monticolagroup,A.medogensisfromA.marmoratusgroup,A.lifanensisandA.viridimaculatusfromA.mantzorumgroup,and the newly described speciesAmolops pallasitatussp.nov.were unresolved,because of the relatively insignificant supports.
Amolops pallasitatussp.nov.forms the sister taxon to(A.medogensis+A.viridimaculatus),while with relatively insignificant supports.Therefore in this study,the new species is not assigned to any recognized species group and further study on this topic will be explored.
AcknowledgementsThis study is supported by Ministry of Science and Technology of China(2014FY210200),The Second National Survey of Terrestrial Wildlife Resources in China and The Second National Survey of Terrestrial Wildlife Resources in Tibet of China.We would like to thank K.JIANG and Y.T.WANG for providing tissue samples,specimens and literature.We thank Y.Y.Wang for provide valuable suggestions.
Appendix I.List of specimens examined in this study.
Amolops aniqiaoensis(n=1):China,Tibet,Mêdog County(type locality):SYNU 1607058
Amolops chayuensis(n=6):China,Tibet,Baxoi County:SYNU 1608048-54
Amolops mantzorum(n=1):China,Sichuan,Tianquan County:SYNU 1807049
Amolops medogensis(n=5):China,Tibet,Mêdog County(type locality):SYNU 1607034,1607040-41,1 607068;China,Tibet,Mêdog County:SYS a006657
Amolops nyingchiensis(n=2):China,Tibet,Zayü County:SYNU 1 608023;Lhünzê County:SYNU 1807055
Amolopssp.(n=3):China,Tibet,Mêdog County:SYNU 1 607069;China,Tibet,Mêdog County,Beibeng Village:SYS a006638-39
Amolops viridimaculatus(n=4):China,Yunnan,Tengchong County,Gaoligong Nature Reserve(type locality):SYS a003753-3754,3812-3813
Amolops xinduqiao(n=3):China,Sichuan,Xinduqiao Town(type locality),SYNU 1506002-04
 Asian Herpetological Research2019年4期
Asian Herpetological Research2019年4期
- Asian Herpetological Research的其它文章
- Molecular Cloning,Characterization and Sequence Analysis of KCNQ4 in Large Odorous Frog,Odorrana graminea
- Geographical Distribution and Morphological Variability of the Rapid Racerunner,Eremias velox(Pallas,1771)(Reptilia,Lacertidae)in the Eastern Periphery of Its Range
- Ecological and Geographical Reasons for the Variation of Digestive Tract Length in Anurans
- Mating Ethogram of a Video-aided Study of Mating and Parturition in Captive Chinese Crocodile Lizards(Shinisaurus crocodilurus)
- Home Range Size and Overlap of the Small Nocturnal Schlegel's Japanese Gecko(Gekko japonicus),Introduced into a City Park in Korea
- Correlation between Climatic Factors and Genetic Diversity ofPhrynocephalus forsythii
