Effects of Siwei Yuganzi decoction on LXRα and CYP7A1 in hyperlipidemic rats
Ru-Yi Yang, Hong-Bin Wang, Pan-Pan Zhou
1Department of Integrative Medicine, Affiliated Hospital of Qinghai University, Xining 810000, China. 2Medical College of Qinghai University, Xining 810000, China.
Abstract
Keywords: Hyperlipidemia,Experimental study,Tibetan medicine,Siwei Yuganzi decoction,LXRα,CYP7A1
Background
Hyperlipidemia (HLP) is a disorder in which lipids in the blood are disturbed due to abnormal lipid metabolism [1].With the increase of high-protein,high-fat diets,especially in the Qinghai plateau of China,people eat more cattle and high-fat mutton in combination with less exercise,which result in excess fat that is not metabolized,causing HLP over time.Studies have shown that as the main risk factor for coronary heart disease,atherosclerosis,and myocardial infarction is lipid metabolism disorder [2-5],the effective control of HLP is key for the prevention of cardiovascular and cerebrovascular diseases.Lipid-lowering therapy can improve the patient condition in relation to coronary heart disease.In the search for lipid-regulating drugs,a long-term exploration has been conducted and remarkable achievements have been made,especially in statins and fibrates as lipid-regulating drugs that prevent the generation of lipids and reduce the morbidity and mortality of cardiovascular and cerebrovascular diseases.However,the adverse reactions and side effects of lipid-regulating drugs limit their wide clinical application.Gastrointestinal discomfort such as nausea,diarrhea,dyspepsia,and constipation,muscle pain,difficulty concentrating,abnormal liver function,and rhabdomyolysis in severe cases can be caused by HLP [6].
A Tibetan medicine compound preparation Siwei Yuganzi decoction (SYD),is an adaptation of a classic Tibetan medicine called Sanguo decoction,which contains Hezi (Terminalia chebula Retz.),Maohezi(Terminalia billerica (Gaertn.) Roxb.) and Yuganzi(Phyllanthus emblica Linn.) and is recorded in the ancient book of Tibetan medicine entitledSibu Jingdian,written by the famous Tibetan medical scientist Yutuo Ningmayundangongbu in 800 C.E.It is an academic and authoritative reference book of Tibetan medicine that integrates the medical practice and theoretical essence of Tibetan medicine.It is the most systematic,complete,and fundamental theoretical system in Tibetan medicine.
The adaption of the Sanguo decoction is involved in many Tibetan medicines and is also used in Indian medicine [7-9].Sanguo decoction contains gallic acid,ellagic acid,ethyl gallate,corilagin,and other organic acids such as tannins and triads,which have a strong antioxidant effect [10].Wanget al.[11] discovered that Yuganzi (Phyllanthus emblica Linn.) regulates lipid metabolism,improves antioxidant capacity,reduces lipid peroxidation,and prevents experimental atheromatous plaque formation in rabbits.Banjareet al.[12] demonstrated that the aqueous extract from Sanguo decoction achieves a lipid-lowering effect by regulating lipid metabolism.
Here,the curative effect of a SYD on an experimental HLP rat model was investigated by observing the changes of cholesterol (TC),triglyceride(TG),low-density lipoprotein cholesterol (LDL-C),and high-density lipoprotein cholesterol (HDL-C) in blood and Liver X receptor α (LXRα) and cholesterol 7α-hydroxylase 1 (CYP7A1) in liver tissue.
Materials
Experimental animals
Sixty Sprague Dawley rats,mail,weighed 200 ± 20 g,were provided by the Experimental Animal Center of Gansu University of Traditional Chinese Medicine [No.SYXK (Gan) 2015-0005].All animal experiments were conducted according to the National Institutes of Health Regulations and approved by the Institutional Animal Care and Use Committee Qinghai University Medical College.The ethical approval number for animal experiments is YJ-SL-2017012/01-YJ-SL-2017012.The medicine and reagent were administered by complete pellet feed and the water met the standard drinking water requirements.All animals were maintained in specific pathogen-free conditions with a temperature of 21 ± 2 °C,a humidity of 45 ±10%,a 12 h light/dark cycle and food and water ad libitum.
Drug
The SYD: Yuganzi fruit (Phyllanthus emblica Linn.)water extract (Batch No: ZL2017061201),Zangyinchen stem leaf (Capillary Worm wood.) water extract (Batch No.: ZL2017082106),Hezi fruit(Terminalia chebula Retz.) water extract (Batch No:ZL2017082201),Maohezi fruit (Terminalia bellirica(Gaertn.)Roxb.) water extract (Batch No:ZL2017082202) were provided by Nanjing Zelang Biological Technology Co.,Ltd.All the drug powder was mixed in the ration of 5:3:3:3.The Chinese patent medicine Xuezhikang capsules (Batch No: 20161110)were produced by Peking University Weixin Biological Technology Co.,Ltd.In the experiment,all the drugs were dissolved with the distilled water to produce the suspension with required concentration and was stored in 4 °C
Reagent
TC,TG,LDL-C,and HDL-C kits were provided by the Yuanmu Company (Shanghai,China).PrimeScriptTM RT reagent kit (for real time) was provided by the TAKARA Company (Japan).SYRB®Premix Ex Taq ™ II (Tli RNaseH Plus) was provided by the TAKARA Company (Japan).TRIzol reagent was provided by the Ambion Company (Shanghai,China).PMSF was provided by the Amresco Corporation (Washington,USA).Western blot and immunoprecipitation cell lysates,BCA protein concentration assay kit,the rabbit primary antibody anti-LXR alpha and anti-CYP7A1 and the goat anti-rabbit secondary antibody IgG/HRP were provided by Biyuntian Biological Engineering Co.,Ltd.(Shanghai,China).Trizma® Base was supplied by VETEC (Missouri,America).Immobilon Western HRP Substrate was supplied by Millipore (Massachusetts,America).PageRuler Prestained Protein Ladder was supplied by Thermo Fisher Scientific (Massachusetts,USA).
Methods
Grouping and handling
A total of 60 rats were weighed and randomly divided into 6 groups: the blank control group,the HLP group,the Chinese patent medicine Xuezhikang positive control group,and the Tibetan medicine SYD treatment group with high-,middle-,and low-dosages,with 10 rats in each group.After one week of adaptive feeding,the rats in the blank control group were given intragastric administration of normal saline (10 mL/kg,twice daily) while those in the other groups were given daily high fat emulsion (cholesterol 2%,lard 10%,egg yolk powder 5%,and Tween-80 0.2%,prepared by emulsion preparation method plus distilled water) to establish the HLP modal [13].Then the rats in the blank control and HLP groups were fed with 0.9%normal saline,those in the Xuezhikang group was fed with Chinese patent medicine Xuezhikang suspension at the dose of 0.11 g/kg/d,and those in the SYD groups were feed with SYD suspension at the dosage of 16.2 g/kg/d (high dosage),10.8 g/kg/d (middle dosage),and 5.4 g/kg/d (low dosage),respectively.After 8 weeks,food was withheld,sodium pentobarbital was used for anesthesia,blood was collected from the abdominal aorta,and the liver was harvested,frozen in liquid nitrogen,and moved to a -80 °C freezer for storage.All animal handling methods in this experiment were in accordance with animal ethics standards.
Serum lipid
The values of serum TC,TG,LDL-C,and HDL-C were determined by the ELISA method,which was performed according to the instructions in the kit.Fifty μL of PBS was added to the blank control wells.To each standard well,50 μL of standards with different concentrations were added and the concentrations of each standard (S0-S5) are as follows: 0,0.5,1,2,4,and 8 mmol/L.Then,10 μL of samples were added to be tested and 40 μL of sample diluent was added to sample wells.Then,100 μL of detection antibody labeled with HRP was added to each well of the standard and sample wells,the reaction wells were sealed with a microplate sealer,and these were incubated in a water bath or incubator at 37 ℃ for 60 min.The plate was washed five times with washing solution.Fifty μL of substrate A and 50 μL of substrate B were added to each well and incubated for 15 min at 37 °C in the dark.Stop solution of 50 μL was added to each well,and within 15 min,the OD value of each well was measured by a THERMO MK3 microplate(USA) at a wavelength of 450 nm.The standard curve was established according to the concentrations of the standards and the OD in the standard wells.The levels of serum lipids were calculated according to the standard curve.
Expression of LXRα and CYP7A1 by RT-PCR
Total RNA was extracted from liver tissue using the TRIzol RNA extraction kit and the concentration of sample RNA was measured.cDNA synthesis: The mixture of RT enzyme,oligo dT primer,and sample RNA was prepared in 0.2 mL of RNase free Eppendorf tubes and put on ice.The cDNA that was obtained at 37 °C after 15 min,85 °C after 5 s,and 4 °C after 60 min were diluted by the addition of 180 μL of distilled water and this was stored at -20 °C.Quantitative PCR reaction: based on the published rat LXRα,CYP7A1,and β-actin cDNA sequences from Genbank,using Primer Premier 5.0 software and Oligo6 software for homology correlation comparison,the regions with higher homology were selected to design amplification primers for LXRα and CYP7A1.The primers were synthesized by Shanghai Yuanmu Biotechnology Company (China) with the following sequences (Table1).
The PCR reaction mixture was prepared in a PCR tube and operated on ice.The mixture was fully mixed and three replicate controls were produced for each sample.The PCR program was progressed with Roche LightCycler® 96 Fluorescence Quantitative PCR Instrument and was set: Stage 1: pre-denaturation at 95 °C for 30 s; Stage 2: PCR reaction at 95 °C for 5 s and 60 ° C for 34 s for 40-45 cycles; Stage 3:dissociation curve at 95 °C for 15 s,60 °C for 60 s,and 95 °C for 15 s.Onboard amplification was used fordetection and the relative expression was calculated using the 2 - ΔΔCt method.
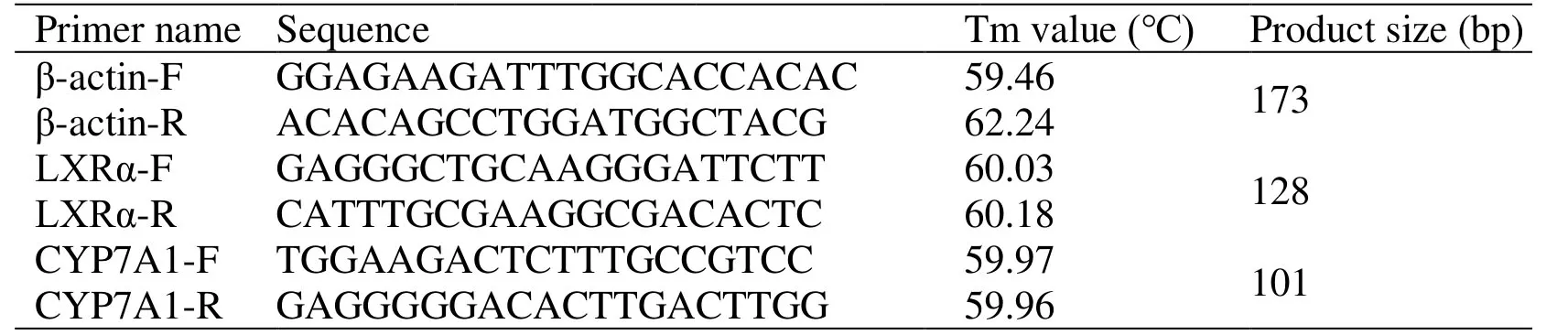
Table1 Designed primer sequences and analysis conditions
The expression of LXRα and CYP7A1 proteins were detected by western blotting
Cellular proteins were extracted using cell lysates containing PMSF with the final concentration of 1 mm/L and quantified using the BCA method.Proteins were transferred to a nitrocellulose membrane in SDS-PAGE at a concentration of 20%by switching on the power supply to 350 mA for 2 h.Ponceau red staining was used to observe the effect of protein imaging.The membranes were blocked by adding non-fat dry milk to a final concentration of 5% (w/v) in 1×TBST and shaken at room temperature for 1 h.The membranes were reacted with rabbit primary antibodies,including CYP7A1(1:1000) and LXRa (1:1000),followed incubation with the goat anti-rabbit IgG/HRP secondary antibody (1:1000).The protein bands were detected by chemiluminescence using a Qinxiang ChemiQ4600 fluorescence chemiluminescence imager (China) and the results were scanned and analyzed using the automatic gel analysis and imaging software scanning system.The protein bands were analyzed using β-actin as an internal reference.
Expression of LXRα and CYP7A1 in liver tissue by immunohistochemistry
A tissue block from a fixed site in the right lobe of the rat liver was immediately placed in 10%formaldehyde and fixed for 48 h.The animal tissues were cut into slices of 0.2-0.5 cm,dehydrated using alcohol,penetrated with xylene,embedded with paraffin,cut into 5 µm thick sections and then dried at 65 °C for 3 h.Paraffin sections were removed after roasting overnight in a 37 °C incubator,placed at room temperature for 5 min,soaked in distilled water for 5 min,and washed 3 times in PBS.EDTA buffer was added,microwaved to boiling,cooled to room temperature,and immersed in 0.1% Triton X-100 for 10 min.This was washed with PBS three times and immersed in 3% hydrogen peroxide RT for 15 min.The tissues were dried with absorbent paper,circled with a histochemical pen,and blocked by immune sheep serum for 30 min.The tissues were reacted with rabbit primary antibodies,including CYP7A1(1:100),LXRa (1:25),followed incubation with goat anti-rabbit IgG/HRP secondary antibody (1:50).Hematoxylin staining solution was counterstained,washed with distilled water,differentiated with hydrochloric acid alcohol (70% ethanol: hydrochloric acid = 99:1) for 20 s,washed with distilled water,and reversed blue with dilute ammonia (1%) for 10 s.After dehydration and clearing,95% ethanol for 5 min,100% ethanol for 5 min,xylene I for 10 min,and xylene II for 10 min,the slides were mounted with neutral gum.Immunohistochemical images were collected by an Olympus BX51T-PHD-J11 microscope (Japan) under 400 × magnification,and LXRα and CYP7A1 in the liver immunohistochemical layer were semiquantitatively analyzed using multifunctional true-color cell image analysis and management system (Image-Pro Plus 6.0,Media Cybernetics,USA).Five fields were selected for each sample in a sequence,the mean absorbance of the stained area in each field was calculated.
Statistical analysis
Statistical software SPSS 17.0 was used for data analysis.Measurement data are expressed as mean ±standard deviation (±s).Analysis of variance was used for comparison.P <0.05 was considered statistically significant.
Results
Effect of SYD on lipid metabolism in rats
The serum levels of TG,TC,and LDL-C (P <0.05)are higher and HDL-C is lower in the HLP group (P<0.05) than those in the blank control group; these findings suggest that a model of HLP in rats is successfully established.The TC,TG,and LDL-C (P<0.05) are lower and HDL-C (P <0.05) is higher in the SYDgroups and Xuezhikang groups than those in the HLP group.The lipid regulating effect of SYD at high dose is superior to that of Xuezhikang (P <0.05) (Table2).
Effect of SYD on LXRα and CYP7A1 mRNA expression in the rat liver
The expression levels of LXRα and CYP7A1 mRNA in the liver in the HLP group are significantly lower than that in the blank control group (P <0.05).The expression levels of LXRα and CYP7A1 mRNA in the liver of SYD groups are significantly higher than those in the HLP group (P <0.05),which indicates that SYD increases the levels of LXRα and CYP7A1 mRNA.The expression levels of LXRα and CYP7A1 mRNA in the liver are higher in the Xuezhikang group than those in the HLP group (P <0.05),which indicates that that the Xuezhikang increases the levels of LXRα and CYP7A1 mRNA.Moreover,the therapeutic effect of the SYD at high dosage is superior to that of the Xuezhikang (P <0.05) (Table3).
Results of western blotting
The bands of LXRα and CYP7A1 protein in the HLP group are significantly thinner and lighter and the average protein gray value is lower than those in the blank control group (P <0.05).The bands of LXRα and CYP7A1 protein in the Xuezhikang and SYD groups are significantly thicker and their levels are higher than those in the HLP group (P <0.05),which indicates that Xuezhikang and SYD can increase the expression levels of LXRα and CYP7A1.Furthermore,the therapeutic effect of SYD at high dose group of is superior to Xuezhikang (P <0.05),which itself is superior to the middle- and low-dose groups (P <0.05) (Figure1,Table4).
Immunohistochemical expression of LXRα and CYP7A1 in the rat liver
The immunohistochemistry of liver tissues show that the expression levels of LXRα and CYP7A1 proteins in the liver tissues in the blank control group are the strongest while those of LXRα and CYP7A1 in the HLP group is the weakest,as it shows a weak aggregation of dark brown particles in the cells.The expression levels of LXRα and CYP7A1 proteins in the liver tissues in the Xuezhikang group and SYD group at high dosage are enhanced,with dark brown particles in the indicated cells that are significantly greater than those in the HLP group.(Figure2).Scattered positive cells are observedaround
theportal area and central vein of the liver in the blank control group and fewer positive cells are observed around the portal area and central vein of the liver in the HLP group,which is significantly less than that in the blank control group (P <0.05).The positive cells in the SYD and Xuezhikang groups are more than those in the HLP group and the expression levels of LXRα and CYP7A1 are increased (P <0.05),which indicates that the Xuezhikang and SYD can increase the expression levels of LXRα and CYP7A1,and that the therapeutic effect of SYD at high-dose group is superior to that of Xuezhikang group (P <0.05) (Table5).
Table2 Effect of SYD on lipid metabolism in experimental hyperlipidemic rats (±s)
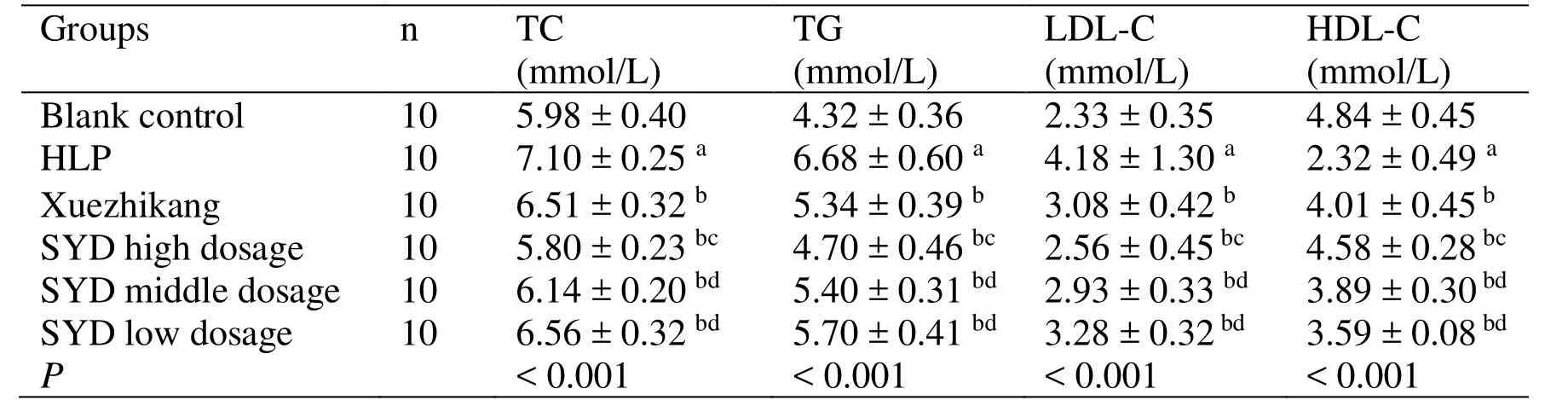
Table2 Effect of SYD on lipid metabolism in experimental hyperlipidemic rats (±s)
Note: a Compared with the blank control group,P < 0.05; b Compared with the HLP group, P < 0.05; c Compared with the Xuezhikang group, P < 0.05; d Compared with the SYD high dose group, P < 0.05.TC,Total cholesterol;TG,Triglyceride; LDL-C,Low-density lipoprotein cholesterol; HDL-C,High-density lipoprotein cholesterol; HLP,Hyperlipidemia; SYD,Siwei Yuganzi decoction.
Groups n TC(mmol/L)TG(mmol/L)LDL-C(mmol/L)HDL-C(mmol/L)Blank control 10 5.98 ± 0.40 4.32 ± 0.36 2.33 ± 0.35 4.84 ± 0.45 HLP 10 7.10 ± 0.25 a 6.68 ± 0.60 a 4.18 ± 1.30 a 2.32 ± 0.49 a Xuezhikang 10 6.51 ± 0.32 b 5.34 ± 0.39 b 3.08 ± 0.42 b 4.01 ± 0.45 b SYD high dosage 10 5.80 ± 0.23 bc 4.70 ± 0.46 bc 2.56 ± 0.45 bc 4.58 ± 0.28 bc SYD middle dosage 10 6.14 ± 0.20 bd 5.40 ± 0.31 bd 2.93 ± 0.33 bd 3.89 ± 0.30 bd SYD low dosage 10 6.56 ± 0.32 bd 5.70 ± 0.41 bd 3.28 ± 0.32 bd 3.59 ± 0.08 bd P < 0.001 < 0.001 < 0.001 < 0.001
Table3 Effect of SYD on LXRα and CYP7A1 mRNA expression levels in rat liver tissues (±s)
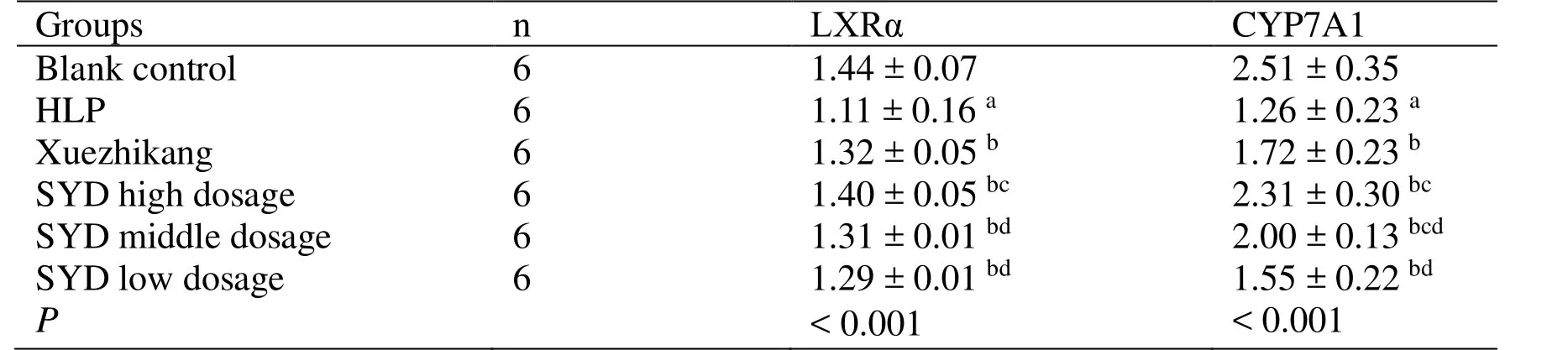
Table3 Effect of SYD on LXRα and CYP7A1 mRNA expression levels in rat liver tissues (±s)
Note: a Compared with the blank control group, P < 0.05; b Compared with HLP group, P < 0.05; c Compared with the Xuezhikang group, P < 0.05; d Compared with SYD high dosage group, P < 0.05.LXRα,Liver X receptor α; CYP7A1,Cholesterol 7α-hydroxylase 1; HLP,Hyperlipidemia, SYD,Siwei Yuganzi decoction.
Groups n LXRα CYP7A1 Blank control 6 1.44 ± 0.07 2.51 ± 0.35 HLP 6 1.11 ± 0.16 a 1.26 ± 0.23 a Xuezhikang 6 1.32 ± 0.05 b 1.72 ± 0.23 b SYD high dosage 6 1.40 ± 0.05 bc 2.31 ± 0.30 bc SYD middle dosage 6 1.31 ± 0.01 bd 2.00 ± 0.13 bcd SYD low dosage 6 1.29 ± 0.01 bd 1.55 ± 0.22 bd P < 0.001 < 0.001
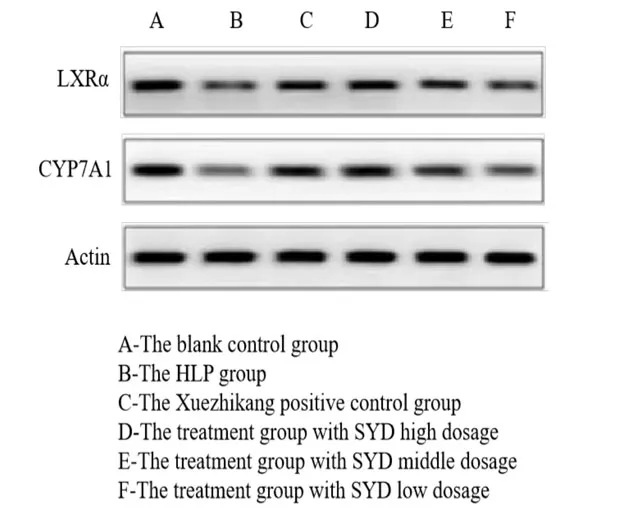
Figure1 Western blotting results of LXRα and CYP7A1 protein in rat liver tissues
Table4 Comparison of mean gray values of LXRα and CYP7A1 protein in rat liver (±s)
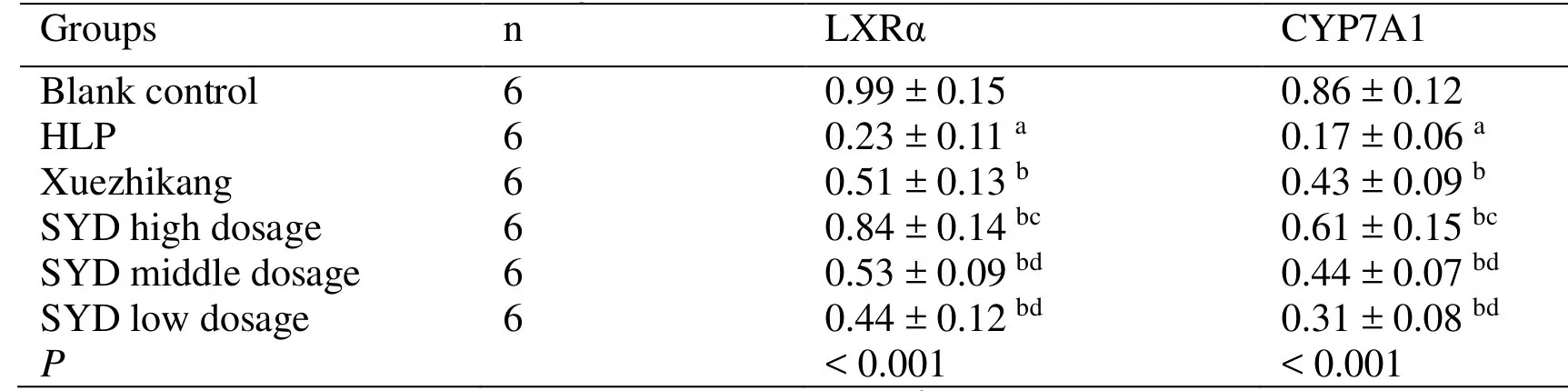
Table4 Comparison of mean gray values of LXRα and CYP7A1 protein in rat liver (±s)
Note: a Compared with the blank control group P < 0.05; b Compared with HLP group, P < 0.05; c Compared with the Xuezhikang group, P < 0.05; d Compared with the SYD high dose group P < 0.05.LXRα,Liver X receptor α; CYP7A1,Cholesterol 7α-hydroxylase 1; HLP,Hyperlipidemia,SYD,Siwei Yuganzi decoction.
Groups n LXRα CYP7A1 Blank control 6 0.99 ± 0.15 0.86 ± 0.12 HLP 6 0.23 ± 0.11 a 0.17 ± 0.06 a Xuezhikang 6 0.51 ± 0.13 b 0.43 ± 0.09 b SYD high dosage 6 0.84 ± 0.14 bc 0.61 ± 0.15 bc SYD middle dosage 6 0.53 ± 0.09 bd 0.44 ± 0.07 bd SYD low dosage 6 0.44 ± 0.12 bd 0.31 ± 0.08 bd P < 0.001 < 0.001
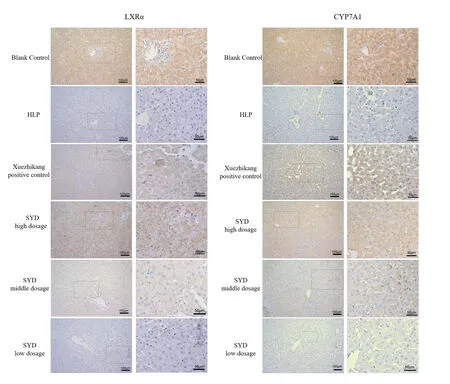
Figure2 Immunohistochemical expression of LXRα in rat liver and immunohistochemical expression of CYP7A1 in rat liver
Discussion
Disturbances in lipid metabolism and turnover are the main causes of HLP,mainly hypercholesterolemia,hypertriglyceridemia,and mixed HLP [14-15].The Chinese patent medicine Xuezhikang is a commonly used Chinese patent medicine.Studies have shown that the Xuezhikang [16] decreases the degradation value of oxidized-LDL macrophages and inhibits the uptake of ox-LDL by macrophages.It also significantly decreases the levels of C-reactive protein [17],which could be via the effect on adiponectin and leptin against atherosclerosis [18].The Xuezhikang can improve the metabolism of lipids by hepatocytes via decreasing the intracellular Ca2+concentration,promoting mitochondrial oxidative phosphorylation,and reducing the generation of intracellular oxygen free radicals while promoting mitochondrial membrane depolarization and preserving mitochondrial function in hepatocytes,thereby counteracting the development and progression of HLP [19].
This experimental study showed that SYDregulated a lipid metabolism disorder and enhanced cholesterol clearance in the body.It reduced TC,TG,and LDL levels,and increased HDL levels.The therapeutic effect of SYD at high dosage was superior to the Xuezhikang groups.PCR,western blotting,and immunehistochemistry showed that the levels of LXRα and CYP7A1 in the liver of rats in the HLP model group were significantly lower than those in the blank control group.SYD at high dosage increased theexpression levels of LXRα and CYP7A1,which was better than that of the Xuezhikang capsule.
Table5 Effect of SY decoction on LXRα and CYP7A1 in the liver tissue of rats with experimental HLP (±s)
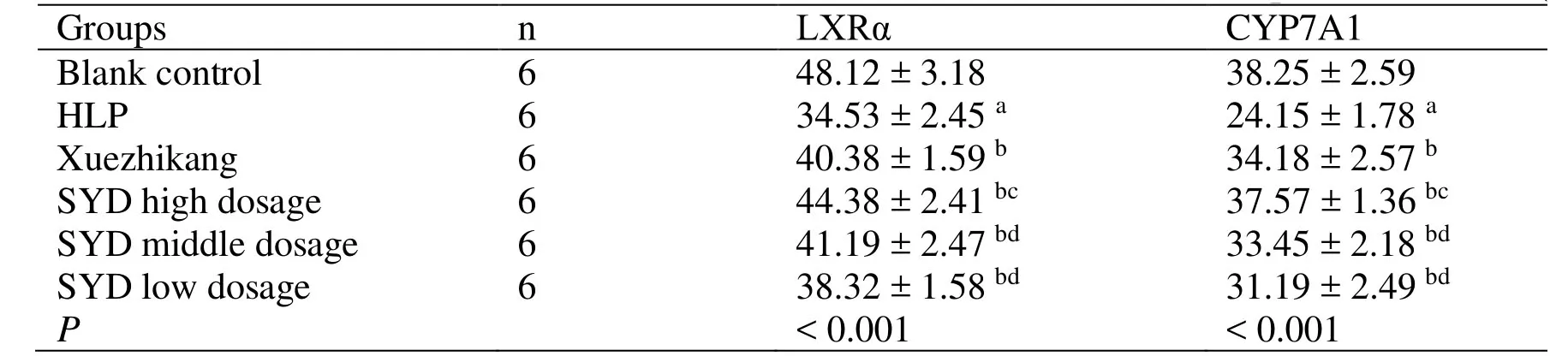
Table5 Effect of SY decoction on LXRα and CYP7A1 in the liver tissue of rats with experimental HLP (±s)
Note: a Compared with the blank control group, P < 0.05; b Compared with the HLP group, P < 0.05; c Compared with the Xuezhikang group, P < 0.05; d Compared with the SYD high dose group, P < 0.05.LXRα,Liver X receptor α; CYP7A1,Cholesterol 7α-hydroxylase 1; HLP,Hyperlipidemia; SYD,Siwei Yuganzi decoction.
Groups n LXRα CYP7A1 Blank control 6 48.12 ± 3.18 38.25 ± 2.59 HLP 6 34.53 ± 2.45 a 24.15 ± 1.78 a Xuezhikang 6 40.38 ± 1.59 b 34.18 ± 2.57 b SYD high dosage 6 44.38 ± 2.41 bc 37.57 ± 1.36 bc SYD middle dosage 6 41.19 ± 2.47 bd 33.45 ± 2.18 bd SYD low dosage 6 38.32 ± 1.58 bd 31.19 ± 2.49 bd P < 0.001 < 0.001
LXRα is highly expressed in liver tissue and is also expressed in other tissues (e.g.,fat and small intestine).LXRα,a sensor of TC,inhibits cholesterol absorption by intestinal epithelial cells after activation,increases cholesterol efflux,and promotes the conversion of cholesterol into bile acids [20].LXRα activation promotes cholesterol transfer to the ileal lumen [21].Therefore,the level of LXRα reflects the metabolic level of TC.CYP7A1 acts as a lipid-lowering target and its transcriptional activation and inhibition cascade network influences the balance of bile acid synthesis and lipid metabolism.CYP7A1 is the rate-limiting enzyme in the synthesis of bile acids from cholesterol and is one of the downstream target genes of LXRα.LXRα can affect the expression of CYP7A1 and promote the conversion of cholesterol to bile acids[22-23].Additionally,elevated CYP7A1 can reduce plasma cholesterol levels [24-25].CYP7A1 is involved in lipolysis and is able to convert TC in the liver into bile acids,excrete them,and reduce hepatic TC content[26].The results of this experiment showed that SYD effectively upregulated the expression levels of LXRα and downstream CYP7A1 in rat livers.On the one hand,it promotes cholesterol transfer to the ileal lumen by up-regulating LXRα.On the other hand,it converts TC in the liver into bile acids and is excreted from the body,thereby reducing the hepatic TC content.
Conclusion
In summary,the hyperlipidemic rat model downregulated the expression levels of LXRα and CYP7A1 proteins and affected lipid metabolism.A Tibetan medicine,SYD,may regulate lipid levels in hyperlipidemic rats by upregulating the expression of LXRα and CYP7A1 proteins.However,the mechanism of SYD in the regulation of blood lipids should be further studied.
 Traditional Medicine Research2019年6期
Traditional Medicine Research2019年6期
- Traditional Medicine Research的其它文章
- Study on the relationship between the structure of bacterial flora on the tongue and types of tongue coating in patients with type 2 diabetes mellitus
- A systematical review of traditional Ayurvedic and morden medical perspectives on Ghrita(clarified butter):a boon or bane
- Effectiveness of health coaching on diabetic patients: A systematic review and metaanalysis
- Treatment of diabetic foot ulcer with medicinal leech therapy and honey curcumin dressing:a case report
- Tu Youyou:A scientist moving forward in controversy
- The research of acupuncture on the treatment of alcohol dependence: hope and challenge
