Qualitative and quantitative phytochemical analysis of selected mosses with different alcoholic solvents
Kehinde O.Olasoji ,Amos M.Makinde ,Bolajoko A.Akinpelu ,Musibau O.Isa
1.Department of Botany,Obafemi Awolowo University,Ile-Ife,Osun State 230103,Nigeria
2.Department of Biochemistry and Molecular Biology,Obafemi Awolowo University,Ile-Ife,Osun State 230103,Nigeria
ABSTRACT This study was conducted to investigate the qualitative and quantitative phytochemical content of the crude extracts of Archidium ohioense,Pelekium gratum,and Hyophila involuta with different alcoholic solvents(ethanol,methanol,Seaman's Schnapps,fresh oil-palm wine,and fresh Raffia-palm wine).The mosses were collected from their natural populations on the central campus of the Obafemi Awolowo University,Ile-Ife,Nigeria.The yield of the extracts was weighed for all the solvents,and the qualitative and quantitative evaluations of the extracts were carried out using standard methods.The results of phytochemical analysis of the crude extracts from the mosses showed the presence of saponins,cardiac glycosides,triterpenes,alkaloids,flavonoids,and steroids.The quantitative phytochemical analysis of the crude extracts showed that ethanolic extracts of Hyophila involuta had the highest flavonoid content(288.37±0.10 mg RE/g),and Raffiapalm-wine extracts of Hyophila involuta had the highest saponin content(224.70±0.02 mg/g),while the methanolic extract of Archidium ohioense had the highest cardiac glycosides content(63.71±0.14 mg/g),and the Raffia-palm wine extract of Hyophila involuta had the highest alkaloids content(102.50±0.12 mg/g).Raffia-and oil-palm wines were observed to be the most effective solvents for all the mosses studied,followed by Seaman's Schnapp,while methanol and ethanol were less effective.The study concluded that the extracts of the mosses studied contain pharmacologically active constituents that can be used for therapeutic purposes.
Keywords:Archidium ohioense;bryophyte;Hyophila involuta;Pelekium gratum;phytoconstituents
1 Introduction
Bryophytes are the second largest group of land plants,after flowering plants,with about 15,000 to 25,000 species worldwide,and are divided into three divisions,the Marchantiophyta(liverworts),Anthocerotophyta(hornworts),and Bryophyta(mosses)(Gradsteinet al.,2001;Asakawaet al.,2013).In West Africa,Nigeria is home to varieties of these bryophytes;and some of the common species in Nigeria includeArchidium ohioenseSchimp ex.C.Mull,Jaegerina scariosa(Lor.)Arz.,Octoblepharum albidumHedw.,Racopilum africanumMitt.,Barbula lambarenensisC.Mull,andPhilonotis hastata(Duby)Wijk&Margad(Akande,1992).Bryophytes are able to produce various secondary metabolites to cope with a number of biotic and abiotic stresses such as predation,ultraviolet radiation,extreme temperature,and microbial decomposition(Xie and Lou,2009).
Bryophytes have been neglected for a long time and considered almost useless as a source of biologically active substances due to their small size and identification problems.But of recent,the interest in bryophyte chemical composition is "increasingly growing",as a high number of biologically active compounds have been found in mosses and liverworts(Asakawaet al.,2013).Biologically active compounds from mosses includes biflavonoids, terpenes, terpenoids(such as di-and triterpenoids),and flavonoids,whereas liverworts are reported to contain a large variety of lipophilic mono-,di-,and sesquiterpenoids,as well as aromatic compounds such as bibenzyls,benzoates,cinnamates,and naphtalenes(Asakawa,2007).Many compounds that have been isolated from bryophytes have shown high biological activity(Üçüncüet al.,2010;Chenget al.,2012).Therefore,extracts of bryophytes are prospects for the search of new pharmaceutically active compounds(Asakawa,2007).This study aimed at evaluating the phytochemical contents and antioxidant activity of some selected mosses,using different alcoholic solvents as the extractant.
2 Materials and methods
2.1 Sample collection
The investigations were carried out on the central campus of the Obafemi Awolowo University(OAU),Ile-Ife,Nigeria,latitude 7.56°N and longitude 4.533°E.The mosses investigated wereArchidium ohioenseSchimp ex.C.Mull.,Hyophila involuta(Hook)Jaeg.andPelekium gratum(Palis)Jaeg.Archidium ohioensespecimens were collected from the base of Hill 2,along Road 8,OAU,Ile-Ife,whileHyophila involuta(Hook)Jaeg.andPelekium gratum(Palis)Jaeg.were collected from the Biological Garden,OAU,Ile-Ife,from 2015 to 2016.
2.2 Preparation of plant materials
Archidium ohioense,Pelekium gratum,andHyophila involutawere collected from their natural populations,separated from attached dirt,washed in a bowl of water,and then air-dried at room temperature.An alcohol meter(US Custom House,alcohol percentage for spirits)was used to determine the level of alcohol in the extractants:Seaman's Schnapps,40%;methanol,100%;ethanol,98%;oil-palm wine,0%;andRaffia-palm wine,0%.Extracts of each of the mosses were then prepared by soaking separately in methanol, ethanol, Seaman's Schnapps, oil-palm wine,andRaffia-palm wine for 48 hours.Each suspension was filtered through two layers of cheese cloth.The filtrates were evaporated to dryness on a rotary vacuum evaporator at 35°C to obtain the residues,termedcrude alcoholic extracts.The crude extracts were weighed to obtain the yield of each extract.
2.3 Qualitative phytochemical screening of the crude extracts
The extracts were screened for the presence of secondary metabolites,such as flavonoids,tannins,alkaloids,saponins,anthraquinones,steroids,cardiac glycosides, xanthoproteins, phlobatannins, and triterpenes,using procedures based on the earlier reports in the literature(Trease and Evans,2002;Oyesiku,2005).
2.3.1 Test for flavonoids
Three methods were used to screen for the presence of flavonoids.
2.3.1.1 Ethanolic potassium hydroxide solution test
Each extract(0.05 g)was dissolved separately in 5.0 mL of distilled water,followed by filtration.Two drops of ethanolic potassium hydroxide solution were added to 1.0 mL of the filtrate.The resulting solution was examined for the formation of a suspension,cloudiness,or precipitation.
2.3.1.2 Ammonium test
Each extract(0.1 g)was extracted with ethyl acetate(10 mL)over a period of 1 hour,followed by filtration.To 1 mL of the filtrate were added two drops of the ammonia solution.The aqueous layer was observed for colour change or precipitate.
2.3.1.3 Aluminium chloride test
Two drops of 1%(w/v)aluminium chloride solution were added to a portion of each filtrate.The presence of yellow colouration indicates the presence of flavonoids.
2.3.2 Test for tannins
Each extract(0.05 g)was dissolved in 20 mL of distilled water in a test tube and then filtered.Two to three drops of 0.1%ferric chloride in a glacial acetic acid solution were added to the filtrate.The mixture was examined for the formation of a brownish green or blue-black precipitate.
2.3.3 Test for alkaloids
Acidic solutions of the extracts were prepared by accurately weighing 50 mg into three test tubes,to each of which 10 mL of 10%(v/v)HCl were added.The test tubes were heated and filtered.1.0 mL of Mayer's reagent and 1.0 mL of Wagner's reagent were added separately to each filtrate;the mixtures were examined for colour change,turbidity,or formation of precipitate.Equal volumes of 10%(v/v)HCl were used as a parallel control.
2.3.4 Test for saponin(frothing test)
Each extract(0.05 g)suspended in 2.0 mL of distilled water in a test tube was vigorously shaken and noted for froth.Then,the test tube was warmed gently at about 70°C for about 10 minutes in a water bath.The mixture was shaken vigorously after warming.Persistence of frothing after warming indicates the presence of saponin.
2.3.5 Test for anthraquinone
Each extract(0.5 g)was boiled in 2.0 mL of dilute sulphuric acid and filtered while it was still hot.To the filtrate,about 2.5 mL of benzene were added;and the mixture was vigorously shaken.The benzene layer was separated;and into half its volume,2 mL of 10%(v/v)ammonia solution were added.The ammonia layer was observed for either a pink,red,or violet colouration.
2.3.6 Test for steroids
Acetic anhydride(2 mL)and H2SO4(2 mL)were added to 0.5 g of each extract and shaken.A colour change from violet or light brown to deep viscous brown indicates the presence of steroids.
2.3.7 Test for cardiac glycosides
Each extract(0.5 g)was added to 2 mL of chloroform;and filtered into a clean test tube.2 mL of concentrated sulphuric acid were carefully added to form a lower layer.A reddish brown colour ring at the chloroform/sulphuric acid interface indicates the presence of a steroidal ring or glycine of the cardiac glycosides.
2.3.8 Test for xanthoproteins
Each extract(0.1 g)was dissolved in 5 mL of distilled water and filtered.The filtrate was shaken with drops of nitric acid and ammonia solution.Formation of a coloured precipitate indicates the presence of xanthoproteins.
2.3.9 Test for phlobatannins
Each aqueous extract(0.5 g)was dissolved in 10 mL of water in a test tube and then filtered.The filtrate(1 mL)was shaken with 10%HCl and then observed for the deposition of a red precipitate,which indicates the presence of phlobatannins.
2.3.10 Test for triterpenes
Each extract(0.5 g)was gently warmed with chloroform(5 mL)and filtered.Concentrated H2SO4(2 mL)was carefully added to the filtrate,and formation of a reddish brown colouration at the interface indicates the presence of triterpenes.
2.4 Quantitative phytochemical evaluation of the crude extracts
2.4.1 Determination of flavonoid content
The concentration of flavonoids in each extract of the selected mosses was estimated spectrophotometrically,according to the procedure of Sunet al.(1999).The extract(0.1 g)was dissolved in 20 mL of 70%(v/v)ethanol to give a final concentration of 5 mg/mL.Each 0.5 mL of the sample was diluted with 4.5 mL of distilled water and transferred with the aid of a pipette into a clean test tube(in triplicate).To each test tube was added 0.3 mL of 5%(w/v)NaNO2,0.3 mL of 10%AlCl3,and 4 mL of 4%(w/v)NaOH.The reaction mixtures were incubated at room temperature for 15 minutes.The absorbance was read at 500 nm against a reagent blank. The standard calibration curve was prepared by pipetting 0.0,0.2,0.4,0.6,0.8,and 1.0 mL of 1 mg/mL rutin into clean,dry test tubes.The volumes were made up to 5 mL with distilled water.Absorbance was taken at 500 nm and was plotted against the concentration to give the standard calibration curve.The concentration of the flavonoids in the extract was extrapolated from the standard calibration curve and expressed as milligrams rutin equivalent per gram of extract(mg RE/g extract).
2.4.2 Determination of saponin content
Estimation of saponin content was carried out according to the methods described by Abdel-Gawadet al. (1999) and Wagneret al. (1984). The extract(40 g)was washed twice with chloroform(50 mL)and then twice with ethylacetate(50 mL).The residue was dissolved in 20%(v/v)ethanol,and the solution was extracted three times(100 mL)with butanol.On evaporation,the obtained residue was taken up in methanol.The residue was dissolved in 50 mL of 50%(v/v)methanol,followed by the addition of diethylether(100 mL)to precipitate crude saponins.The upper diethylether layer was carefully removed with the aid of a separating funnel;the residue was dissolved in 10 mL of methanol and poured into diethylether(200 mL).The upper diethylether was again removed,and the residue was dissolved as described earlier and precipitated by the addition of diethylether.The precipitate was dried in the oven to a constant weight.The percentage of saponin content of the extract was calculated using the following expression:
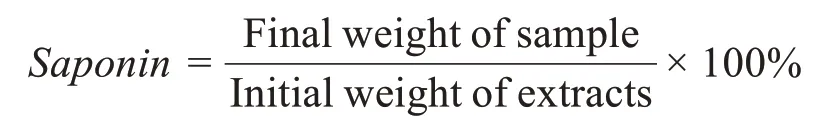
2.4.3 Determination of alkaloids content
Estimation of alkaloids content of each selected moss was carried out according to the procedure described in the literature(Kamet al.,1999;Yanget al.,1999).The extract(40 g)was dissolved in 200 mL of 5%(v/v)HCl solution,followed by successive partitioning of the acidic filtrate with benzene(100 mL×3),chloroform(100 mL×3),and ethyl acetate(100 mL×3)to remove nonalkaloid materials.The acidic aqueous solution was basified with an ammonia solution of pH 12,followed by the extraction of released alkaloids with chloroform(150 mL×5).The chloroform extracts were combined,followed by evaporation at 35°C to dryness under reduced pressure,and weighed.The alkaloids content of the extract was calculated as follows:

2.4.4 Determination of cardiac glycosides content
Cardiac glycosides content in the sample was evaluated using a reagent as described by El-Olemyet al.(1994).Fine powder(1 g)of each moss sample was soaked in 10 mL of 70%alcohol(ethanol)for 2 hours and then filtered.The filtrate obtained was then purified by adding 60 mL of distilled water and 10 mL of 12.5%lead acetate(to precipitate resins,tannins,and pigment).The mixture was properly shaken with distilled water and filtered through a Whatman No.1 filter paper.The filtrate(50 mL)was transferred using a pipette into a 100-mL volumetric flask,and 10 mL of 4.77%Na2HPO4solution were added to the filtrate(to precipitate the excess lead ions)and was properly mixed until a clear solution was obtained.The clear filtrate(10 mL)was transferred into a clean,dry Erlynmeyer flask;and 10 mL of freshly prepared Baljet's reagent(containing 95 mL aqueous picric acid+5 mL of 10%aqueous NaOH)were added in three different test tubes.The mixture was allowed to stand for one hour;thereafter,the solution was diluted with 20 mL of distilled water and mixed using an automatic burette.The intensity of the colour obtained was read against the blank at 495 nm,using an Outrao microplate reader.The difference between the intensity of colours of the experimental and blank(distilled water and Baljet's reagent)samples gives the absorbance and is proportional to the concentration of the glycosides.%of cardiac glycoside in 100 g extract=
2.5 Statistical analysis
Values are expressed as the mean±SEM(standard error of the mean)of three consecutive readings.The statistical significant difference was analyzed using one-way analysis of variance(ANOVA)with SAS software(SAS 9.1.2;SAS Institute Inc.,Cary,NC,USA),and the statistical difference between means was determined by Duncan's multiple-range test atp<0.05.
3 Results
3.1 Percentage yield
The percentage yields of crude extract fromArchidium ohioense(175 g),Pelekium gratum(40.25 g),andHyophila involuta(58 g)soaked in ethanol,methanol,Seaman's Schnapps,oil-palm wine,andRaffiapalm wine are shown in Table 1.

Table 1 Percentage yield of crude extract from Archidium ohioense,Pelekium gratum,and Hyophila involuta
3.2 Phytochemical screening
The preliminary phytochemical screening showed the presence of alkaloids,terpenoids,flavonoids,saponins,steroids,and cardiac glycosides,while anthraquinones,tannins,and phlobatannins were not detected in any of the plant extracts(Table 2).The quantitative phytochemical analysis is shown in Tables 3-6.
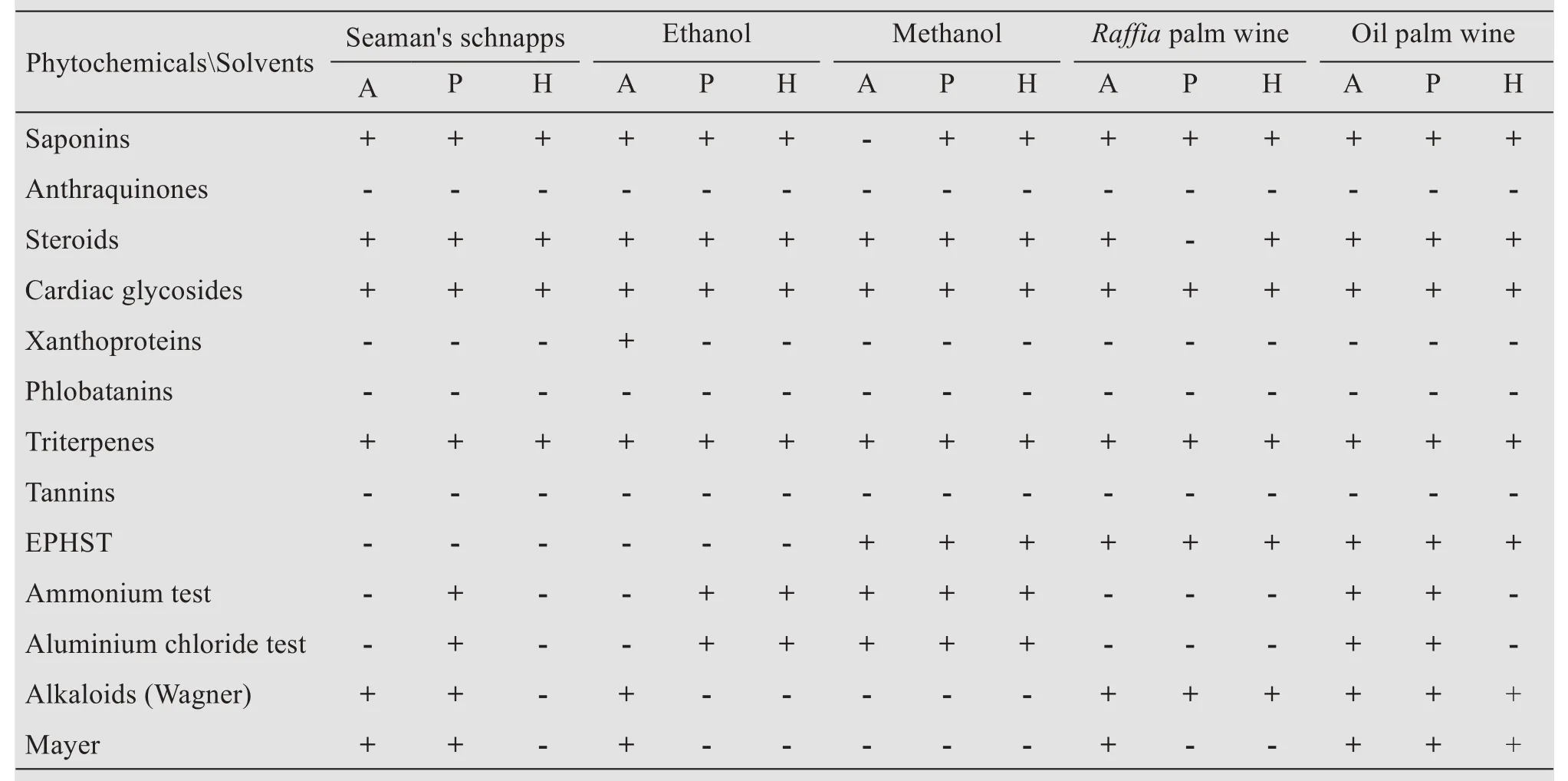
Table 2 Phytochemical constituents in extracts of Archidium ohioense,Pelekium gratum,and Hyophila involuta
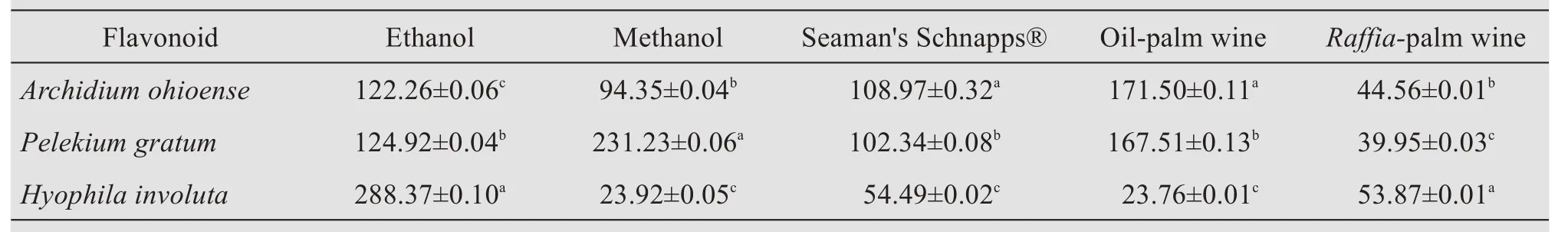
Table 3 Quantitative evaluation of flavonoid per solvent of each plant's samples(mg RE/g)
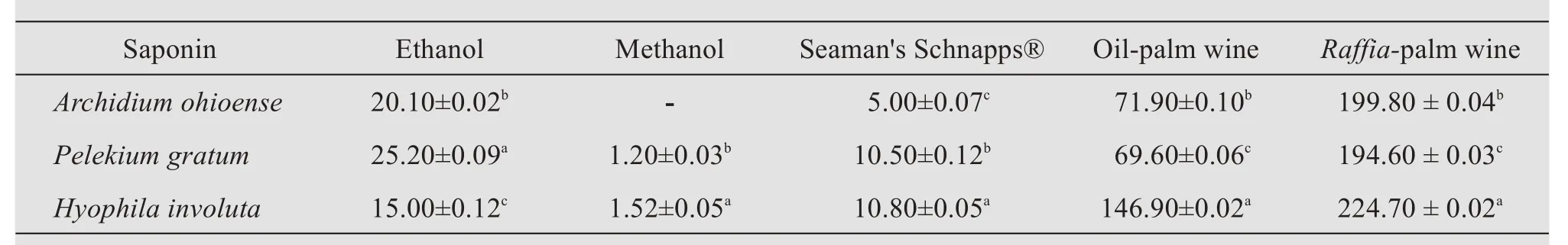
Table 4 Quantitative evaluation of saponin per solvent of each plant's samples(mg/g)
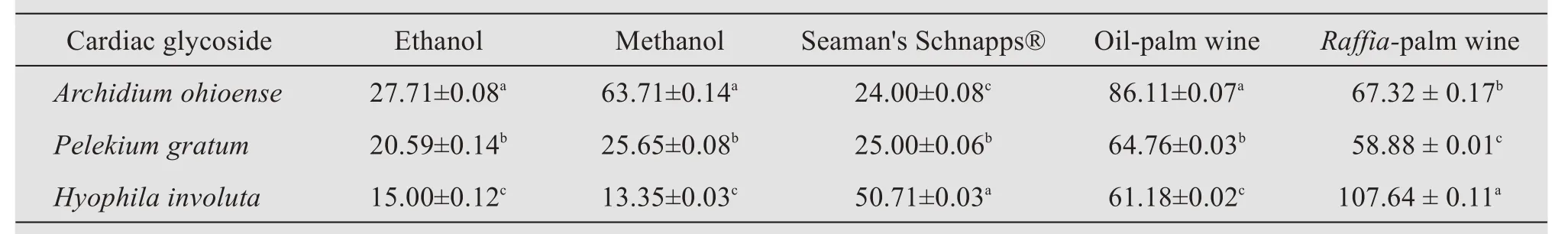
Table 5 Quantitative evaluation of cardiac glycoside per solvent of each plant's samples(mg/g)
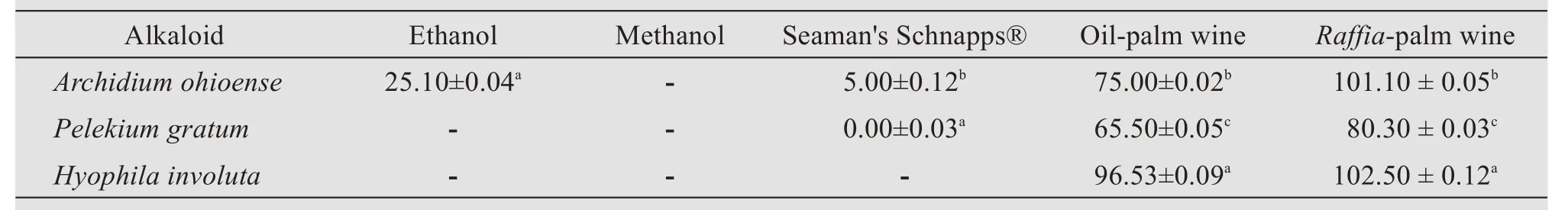
Table 6 Quantitative evaluation of alkaloid per solvent of each plant's samples(mg/g)
4 Discussion
The phytochemical investigations of the crude alcoholic extracts of the mosses studied indicated the presence of pharmacologically active compounds such as flavonoids,terpenoids,alkaloids,steroids,cardiac glycosides,and saponins.These results agreed with similar research done by Isaet al.(2014),who reported the presence of steroids,triterpenes,and cardiac glycosides in methanol and chloroform extracts ofArchidium ohioenseandPelekium gratum.The results of this study are in contrast with the work of Ramyaet al.(2015),who reported the absence of terpenoids, saponins, cardiac glycosides, and phenolic compounds in ethanolic extracts ofHyophila involuta.These differences may be due to geographical location and environmental factors.The presence of specific phytochemicals in these mosses adds to their medicinal properties.
Saponins have been reported to possess expectorant action,which is very relevant in the treatment of upper respiratory tract inflammation(Kamel,1991).Steroidal compounds are of importance in pharmacy due to their relationship with compounds such as sex hormones(Okwu,2001).Plants that possess alkaloids are pharmacologically active,as they have physiological effects and also serve as therapeutic and anti-malaria drugs(Muruganet al.,2012).Cardiac glycoside is being used for the treatment of heart diseases(Pawar and Arumugam,2011).Akinpeluet al.(2017)reported that the presence of these phytochemicals in extracts(ethyl acetate,acetone,and chloroform)ofArchidium ohioensemight be responsible for the reported anti-inflammatory and anti-sickling activities of the plant.In a study by Ramyaet al.(2015),the percentage yield of the ethanolic extract ofHyophila involutawas reported to be 1.6 g.This value was lower than the percentage yield of ethanolic extract(2.0 g)recorded in this study.This difference could be a result of environmental factors such as weather conditions and geographical location;and the polarity of the solvents used may have an effect on the yield of extracts(El-Mahmood,2009).
The quantity of the crude-yield extracts showed that ethanolic extracts ofHyophila involutahad the highest flavonoid content,when compared to other alcoholic extracts used for extraction;and this value of 288.37 mg/g tends to agree with the work of Karimet al.(2014),who reported that ethanolic extracts ofSphagnum cuspidatumcontained the highest flavonoid contents,as compared to aqueous and 80%methanol extracts.The different alcoholic solvents used as extractants ofArchidium ohioense,Pelekium gratum,andHyophila involutayielded different proportions of extracts.The higher yield of theRaffia-palm wine over other solvents used in the study could be due to their difference in polarity.The extraction yield and biological activity of extracts have been reported to be highly dependent on solvent polarity(Zohra and Fawzia,2011).
In the present study,the detection of phytoconstituents depends on the solvent medium used for extraction and the ability of the solvent to dissolve the phytochemicals present in the crude extracts(Isaet al.,2014).With respect to the alcoholic solvents used for extraction in this study,Raffia-and oil-palm wine extracts were discovered to be the most effective extractants for all the mosses studied,followed by Seaman's Schnapps,methanol,and ethanol.
This study shows that the mosses studied contain pharmacologically active constituents that may be responsible for their therapeutic uses and also supports the use of plants in the formulation of new antioxidant drugs.
 Sciences in Cold and Arid Regions2019年5期
Sciences in Cold and Arid Regions2019年5期
- Sciences in Cold and Arid Regions的其它文章
- Editors-in-Chief Guodong Cheng and Ximing Cai
- Origin and advances in implementing blowing-snow effects in the Community Land Model
- Analysis of chaotic climatic process in the Tarim River Basin(I)
- Seed germination and seedling growth of Pycnanthus angolensis(Welw.)Warb.,African false nutmeg
- Soil hydraulic conductivity and its influence on soil moisture simulations in the source region of the Yellow River―take Maqu as an example
- Features on N/P ratio of plants with different functional groups between two types of steppe in semi-arid area
