Increasing Fatty Acids in Rice Root Improves Silence of Rice Seedling to Salt Stress
Liu Ling, Chen Jin, Tan Yanning, Zhou Tianshun, Ouyang Ning, Zeng Jia, Yuan Dingyang, , Duan Meijuan
Letter
Increasing Fatty Acids in Rice Root Improves Silence of Rice Seedling to Salt Stress
Liu Ling1, #, Chen Jin2, 3, #, Tan Yanning3, 4, Zhou Tianshun1, Ouyang Ning1, Zeng Jia2, Yuan Dingyang1, 3, 4, Duan Meijuan5
(1Long Ping Branch, Graduate School of Hunan University, Changsha 410125, China;2College of Bioscience and Biotechnology, Hunan Agricultural University, Changsha 410128, China;3Hunan Academy of Agricultural Sciences, Changsha 410125, China;4State Key Laboratory of Hybrid Rice, Hunan Hybrid Rice Research Center, Changsha 410125, China;5College of Agronomic, Hunan Agricultural University, Changsha 410128, China;#These authors contributed equally to this work)
Salt stress is one of the major abiotic stresses around the world, and salt salinity is one of the major constrains affecting rice production (Tu et al, 2014; Reddy et al, 2017). According to the statistics, more than one billion hectares of the land in the major continents are affected by salinity (Fageria et al, 2012; Zhou et al, 2018). Rice is a salt sensitive crop, considered more sensitive to salt stress during early stage (Hasanuzzaman et al, 2009). Understanding the method to improve plant salt tolerance is a potential way to enhance agriculture productivity in the future (Jing and Zhang, 2017). Polyunsaturated fatty acid (PUFA) on plasma membrane plays important roles in salt tolerance through enhancing the activity of Na+/H+transporters (López-Pérez et al, 2009). Chen et al (2018) has also found that fatty acids especially linoleic acid have closely relationship with salt stress by maintaining the stable of cell membrane. In plants, fatty acids are mainly contained in the seeds of dicots, used as the reserve of carbon source (Li-Beisson et al, 2013). The most important form of fatty acids is triacylglycerol (TAG). Acyl-CoA:diacylglycerol acyltransferase1 (DGAT1) catalyses the final step of TAG synthesis (Zhang M et al, 2009). Fatty acids are also synthesized in monocot (Liu, 2011), but their functions are still not clear.
In order to understand the relationship of fatty acids and salt tolerance in rice during the seedling stage, we firstly put rice seeds on 1/2 MS medium containing different NaCl concentrations (0, 20, 40, 60, 80 and 100 mmol/L NaCl). One week later, we found that seeds can still germinate and grow almost normally under 20 mmol/L NaCl treatment (Fig. 1-A). When the NaCl concentration rose to 40 mmol/L, the growth of the plants was obviously inhibited, and the root cannot grow normally any more. Finally, when the NaCl concentration reached 100 mmol/L, the germinating was totally inhibited, as is shown in Fig. 1-A. The results showed that rice roots in the germinating and seedling stage were quite sensitive to salt stress, and 40 mmol/L NaCl was high enough to inhibit the germinating.
In order to check the changes of fatty acids in rice roots corresponding to the treatment of different concentrations of NaCl, we analyzed the changes of lipid profile with thin layer chromatography (TLC) assay. Accompanied with the increasing concentration of NaCl, the concentration of triacylglycerol (TAG) was also increased, especially under 40 mmol/L NaCl treatment (Fig. 1-B), which indicated that the change of fatty acids in rice root was consistent with salt treated root phenotype.
In order to study the function of fatty acids in plants under high level NaCl, we next tried to enhance the fatty acid content in rice root of DGAT1 transgenic rice with the over-expression ofdriven bypromoter in Nipponbare. With sucrose induce experiment, we found that the expression ofwas induced from 30 mmol/L sucrose and then increased accompany with the increasing of sucrose concentration (Supplemental Fig. 1-A). Fatty acid content assay showed thatrice root contained much higher fatty acids compared with the control after treatment with 30 mmol/L sucrose (Supplemental Fig. 1-B), and the increased fatty acids did not affect the architecture of rice root, as is shown in Fig. 1-C.
After knowing the over-expression ofin rice root can increase fatty acid content, the function of fatty acids in salt stress process was performed. After growing both wild-type and transgenic seeds on 1/2 MS with 30 mmol/L sucrose medium for one week, we found that the transgenic rice root could germinate and elongation on both medium with or without 100 mmol/L NaCl (Fig. 1-C), however, wild-type rice was almost inhibited under 100 mmol/L NaCl with normal germination and growth on medium without NaCl (Fig. 1-C). Fatty acid assay using TLC showed that DGAT1 rice root with the treatment of 100 mmol/L NaCl had the biggest staining spot (Fig. 1-D). Gas chorography (GC) result indicated that DGAT1 rice had much higher fatty acids in root than the wild-type under 100 mmol/L NaCl (Fig. 1-E). Therefore, we speculated that synthesized fatty acids in rice root were mainly used to increase the salt resistance. As reported before, higher amount of fatty acids can improve the stable of plasma membrane, and then cells has higher ability to resistant Na+poison caused by NaCl (Zhang et al, 2012; Chen et al, 2018).
We also checked the expression levels of genes corresponding to salt stress and fatty acid synthesis. The primers used are shown in Supplemental Table 1. The results showed that with the increasing concentration of NaCl, the genes which are resistance to salt stress were up-regulated gradient, including,and(Fig. 2-A, -C and -D) (Zhang L et al, 2009; Jiang et al, 2012), and the salt sensitive gene, such as(Zhang L et al, 2009), was down-regulated(Fig. 2-B). Meanwhile, the relative expression levels of fatty acid-related genes, such as,,and, were increased (Fig. 2-F, -G, -I and -J). In DGAT1 rice, even though the expression levels of salt-resistance genes were also up-regulated with the treatment of 100 mmol/L NaCl compared with untreated, their expression levels were lower than those in NaCl-treated Nipponbare (Fig. 2). The salinity sensitive gene () had almost the same expression level in NaCl-treated DGAT1 rice root compared with untreated one, but much higher than Nipponbare rice root treated with NaCl (Fig. 2-L). The results indicated that the root with higher expression ofwas also not as sensitive to salinity as wild type. The speculated reason is that the increased fatty acid content in DGAT1 rice root improves the membrane liquidity, which increases the speed of Na+transportation and decreases the Na+concentration in innercell (Zhang et al, 2012). Further result showed that the expression levels of fatty acid-related genes were higher in NaCl-treated DGAT1 rice root than Nipponbare (Fig. 2).
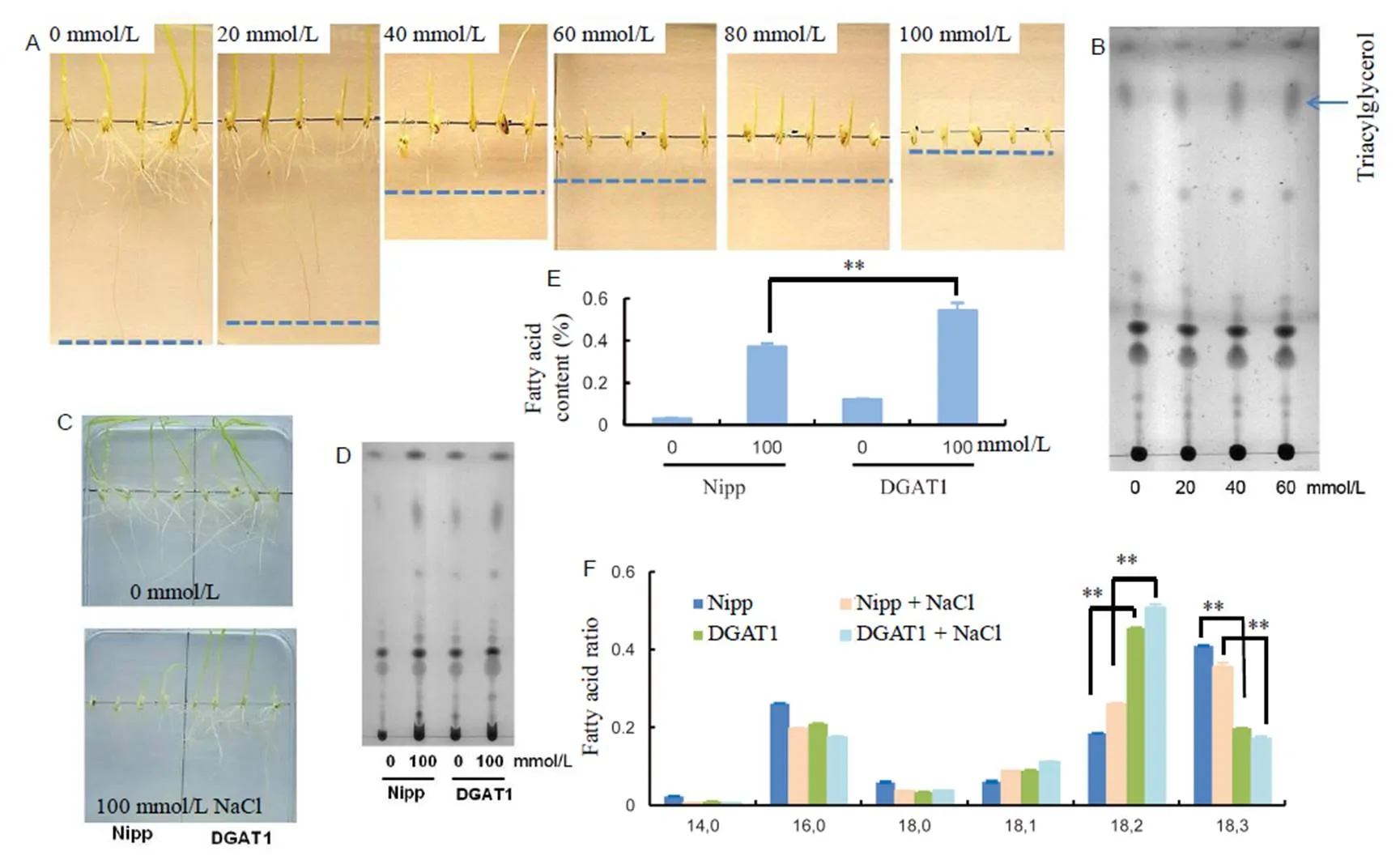
Fig. 1. Property of fatty acids in roots of rice seedlings under salt stress.
A, Root lengths of wild type rice treated with different concentrations of NaCl. B, Thin layer chromatography (TLC) analysis of fatty acids in rice roots under different NaCl concentrations. The arrow shows triacylglycerol. C, Seed germination and root elongation. D, TLC analysis of fatty acids in rice roots treated with or without 100 mmol/L NaCl. E, Gas cholography analysis of Nipp and DGAT1 rice roots treated with or without 100 mmol/L NaCl. F, Fatty acid ratio in rice roots with or without 100 mmmol/L NaCl treatment. Nipp, Nipponbare.
**,< 0.01 by the Student’stest with the comparison between DGAT1 and Nipp rice roots treated with or without NaCl. Values are Mean ± SD (= 3).
With the comparison of fatty acid compositions in rice root, we found that salt stress can inhibit the conversation of linoleic acid (18:2) into linolenic acid (18:3) in wild type rice root (Fig. 1-F), NaCl-treated Nipponbare rice root had significantly higher ratio of linoleic acid than untreated one, and the ratio of linolenic acid was lower. When analyzing the ratio of different fatty acid compositions in DGAT1 rice root, the interesting result was that DGAT1 rice root had much higher linoleic acid (18:2) ratio, especially after treatment with 100 mmol/L NaCl (Fig. 1-F). The result indicated that linoleic acid plays an important role in the process of salt stress tolerance. It has been reported linoleic acid can maintain cell membrane stability (Zhang et al, 2012; Chen et al, 2018), and therefore, the increased ratio of linoleic acid in rice root may increase the salt stress resistance. The collection of linoleic acid in DGAT1 rice root (Fig. 1-F) partially explained the function mechanism ofin tolerating salt stress. The increasedexpression level in NaCl-treated rice root (Fig. 2-F) also certificated the relationship of linoleic acid and salt stress, as certificated before,is crucial for the synthesis of linoleic acid (Dar et al, 2017).
In summary, we identified thatimproves the resistance of rice seedlings to salt stress through increasing the content of fatty acids in seedling roots. The higher ratio of linoleic acid in DGAT1 rice root also explains the resistant mechanism of DGAT1 rice root to salt stress. Our results provided a new insight into the improvement of the resistance of rice to salt stress and made it possible to grow rice and other monocot plants in barren land.

Fig. 2. qRT-PCR analysis of genes responsible for salt stress and fatty acids synthesis.
A to J, Relative expression levels (REL) of genes in rice roots under different NaCl treatments. K to T, Relative expression levels in Nipponbare (Nipp) and DGAT1 rice roots treated with 100 mmol/L NaCl. Values are Mean ± SD (= 3).was used as a housekeeping gene.
Acknowledgements
This study was supported by the Hunan Science and Technology Major Project (Grant No. 2018NK1010) and National Natural Science Foundation of China (Grant No. 31771767). We thank Yuan Guilong, Ding Jia and Yuan Guangjie for their critical comments and advice.
Supplemental data
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/journal/ 16726308; http://www.ricescience.org.
Supplemental File 1. Materials and methods used in this study.
Supplemental Fig. 1. Expression ofand fatty acid content analysis in DGAT1 rice root supplied with different concentration sucrose.
Supplemental Table 1. Primers used in this study.
Chen X L, Zhang L J, Miao X M, Hu X W, Nan S Z, Wang J, Fu H. 2018. Effect of salt stress on fatty acid and α-tocopherol metabolism in two desert shrub species., 247(2): 499–511.
Dar A A, Choudhury A R, Kancharla P K, Arumugam N. 2017. Thegene in plants: Occurrence, regulation, and role.,8: 1789.
Fageria N K, Stone L F, Santos A B D. 2012. Breeding for salinity tolerance.: Fritsche-Neto R, Borém A. Plant Breeding for Abiotic Stress Tolerance. Berlin: Springer-Verlag: 103–122.
Hasanuzzaman M, Fujita M, Islam M N, Ahamed K U, Nahar K. 2009. Performance of four irrigated rice varieties under different levels of salinity stress., 6(2): 85–90.
Jiang S Y, Bhalla R, Ramamoorthy R, Luan H F, Venkatesh P N, Cai M, Ramachandran S. 2012. Over-expression ofincreases drought and salt tolerance in transgenic rice plants., 21(4): 785–795.
Jing W, Zhang W H. 2017. Research progress on gene mapping and cloning for salt tolerance and variety improvement for salt tolerance by molecular marker-assisted selection in rice., 31(2): 111–123. (in Chinese with English abstract)
Li-Beisson Y, ShorroshB, Beisson F, Andersson M X, Arondel V, Bates P D, Baud S, Bird D, DeBono A, Durrett T P, Franke R B, Graham I A, Katayama K, Kelly A A, Larson T, Markham J E, Miquel M, Molina I, Nishida I, Rowland O, Samuels L, Schmid K M, Wada H, Welti R, Xu C C, Zallot R, Ohlrogge J. 2013. Acyl-lipid metabolism., 11: e0161.
Liu K S. 2011. Comparison of lipid content and fatty acid composition and their distribution within seeds of 5 small grain species., 76(2): 334–342.
López-Pérez L, Martínez-Ballesta M C, Maurel C, Carvajal M. 2009. Changes in plasma membrane lipids, aquaporins and proton pump of broccoli roots, as an adaptation mechanism to salinity., 70(4): 492–500.
Reddy I N B L, Kim B K, Yoon I S, Kim K H, Kwon T R. 2017. Salt tolerance in rice: Focus on mechanisms and approaches., 24(3): 123–144.
Tu Y, Jiang A M, Gan L, Hossain M, Zhang J M, Peng B, Xiong Y G, Song Z J, Cai D T, Xu W F, Zhang J H, He Y C. 2014. Genome duplication improves rice root resistance to salt stress., 7(1): 15.
Zhang J T, Liu H, Sun J, Li B, Zhu Q, Chen S L, Zhang H X. 2012.fatty acid desaturase FAD2 is required for salt tolerance during seed germination and early seedling growth., 7(7): e30355.
Zhang L, Tian L H, Zhao J F, Song Y, Zhang C J, Guo Y. 2009. Identification of an apoplastic protein involved in the initial phase of salt stress response in rice root by two-dimensional electrophoresis., 149(2): 916–928.
Zhang M, Fan J L, Taylor D C, Ohlrogge J B. 2009. DGAT1 and PDAT1 acyltransferases have overlapping functions intriacylglycerol biosynthesis and are essential for normal pollen and seed development., 21(12): 3885–3901.
Zhou G Y, Zhai C J, Deng X L, Zhang J, Zhang Z L, Dai Q G, Cui S Y. 2018. Performance of yield, photosynthesis and grain quality ofrice cultivars under salinity stress in micro-plots., 32(2): 146–154. (in Chinese with English abstract)
Duan Meijuan (duanmeijuan@163.com); Yuan Dingyang (yuandingyang@hhrrc.ac.cn)
22 August 2018;
10 January 2019
Copyright © 2019, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2019.01.005
- Rice Science的其它文章
- Genetic Diversity and Allelic Frequency of Selected Thai and Exotic Rice Germplasm Using SSR Markers
- Genome-Wide Association Analysis and Allelic Mining of Grain Shape-Related Traits in Rice
- Impact of Rice-Catfish/Shrimp Co-culture on Nutrients Fluxes Across Sediment-Water Interface in Intensive Aquaculture Ponds
- Soil Nitrogen Distribution and Plant Nitrogen Utilization in Direct-Seeded Rice in Response to Deep Placement of Basal Fertilizer-Nitrogen
- Characterization of a Novel Gain-of-Function Spotted-Leaf Mutant with Enhanced Disease Resistance in Rice
- Application of Micronutrients in Rice-Wheat Cropping System of South Asia

