Characterization of a Novel Gain-of-Function Spotted-Leaf Mutant with Enhanced Disease Resistance in Rice
Chen Ting, Chen Zheng, Atul Prakash Sathe, Zhang Zhihong, Li Liangjian, Shang Huihui,Tang Shaoqing, Zhang Xiaobo, Wu Jianli
Research Paper
Characterization of a Novel Gain-of-Function Spotted-Leaf Mutant with Enhanced Disease Resistance in Rice
Chen Ting, Chen Zheng, Atul Prakash Sathe, Zhang Zhihong, Li Liangjian, Shang Huihui,Tang Shaoqing, Zhang Xiaobo, Wu Jianli
()
We here reported the identification and characterization of a novel gain-of-function() mutant from an ethylmethylsulfone (EMS)-induced rice cultivar IR64.displayed reddish- brown lesions that firstly appeared on the leaf tips at the early tillering stage and spread gradually downward to cover the whole leaf blades that wilted subsequently. The lesion development was light- dependent under natural conditions.exhibited impaired photosynthetic capacity with decreased chlorophyll content and lowered photosynthetic parameters which ultimately led to the poor performance of agronomic traits. Severe cell death occurred inin accompany with increased malonaldehyde level and membrane ion leakage rate, elevated reactive oxygen species (ROS) accumulation, altered ROS scavenging activities, increased DNA fragmentation and decreased soluble protein levels. Defense responses were activated inwith enhanced resistance to rice bacterial blight, up-regulation of defense response genes and altered endogenous hormone levels. The spotted-leaf phenotype is controlled by a single dominant nuclear gene localized to a 305 kb region between RM5490 and InDel42 on the short arm of chromosome 7. The data suggested thatis a novel gain-of-function spotted-leaf mutant with enhanced bacterial disease resistance and immunity-associated premature leaf senescence and would provide the basis for cloning of the target gene.
defense response; rice; reactive oxygen species; senescence; spotted-leaf; disease resistance
Hypersensitive response (HR) is a type of programmed cell death (PCD) induced by pathogen invasion. This genetically complicated and tightly controlled cell death prevents further pathogen infection (Greenberg et al, 1994, 1997) and plays a critical role in plant development and responses to biotic/abiotic stresses (Williams and Dickman, 2008). Plant lesion mimic or spotted-leaf mutants that produce spontaneous HR-like lesions without obvious biotic/abiotic stresses (Huang et al, 2010) have been identified in many plant species and have facilitated the study on mechanisms underlying lesion formation and plant responses to biotic/abiotic stresses especially pathogen infection (Jiao et al, 2012; Zhang et al, 2018).
The first rice (L.) spontaneous spotted- leaf mutant was discovered by Sekiguchi and the mutant was later named sekiguchi lesion (), which is controlled by a single recessive gene (Kiyosawa, 1970). Since then, a large number of spotted-leaf mutants have been identified and more than a dozen of genes responsible for the corresponding phenotypes have been isolated (Yamanouchi et al, 2002; Zeng et al, 2004; Mori et al, 2007; Qiao et al, 2010; Tang et al, 2011; Wang S H et al, 2015; Wang Z H et al, 2015; Liu et al, 2017; Sun et al, 2017a, b; Wang et al, 2017; Xu et al, 2018; Zhang et al, 2018; Song et al, 2019). Most spotted-leaf mutants show HR-like lesions with cell death at/around the lesions such as(Mori et al, 2007),(Qiao et al, 2010) and(Zhang et al, 2018), while a few mutants develop lesions probably due to the accumulation of unknown pigments rather than dead cells (Huang et al, 2011). It has been shown that cell death in spotted-leaf mutants is mainly resulted from elevated reactive oxygen species (ROS) accumulation including hydrogen peroxide (Qiao et al, 2010; Huang et al, 2016; Chen et al, 2018), singlet oxygen and superoxide anion (Qiao et al, 2010), which are widely known to kill the cells when present in an excessive level. The cell death is accompanied by a series of changes in physiological and biochemical indicators such as DNA degeneration, increased membrane ion leakage and reduced soluble protein content (Wang et al, 2017; He et al, 2018). However, the most prominent character of spotted-leaf mutants is shown by enhanced disease resistance to various pathogens such as(Yin et al, 2000; Wu et al, 2008) andpv.(Chen et al, 2018). The elevated defense responses are usually accompanied with deposition of callose (Takahashi et al, 1999; Qiao et al, 2010), up-regulated expression of pathogenesis-response genes (Feng et al, 2013), and elevated level of phytohormones (Zhang et al, 2018). Similar observations have been noticed in many other mutants such asin(Dietrich et al, 1994, 1997),in(Büschges et al, 1997) andin(Gray et al, 1997). These altered defense responses including broad-spectrum resistance make spotted-leaf mutants an ideal source for characterizing the mechanisms underlying plant innate immunity.
The majority of spotted-leaf mutants identified so far are genetically recessive in nature (Huang et al, 2010). A few of them are controlled by dominant mutations, for example,and, both belong to viable gain-of-function mutations (Mori et al, 2007; Wu et al, 2008; Chen et al, 2018). In contrast, some dominant mutations are homozygous lethal. For example, the ricemutation shows severe growth arrest and the mutant plants die within three weeks (Tang et al, 2011). The homozygousmutant is dwarf with numerous brown lesions over the entire leaf blade and dies approximately 30 d after sowing owing to significantly increased level of hydrogen peroxide (Takahashi et al, 1999). Homozygous lethal mutants have also been reported in several other plant species. Ainsertion inencoding a peroxisomal protein results in abnormal embryos and homozygous lethal plants (Sparkes et al, 2003). Homozygousinis also seedling lethal, however, the mutant displays no increased resistance to plant pathogens despite showing lesions (Salt et al, 2011). The dominant maize mutantexhibits HR-like necrotic lesions, and homozygous plants are yellow-leaved and lethal at the seedling stage (Hu et al, 1998). The mechanism of homozygous lethal is still largely unknown, and thus, identification of such novel mutants would facilitate the understanding of the mechanisms involved.
We have reported the identification of a spotted-leaf mutant () from ethyl methanesulfonate (EMS) mutagenesis of anrice cultivar IR64 (Wu et al, 2005). In this study, homozygous plants were lethal at the seedling stage while heterozygous plants (hereafter refers to) displayed spontaneous necrotic lesions, lowered photosynthetic capacity and poor performance of agronomic traits. In addition,, a novel mutant, displayed enhanced disease resistance topv.accompanied by the upregulation of defense-related genes probably by activating jasmonate signaling pathway.
Materials and Methods
Rice materials
The spotted-leaf phenotype has been stably inherited over multiple generations under the field and greenhouse conditions in Hangzhou and Lingshui, China., as the female parent, was crossed to a normal green leafcultivar Moroberekan, and the resultant F1plants and F2individuals were grown in the paddy field at the China National Rice Research Institute (CNRRI) for genetic analysis and gene mapping.
Agronomic trait evaluation
The wild-type IR64 andwere grown in the paddy field at CNRRI from May to October 2018 with a conventional management of water and fertilizer. The major agronomic traits including plant height, panicle length, number of tillers per plant, number of filled grains per panicle, seed-setting rate and 1000-grain weight were measured from three-randomly chosen individual plants at full maturity. The means from three replicates were used for Student’stest with the Excel 2010 software.
Shading treatment
At the tillering stage, the leaves without lesions inand IR64 were shaded respectively with a piece of 2 cm aluminum foil for 3 d under natural field conditions. Then, the foil was removed and light was reinstated. In addition, theleaves with lesions were shaded for 7 d. Lesion development was documented by a scanner (HP scanner jet 4010, Shanghai, China).
Measurement of photosynthetic pigments and photosynthetic parameters
The upper fresh leaves ofand IR64 at the tillering and heading stages were used for pigment extraction. The contents of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl T) and carotenoid were determined according to Arnon (1949) and Wellburn (1994). Absorption values were measured using a SpectraMax i3x Multi-Mode Microplate Reader (Molecular Devices, Sunnyvate, CA, USA). Photo- synthetic parameters including the net photosynthetic rate (), stomatal conductance (), transpiration rate () and intercellular CO2concentration () were measured on the flag leaves ofand IR64 at 9:00–10:00 am under natural field conditions using LI-6400XT portable photosynthesis system (LI-COR, Lincoln, NB, USA). All the measurements were taken at the saturation irradiance with an incident photo- synthetic photo flux density (PPFD) of 1 200 µmol/(m2·s) and an airflow rate at 500 µmol/s. The mean values from three measurements were used for Student’stest with the Excel 2010 software.
Histochemical analysis
The leaves with lesions inand leaves of IR64 at the tillering stage were collected for histochemical assay. The cell death was detected by trypan blue staining according to Yin et al (2000). H2O2accumulation was detected by 3,3-diaminobenzidine (DAB) staining (Thordal-Christensen et al, 1997). Nitroblue tetrazolium (NBT) staining for O2-accumulation was carried out as described previously (Qiao et al, 2010). The pictures were recorded using a scanner (HP scanner jet 4010, Shanghai, China).
TUNEL assay and membrane ion leakage determination
The leaves with lesions inand leaves of IR64 at the tillering stage were used for a terminal deoxyribo- nucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay by using a FluoresceinCell Death Detection Kit following the manufacturer’s instructions (Roche, Basel, Switzerland). Samples were photographed using a confocal laser scanning microscope (Ceise, Jena, Germany). Membrane ion leakage of flag leaves inand IR64 were determined according to He et al (2018). The membrane ion leakage was measured using a DDS-307A conductivity meter (LeiCi, Hangzhou, China). The means from three measurements were used for Student’stest with the Excel 2010 software.
Determination of enzymatic activity
Activities of anti-oxidative enzymes, including catalase (CAT), ascorbate peroxidase (APX), peroxidase (POD) and superoxide dismutase (SOD), as well as the contents of hydrogen peroxide (H2O2), malonaldehyde (MDA) and soluble proteins, were determined following the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Absorption values were measured using a SpectraMax i3x Multi- Mode Microplate Reader (Molecular Devices, Sunnyvate, CA, USA). The means from three measurements were used for Student’stest with the Excel 2010 software.
Disease resistance evaluation
The leaves of 7-week-old plants from, IR64 and the susceptible control Jingang 30 (JG30) were inoculated withpv.strain PXO99 (Philippine race 6) and PXO86 (Philippine race 2). The bacterial strains were cultured on a peptone sucrose agar medium. Six fully expanded leaves per genotype were inoculated with a bacterial suspension (600= 1.0) by a leaf clipping method (Kauffman et al, 1973). The lesion length was measured 14 d after inoculation. The mean lesion length values from six leaves were used for Student’stest with the Excel 2010 software.
Gene expression analysis
Total RNA was extracted from leaves ofand IR64 using NucleoZOL Reagent Kit according to the manufacturer’s instructions (Macherey-Nagel, Düren, Germany). RNA was reverse-transcribed using the ReverTra Ace qPCR RT Master Mix with genomic DNA (gDNA) Remover Kit (Toyobo, Osaka, Japan). The quantitative real-time PCR (qRT-PCR) was carried out using the FastStar Essential DNA Green Master Kit (Roche, Basel, Switzerland) and performed on a Thermal Cycler Dice Real Time System II (Takara, Kusatsu, Japan), following the method described previously (Chen et al, 2018). The ricewas used as an internal control. The means from three replicates were used for Student’stest with the Excel 2010 software. The primers of defense response genes used for qRT-PCR are listed in Supplemental Table 1.
Hormone level determination
The flag leaves ofand IR64 at the heading stage were used for determination of the levels of hormones including salicylic acid (SA), jasmonic acid (JA), indole acetic acid (IAA) and abscisic acid (ABA) by Zoonbio Biotechnology Co., Ltd, Nanjing, China, following the method described previously (Zhang et al, 2018). The means from three replicates were used for Student’stest with the Excel 2010 software.
Genetic analysis and gene mapping
The F1plants were grown in the paddy field at CNRRI for determining the dominant/recessive nature of the target gene(s). The F2individuals were used for segregation analysis and gene mapping. Equal amounts of leaves from each of ten Moroberekan (the wild type, WT) and tenplants were collected for DNA extraction to form a WT DNA pool and a mutant-type DNA pool, respectively. The DNA of the parents and F2normal green individuals were extracted following the method of Edwards et al (1991). Simple sequence repeat (SSR) markers were obtained from thewebsite (http://www.gramene.org/) while insertion/deletion (InDel) markers were designed using the Primer 5.0 software after comparison of the sequences between thecultivar Nipponbare and thecultivar 93-11 on thewebsite (http://plants.ensembl.org/ index.html, http://gramene.org/genome_browser/index. html). PCR was performed in a total volume of 10 μL reaction buffer containing 50 ng template DNA, 1.0 μmol/L each primer, 5.0 μL of 2× PCR mixture (TsingKe Biological Technology, Hangzhou, China). The reaction was performed on a Biometra Thermal Cycler (Hiomedizinische Analytik, Germany): Pre- denaturation at 94 ºC for 2 min, followed by 35 cycles of 94 ºC for 30 s, 55 ºC for 30 s and 72 ºC for 30 s, with a final extension at 72 ºC for 5 min. The PCR products were run on 6% non-denaturing polyacrylamide gel electrophoresis (PAGE) and detected using silver staining. The primers were synthesized by TsingKe Biological Technology (Hangzhou, China). The primers for gene mapping are listed in Supplemental Table 2.
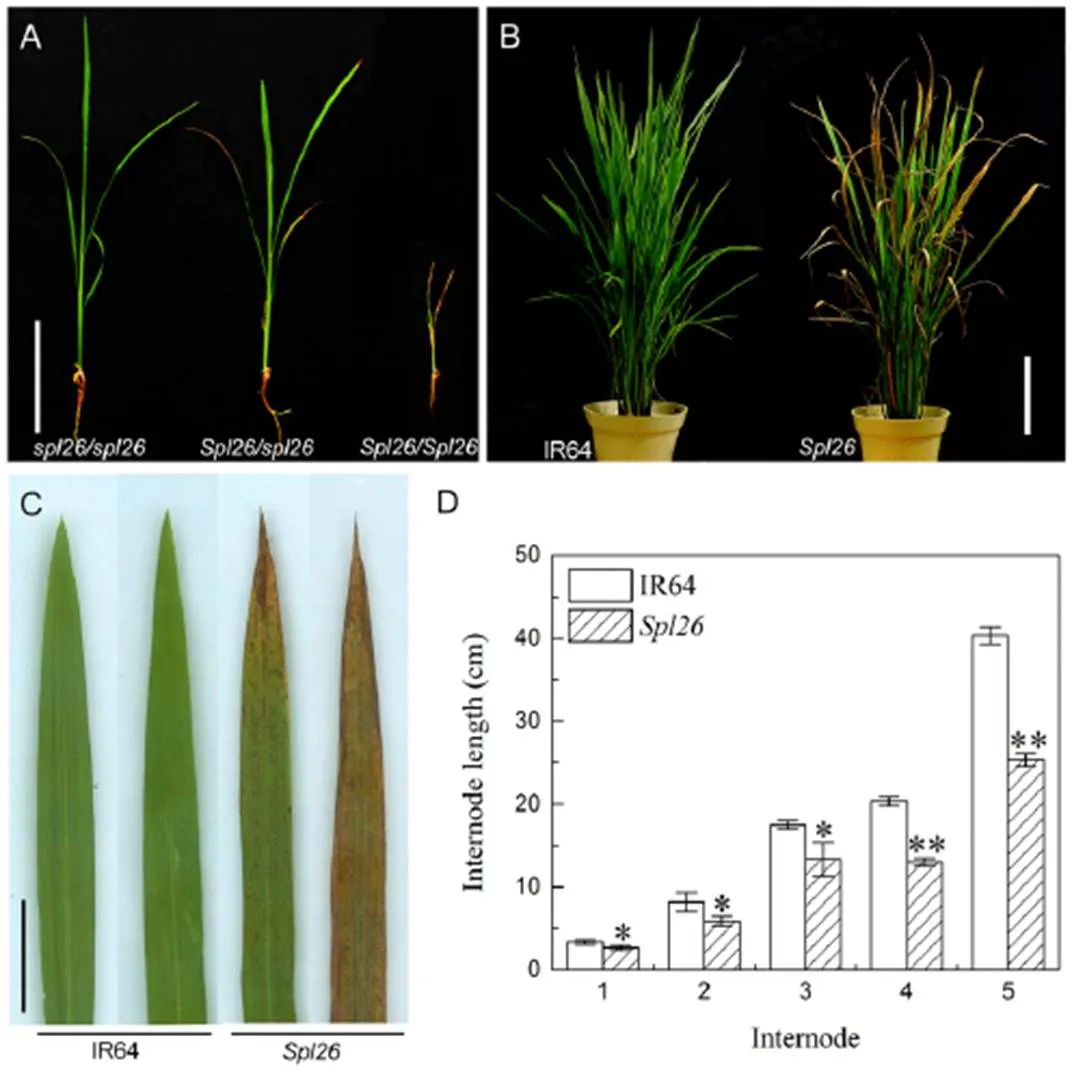
Fig. 1. Phenotypes ofand the wild-type IR64.
A, Phenotypes ofplants grown under normal field conditions for 19 d./is a wild-type,/is a heterozygous plant, and/is a homozygous plant. Scale bar = 20 cm. B, IR64 andat the tillering stage. Scale bar = 20 cm. C, Leaves ofand IR64. Scale bar = 2 cm. D, Internode length of IR64 and.
Values are Mean ± SD (= 3). * and ** indicate significant differences at the 0.05 and 0.01 levels by the Student’stest, respectively.
Results
Phenotypic characterization of Spl26
displayed three types of phenotypes under natural field conditions in Hangzhou, China. The homozygous plant (/) developed brown leaf lesions which quickly merged, wilted and died in three weeks after germination (Fig. 1-A). The heterozygous plant (/, hereafter refers to) exhibited reddish-brown spots/lesions on leaf tips approximately 23 d after sowing. The lesions then spread gradually downward to cover the whole leaf blade. Subsequently, the color of lesions deepened and lesions merged, and the older leaves turned yellow and wilted, resulting in partial or overall leaf death at the tillering stage (Fig. 1-A to -C). The other type of homozygous plants (/) showed a similar phenotype to the wild-type (WT) IR64 (Fig. 1-A to -C). As the homozygous plants (/) were seedling lethal, the heterozygousand WT plants were used in subsequent experiments. Under the paddy field conditions, performance of agronomic traits including plant height, panicle length, number of tillers per plant, number of filled grains per panicle, seed-setting rate and 1000-grain weight were significantly decreased incompared with WT (Table 1). In addition, the heading date ofwas about 10 d later than that of IR64. The reduced plant height forwas due to the shortened length of the panicle, along with shortened lengths of all internodes compared with WT (Fig. 1-D). The results suggested that the spotted-leaf mutation also significantly affected the agronomic performance.
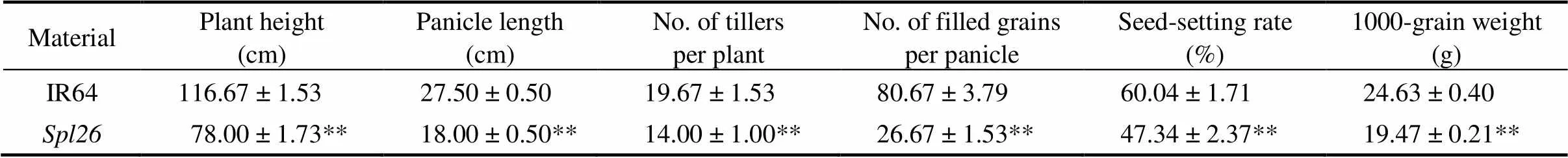
Table 1.Comparison of agronomic traits between IR64 and Spl26.
Data represent Mean ± SD (= 3). ** indicates significant difference at≤ 0.01 by the Student’stest.
Lesion occurrence in Spl26 is light-dependent
Lesion initiation of many lesion mimic mutants is light-dependent (Huang et al, 2010). To determine the effect of light onunder field conditions, the leaves ofwithout lesions were covered with a piece of 2 cm aluminum foil for 3 d. The results showed that no obvious changes were observed before and after shading treatment in WT (Fig. 2-A and -C). Reddish-brown lesions did not appear in the shaded leaf area, while a mass of lesions emerged in the unshaded leaf area of(Fig. 2-B and -D). After removing the foil and reinstating the light for 7 d, new reddish-brown lesions occurred in the shaded leaf area of(Fig. 2-E). In addition, the lesions that developed earlier would not disappear after shading for 7 d (Fig. 2-F). These results suggested that the lesion initiation and development inwas light inducible.
Photosynthetic alterations of Spl26
To explore whether the photosynthetic capacity ofwas changed, we examined the contents of chlorophyll and carotenoid at different growth stages. At the tillering stage, the contents of Chl a, Chl b, Chl T and carotenoid inwere significantly decreased in contrast to IR64, while the ratios of Chl a and Chl b were similar between the two genotypes (Fig. 3-A). A similar trend was detected for the pigment contents betweenand IR64 at the heading stage (Fig. 3-B). These results indicated that the decrease of photosynthetic pigments inmight be caused by necrotic lesions, which led to reduction of the number of living cells containing chloroplasts.

Fig. 2. Effect of light on the formation of lesion in.
A, IR64 before shading. B,before shading. C, IR64 shaded for 3 d. D,shaded for 3 d. E,reinstated for 7 d. F,leaf with lesions shaded for 7 d. Shaded areas are boxed. Scale bar = 2 cm.
We then measured the photosynthetic parameters of the flag leaves inand IR64 at the heading stage. We found that,andinwere significantly lower than those in IR64 (Fig. 3-C to -E), whileshowed no significant difference betweenand IR64. The results suggested that the lowered levels of chlorophyll contents and photo- synthetic parameters indeed resulted in the lower photosynthetic capacity in, resulting in the poorer agronomic performance.
HR-like necrosis and cell death occurred in Spl26
To detect whether cell death occurred in, we first carried out trypan blue staining. The leaves from IR64 andwith apparent lesions were stained with trypan blue at the tillering stage. The staining results showed that a few blue precipitate were observed on leaves of IR64. In contrast, a large number of blue precipitate were observed on leaves of(Fig. 4-A and -B), indicating that severe cell death occurred in. We next examined the membrane ion leakage ofand IR64 at the heading stage. The results showed that the value of membrane ion leakage ofwas significantly higher than that of IR64 (Fig. 4-C), suggesting that irreversible membrane damage occurred in. Further, we performed TUNEL assay for detection of DNA fragmentation, an indicator of apoptosis. The green signals indicated that the exposed 3-OH of DNA fragments were labeled with fluorescein-labeled dUTP under the catalysis of terminal deoxynucleotidyl transferase. The results showed that a large number of labeled nuclei (green) were detected as TUNEL positive signals in, whereas a few number of labeled nuclei were found in IR64 (Fig. 4-D), indicating the presence of numerous degenerated DNA fragments in. Taken together, these results revealed that HR-like necrosis with cell death occurred in.
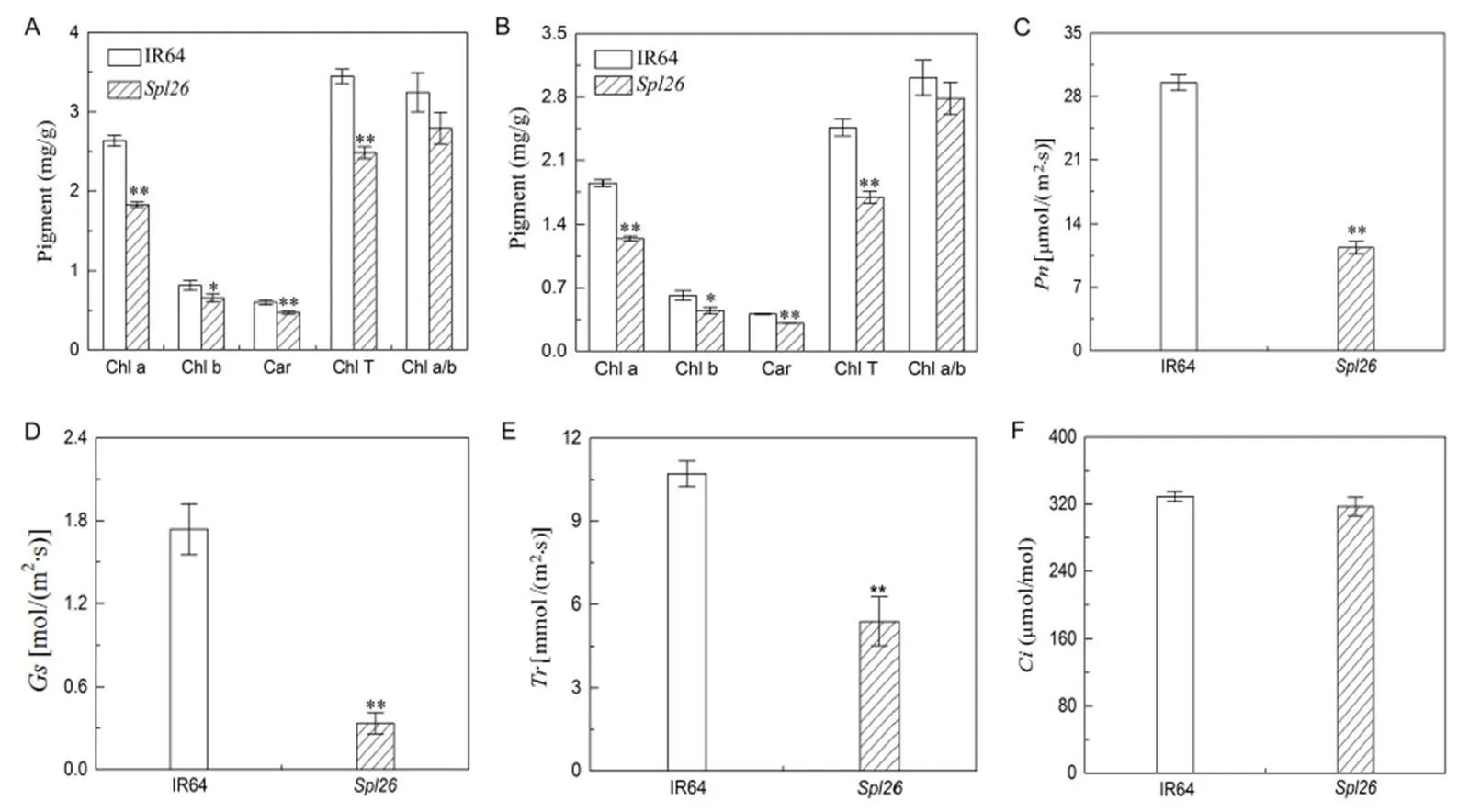
Fig. 3.Photosynthetic pigment contents and photosynthetic parameters ofand IR64.
A, Photosynthetic pigment contents ofand IR64 at the tillering stage. B, Photosynthetic pigment contents ofand IR64 at the heading stage. C, Net photosynthetic rate (). D, Stomatal conductance (). E, Transpiration rate (). F, Intercellular CO2concentration ().
Values are Mean ± SD (= 3). * and ** indicate significant differences at the 0.05 and 0.01 levels by the Student’stest, respectively.

Fig.4. Determination of cell death indicators inand IR64.
A, Trypan blue staining of IR64 at the tillering stage. Scale bar = 1 cm. B, Trypan blue staining ofat the tillering stage. Scale bar = 1 cm. C, Membrane ion leakage rate at the heading stage. D, Terminal deoxyribonucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay at the tillering stage. Blue signal represents 40,6-diamino- phenylindole (DAPI). Green color represents positive result. (a) and (d) are DAPI staining; (b) and (e) are TUNEL signal; (c) and (f) are merged images of (a/b) and (d/e), respectively. Scale bar = 50 μm.
Values are Mean ± SD (= 3). ** indicates significant differences at≤ 0.01 by the Student’stest.
ROS accumulation with decreased activities of ROS scavenging enzymes in Spl26
Excessive accumulation of ROS results in pernicious effect on cells. To determine the accumulation of ROS in, we first carried out DAB staining, a histochemical indicator of H2O2accumulation. We found that the brown precipitate appeared in and around the lesions in, while the leaves of IR64 were clear, indicating that there was a large amount of H2O2accumulation in(Fig. 5-A and -B). In agreement with DAB staining, the content of H2O2inwas significantly higher than that in IR64 (Fig. 5-E). We then carried out NBT staining, a histochemical indicator of O2-accumulation, the results showed that more blue spots were observed on leaves ofcompared with IR64 (Fig. 5-C and -D). These results indicated that the accumulations of both H2O2and O2-led to the cell death in.
To investigate the potential mechanism of ROS accumulation in, we measured the activities of anti-oxidative enzymes including POD, SOD, CAT and APX, which are usually activated to remove excessive ROS under oxidative stress. Our results showed that the activity of CAT was significantly lower inthan in IR64 (Fig. 5-F), while the APX activity inwas similar to that in IR64 at the heading stage (Fig. 5-G). In contrast, the activities of POD and SOD were significantly increased inat the heading stage compared with IR64 (Fig. 5-H and -I). In addition, the MDA content was significantly higher inthan in IR64 (Fig. 5-J), while the content of soluble proteins was apparently decreased incompared with IR64 (Fig. 5-K). These results suggested that the accumulation of ROS was caused by decreased ROS scavenging ability and ultimately led to the HR-like necrosis and cell death in.

Fig. 5.Determination of reactive oxygen species (ROS)-associated parameters inand IR64.
A, 3,3-diaminobenzidine (DAB) staining of IR64 at the tillering stage. Scale bar = 2 cm. B, DAB staining ofat the tillering stage. Scale bar = 2 cm. C, Nitroblue tetrazolium (NBT) staining of IR64 at the tillering stage. Scale bar = 2 cm. D, NBT staining ofat the tillering stage. Scale bar = 2 cm. E, H2O2content. F, Catalase (CAT) activity. G, Ascorbate peroxidase (APX) activity. H, Peroxidase (POD) activity. I, Superoxide dismutase (SOD) activity. J, Malonaldehyde (MDA) content. K, Soluble protein content.
Values are Mean ± SD (= 3). ** indicates significant difference at the 0.01 level by the Student’stest.
Enhanced disease resistance and activation of defense responses in Spl26
Classical spotted-leaf or lesion mimic mutants show enhanced resistance to pathogen infection. To evaluate the disease resistance in, we inoculated the 7-week-old plants of, IR64 and the susceptible control JG30 with virulent strains PXO99 and PXO86 ofpv.(). The results showed that JG30 was susceptible both to PXO99 and PXO86. The disease index and lesion length ofwere significantly reduced when compared to the wild- type IR64 (Fig. 6-A to -C). The results demonstrated that the mutation inindeed induced significantly enhanced resistances to PXO99 and PXO86.
It has been shown that enhanced disease resistance is associated with the activation of defense response genes during pathogen infection (Mizobuchi et al, 2002; Wang et al, 2015). To test this possibility in, we measured the expression of nine defense signaling-related genes (,,,,,,,and) by qRT-PCR. Our results showed that the mRNA levels of these genes inwere significantly increased by 4.4-, 19.6-, 24.3-, 9.7-, 5.8-, 3.5-, 13.3-, 10.2- and 44.8-fold, respectively, compared with IR64 (Fig. 6-D). The up-regulation of defense response genes clearly demonstrated that the enhanced disease resistance ofwas accompanied by the activation of defense response.

Fig. 6.Evaluation of disease resistance and expression of defense response genes inand IR64.
A, Reaction to PXO99. 1–2, JG30; 3–4, IR64; 5–6,. Scale bar = 5 cm. B, Disease index (lesion length/leaf length ratio). Different lowercase letters above the bars indicate difference at≤ 0.05 by the Duncan’s test. C, Lesion length. Different letters indicate a statistical difference at≤ 0.05 by the Duncan’s test. D, Expression of defense response genes inand IR64. The expression level of each gene in IR64 was normalized to 1. ** indicates significant difference at≤ 0.01 by the Student’stest.
Values are Mean ± SD (= 3).
To further understand whether the upregulated expression of defense genes was associated with the levels of endogenous plant hormones in, we determined the levels of several major hormones such as SA, JA, IAA and ABA in the flag leaves ofand IR64. As shown in Fig. 7, the contents of JA, IAA and ABA inwere increased by 10.0-, 6.1- and 3.8-fold compared with those in IR64, respectively, whereas the SA content decreased by 70.7% in. The results suggested that the enhanced disease resistance ofwas probably resulting from the activation of JA-mediated signaling pathway.
Genetic analysis and gene mapping of Spl26
To determine the inheritance pattern of the spotted- leaf phenotype of, we crossedwith a normal green leaf cultivar Moroberekan. We obtained a total of 28 F1plants which morphologically segregated into two categories. Thirteen out of 28 F1plants showed reddish-brown lesions similar towhile the remaining 15 F1plants were green-leaved similar to the wild type, indicating thatwas heterozygous in nature. In addition, in the 329 plants derived fromselfed seeds, 85 plants were normal green-leaved, 149 plants were reddish-brown leaved and 95 plants were brown-spotted and lethal, with the segregation ratio of normal green : reddish brown : lethal fitting to 1 : 2 : 1 (χ2= 1.87 < χ20.05= 5.99,= 2,= 0.17), indicating thatwas heterozygous in nature and controlled by a single dominant gene. In an F2mapping population derived from/ Moroberekan, we found that 1286 plants were reddish-brown leaved and 657 plants were normal green-leaved. The segregation ratio of reddish brown : normal green fitted to 2 : 1 (χ2= 0.20 < χ20.05= 3.84,= 1,= 0.65). These results suggested that the spotted-leaf phenotype ofwas controlled by a single dominant nuclear gene.

Fig. 7.Contents of hormones inand IR64.
SA, Salicylic acid; JA, Jasmonic acid; IAA, Indole acetic acid; ABA, Abscisic acid.
Hormone level in IR64 was normalized to 1. Values are Mean ± SD (= 3). ** indicates significant difference at≤ 0.01 by the Student’stest.
To locate the mutation, we carried out polymorphism survey betweenand Moroberekan using 1064 SSR markers covering all the 12 chromosomes. A total of 487 SSR markers showing polymorphism were identified, which then were used for genotyping of two DNA pools from reddish-brown and WT F2individuals, respectively. RM6697 and RM5752 from chromosome 7 were polymorphic between the DNA pools, indicating that the mutation was likely located to chromosome 7. Subsequently, 36 randomly-selected F2WT plants from the cross were used to confirm whether RM6697 and RM5752 were linked to the mutation. Four and three recombinants each were identified with RM6697 and RM5752, respectively, indicating that the mutation was localized to the short arm of chromosome 7. To further delimit the locus, 865 individual F2WT plants were genotyped. Finally, the mutation locus, tentatively named, was narrowed down to a 524 kb region between RM5490 and RM5752 (Fig. 8).
To further fine-map the mutation, InDel markers were designed according to sequence differences between(93-11) and(Nipponbare) rice. Polymorphic InDel markers were used for the genotyping of 865 WT individuals. Eventually,was delimited to a 305 kb region between RM5490 and InDel42, with 4 and 11 recombinants for each marker, respectively (Fig. 8). Based on the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/), 55 candidate genes were predicted in the 305 kb region, including 48 open reading frames (ORFs), three transposon proteins and four retrotransposon proteins. Isolation and identification of the targetfor spotted-leaf phenotype is currently underway.
Discussion
Lethal phenotype of homozygous Spl26
In the present study, we isolated a novel spotted-leaf mutant,, with reddish-brown lesions and wilted leaf blades in its life cycle. The lesion mimic trait was controlled by a dominant mutation localized to chromosome 7.was maintained in a heterozygous form (/) that imposed a great negative effect on major agronomic traits such as the plant height, plant tiller number and seed-setting rate. Unlike the viable homozygousin rice (Chen et al, 2018), the homozygous mutation (/) plants were unable to survive and died at the seedling stage. These results suggested that the dominant gain-of- function allele displayed a dosage effect ranging from arrested growth to lethality. It has been shown that thehomozygous mutantis also lethal at the seedling stage and exhibits more severe necrotic cell death than the heterozygous mutants at the cellular level (Salt et al, 2011). Similarly, the dominant maize mutantexhibits HR-like necrotic lesions, and the homozygous plants are yellow-leaved and lethal at the seedling stage (Hu et al, 1998).encodes a uroporphyrinogen decarboxylase (UROD), which is a key enzyme in the biosynthetic pathway of chlorophyll and heme metabolism in plants. The mutation causes an excessive accumulation of photoexcitable uroporphyrin leading to the generation of reactive oxygen species that ultimately kill the cells (Hu et al, 1998). Likeand, thelesion development was also light dependent, whether there is similar mechanism involving in cell death and plant survival has yet to be investigated.

Fig. 8.Map-based isolation of.
A,was located on the short arm of chromosome 7 between RM6697 and RM5752. B, Fine mapping ofbased on 865 wild-type F2individuals.
HR-like PCD is associated with premature leaf senescence in Spl26
It has been shown that several HR-like PCDs in spotted-leaf mutants are accompanied by premature leaf senescence phenotype, to which the mechanism is largely unknown (Qiao et al, 2010). In a study ofin rice, this phenomenon is termed immunity- associated senescence asexhibits a darkness- accelerated premature leaf senescence accompanied by the loss of chlorophyll, breakdown of chloroplasts, down-regulation of photosynthesis-related genes, up-regulation of senescence associated genes, as well as increased level of endogenous ABA which is widely considered to trigger senescence in plants (Xu et al, 2018). Similarly,exhibits significantly decreased chlorophyll content, increased MDA level, membrane ion leakage rate, DNA fragmentation and lowered soluble protein content that are prominent indicators manifested on the progression of senescence (He et al, 2018). Furthermore, decreased,andinmight be a result of ongoing leaf senescence or decreased expression of photosynthesis- related genes. It has been shown that disturbed ROS signaling and ROS scavenging enzyme activity is an important factor associated with leaf senescence (Strecker et al, 2010; Zhou et al, 2013). In, elevated H2O2level is likely associated with the decreased CAT activity, the major H2O2-scavenging enzyme, as well as increased activity of SOD which catalyzes the dismutation of O2-to generate H2O2(Miller et al, 2010). This could well explain why the signal of DAB staining was stronger than that of NBT staining in. In plants, POD has been shown to catalyze the oxidation of cinnamyl alcohols for lignin condensation, and the activity of POD is generally higher in aging tissues than in young tissues (Schlimme et al, 2002). Therefore, increased POD activity may reflect the progression of senescence in. Nevertheless, the detailed relationship between the mutation and accumulation of ROS and altered enzymatic activities are yet to be further studied.
Enhanced disease resistance of Spl26 may be mediated by JA signaling pathway
Typical spotted-leaf mutants show enhanced disease resistance in accompany with elevated expression level of defense-associated genes and altered cellular level of hormones (Wang et al, 2015; Xu et al, 2018; Zhang et al, 2018). In this study, the levels of OsSPL26-mediated disease resistance to PXO99 and PXO86, two virulentstrains to the wild type IR64, were significantly increased in accompany with upregulated defense gene expression and elevated internal hormone levels. Highly increased level of cellular JA inis consistent with the upregulation of,,,and, which are important contributors in JA signaling pathway (Shen et al, 2011). Similarly, themutantaccumulates a high level of JA and exhibits increased disease resistance to(Liu et al, 2012). In contrast, impaired JA synthesis and signal transduction would result in increased susceptibility to the fungal root pathogeninfection as shown by themutant, which is unable to accumulate jasmonate (Vijayan et al, 1998). Furthermore, the expression of SA-synthesis-associated genesandand the down-stream transcription cofactorwere all significantly activated in. Over- expression ofwould lead to increased resistance both inand rice (Fu et al, 2012; Liu et al, 2017). It is generally considered that JA and SA interact in an antagonistic manner, consistent inas shown by increased JA and decreased SA levels although this is not always the case whereregulates defense-related genes but not SA and JA levels (Yuan et al, 2007). Furthermore, increasedexpression might be partially responsible for arrested plant growth and development inas overexpression ofsuppresses the auxin signaling (Li et al, 2016). However, the mechanism remains to be elucidated since the IAA level inis apparently increased in contrast to the report by Li et al (2016). Furthermore, ABA plays a critical role in leaf senescence (Lim et al, 2007). The increased levels of ABA inmight partially contribute to leaf senescence along with the decrease of photosynthetic pigments, the increase of MDA content, DNA fragmentation and ROS accumulation. Nevertheless, we concluded that OsSPL26-mediated increase of bacterial resistance was likely conferred by activating the JA signaling pathway.
Acknowledgement
This work was supported by the Ministry of Science and Technology of China (Grant No. 2016YFD0101104).
Supplemental DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Table 1. List of primers for quantitative real-time PCR.
Supplemental Table 2. List of primers for gene mapping.
Arnon D I. 1949. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in., 24(1): 1–15.
Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, van Daelen R, van der Lee T, Diergaarde P, Groenendijk J, Töpsch S, Vos P, Salamini F, Schulze-Lefert P.1997. The barleygene: A novel control element of plant pathogen resistance., 88(5): 695–705.
Chen Z, Chen T, Sathe A P, He Y Q, Zhang X B, Wu J L.2018. Identification of a novel semi-dominant spotted-leaf mutant with enhanced resistance topv.in rice., 19(12): 3766.
Dietrich R A, Delaney T P, Uknes S J, Ward E R, Ryals J A, Dangl J L. 1994.mutants simulating disease resistance response., 77(4): 565–577.
Dietrich R A, Richberg M H, Schmidt R, Dean C, Dangl J L.1997. A novel zinc finger protein is encoded by thegene and functions as a negative regulator of plant cell death., 88(5): 685–694.
Edwards K, Johnstone C, Thompson C.1991. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis., 19(6): 1349.
Feng B H, Yang Y, Shi Y F, Shen H C, Wang H M, Huang Q N, Xu X, Lü X G, Wu J L. 2013. Characterization and genetic analysis of a novel rice spotted-leaf mutantwith broad-spectrum resistance topv.., 55(5): 473–483.
Fu Z Q, Yan S P, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel S H, Tada Y, Zheng N, Dong X N. 2012. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants., 486: 228–232.
Gray J, Close P S, Briggs S P, Johal G S. 1997. A novel suppressor of cell death in plants encoded by thegene of maize., 89(1): 25–31.
Greenberg J T, Guo A, Klessig D F, Ausubel F M.1994. Programmed cell death in plants: A pathogen-triggered response activated coordinately with multiple defense functions., 77(4): 551–563.
Greenberg J T. 1997. Programmed cell death in plant-pathogen interactions., 48: 525–545.
He Y, Zhang Z H, Li L J, Tang S Q, Wu J L.2018. Genetic and physio-biochemical characterization of a novel premature senescence leaf mutant in rice (L.)., 19(8): 2339.
Hu G S, Yalpani N, Briggs S P, Johal G S. 1998. A porphyrin pathway impairment is responsible for the phenotype of a dominant disease lesion mimic mutant of maize., 10(7): 1095–1105.
Huang Q N, Yang Y, Shi Y F, Chen J, Wu J L.2010. Spotted-leaf mutants of rice ()., 17(4): 247–256.
Huang Q N, Shi Y F, Yang Y, Feng B H, Wei Y L, Chen J, Baraoidan M, Leung H, Wu J L. 2011. Characterization and genetic analysis of a light- and temperature-sensitive spotted-leaf mutant in rice., 53(8): 671–681.
Huang Q N, Shi Y F, Zhang X B, Song L X, Feng B H, Wang H M, Xu X, Li X H, Guo D, Wu J L. 2016. Single base substitution inis responsible for premature senescence and death phenotype in rice., 58(1): 12–28.
Jiao B B, Wang J J, Zhu X D, Zeng L J, Li Q, He Z H. 2012. A novel protein RLS1 with NB-ARM domains is involved in chloroplast degradation during leaf senescence in rice., 5(1): 205–217.
Kauffman H E, Reddy A P K, Hsieh S P V, Merca S D. 1973. An improved technique for evaluating resistance of rice varieties to., 57: 537–541.
Kiyosawa S.1970. Inheritance of a particular sensitivity of the rice variety, Sekiguchi-Asahi, to pathogens and chemicals, and linkage relationship with blast resistance., 21: 61–71.
Li L F, Xiong Y Y, Ouyang L J, Peng X S, Chen X R, He X P, Fu J R, Bian J M, Hu L F, Xu J, He H H, Sun X T, Zhu C L. 2018. Identification and gene mapping of white stripe leaf and white panicle mutantin rice., 32(6): 538–548. (in Chinese with English abstract)
Li X Z, Yang D L, Sun L, Li Q, Mao B Z, He Z H. 2016. The systemic acquired resistance regulatorattenuates growth by repressing auxin signaling through promoting IAA- amido synthase expression., 172(1): 546–558.
Lim P O, Kim H J, Nam H G. 2007. Leaf senescence., 58: 115–136.
Liu Q E, Ning Y S, Zhang Y X, Yu N, Zhao C D, Zhan X D, Wu W X, Chen D B, Wei X J, Wang G L, Cheng S H, Cao L Y. 2017.negatively regulates cell death and immunity by degradingin rice., 29(2): 345–359.
Liu X Q, Li F, Tang J Y, Wang W H, Zhang F X, Wang G D, Chu J F, Yan C Y, Wang T Q, Chu C C, Li C Y. 2012. Activation of the jasmonic acid pathway by depletion of the hydroperoxide lyase OsHPL3 reveals crosstalk between the HPL and AOS branches of the oxylipin pathway in rice., 7(11): e50089.
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. 2010. Reactive oxygen species homeostasis and signaling during drought and salinity stresses., 33(4): 453–467.
Mizobuchi R, Hirabayashi H, Kaji R, Nishizawa Y, Satoh H, Ogawa T, Okamoto M. 2002. Differential expression of disease resistance in rice lesion-mimic mutants., 21(4): 390–396.
Mori M, Tomita C, Sugimoto K, Hasegawa M, Hayashi N, Dubouzet J G, Ochiai H, Sekimoto H, Hirochika H, Kikuchi S. 2007. Isolation and molecular characterization of amutant by modified activation-tagging in rice., 63(6): 847–860.
Qiao Y L, Jiang W Z, Lee J H, Park B S, Choi M S, Piao R H, Woo M O, Roh J H, Han L Z, Paek N C, Seo H S, Koh H J. 2010.encodes a clathrin-associated adaptor protein complex 1, medium subunit μ1 (AP1M1) and is responsible for spotted leaf and early senescence in rice ()., 185(1): 258–274.
Salt J N, Yoshioka K, Moeder W, Goring D R. 2011. Altered germination and subcellular localization patterns for PUB44/ SAUL1 in response to stress and phytohormone treatments., 6(6): e21321.
Schlimme M, Blaschke L, Lagrimini L M, Polle A. 2002. Growth performance and lignification in tobacco with suppressed apoplastic anionic peroxidase activity under ambient and elevated CO2concentrations., 163(5): 749–754.
Shen X L, Liu H B, Yuan B, Li X H, Xu C G, Wang S P. 2011.negatively regulates rice bacterial resistance via activation of ethylene biosynthesis., 34(2): 179–191.
Song G, Kwon C T, Kim S H, Shim Y, Lim C, Koh H J, An G, Kang K, Paek N C. 2019. The rice() encodes a plant spastin that inhibits ROS accumulation in leaf development and functions in leaf senescence., 9: 1925.
Sparkes I A, Brandizzi F, Slocombe S P, El-Shami M, Hawes C, Baker A.2003. Annull mutant is embryo lethal, implicating peroxisomes in an essential role during plant embryogenesis., 133(4): 1809–1819.
Strecker V, Mai S, Muster B, Beneke S, Bürkle A, Bereiter-Hahn J, Jendrach M.2010. Aging of different avian cultured cells: Lack of ROS-induced damage and quality control mechanisms., 131(1): 48–59.
Sun L T, Wang Y H, Liu L L, Wang C M, Gan T, Zhang Z Y, Wang Y L, Wang D, Niu M, Long W H, Li X H, Zheng M, Jiang L, Wan J M. 2017a. Isolation and characterization of a leaf spotted leaf 32 mutant with early leaf senescence and enhanced defense response in rice., 7: 41846.
Sun L T, Lin T Z, Wang Y L, Niu M, Hu T T, Liu S J, Wang Y H, Wan J M. 2017b. Phenotypic analysis and gene mapping of a white stripe mutantin rice., 31(4): 335–363. (in Chinese with English abstract)
Takahashi A, Kawasaki T, Henmi K, Shii K, Kodama O, Satoh H, Shimamoto K. 1999. Lesion mimic mutants of rice with alterations in early signaling events of defense., 17(5): 535–545.
Tang J Y, Zhu X D, Wang Y Q, Liu L C, Xu B, Li F, Fang J, Chu C C. 2011. Semi-dominant mutations in the CC-NB-LRR-typegene,, lead to constitutive activation of defense responses in rice., 66(6): 996–1007.
Thordal-Christensen H, Zhang Z G, Wei Y D, Collinge D B. 1997. Subcellular localization of H2O2in plants: H2O2accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction., 11(6): 1187–1194.
Vijayan P, Shockey J, Levesque C A, Cook R J, Browse J. 1998. A role for jasmonate in pathogen defense of., 95: 7209–7214.
Wang S, Lei C L, Wang J L, Ma J, Tang S, Wang C L, Zhao K J, Tian P, Zhang H, Qi C Y, Cheng Z J, Zhang X, Guo X P, Liu L L, Wu C Y, Wan J M. 2017., encoding an eEF1A-like protein, negatively regulates cell death and defense responses in rice., 68(5): 899–913.
Wang S H, Lim J H, Kim S S, Cho S H, Yoo S C, Koh H J, Sakuraba Y, Paek N C. 2015. Mutation of() impairs abscisic acid responsive signaling and delays leaf senescence in rice., 66(22): 7045–7059.
Wang Z H, Wang Y, Hong X, Hu D H, Liu C X, Yang J, Li Y, Huang Y Q, Feng Y Q, Gong H Y, Li Y, Fang G, Tang H R, Li Y S. 2015. Functional inactivation of UDP--acetylglucosamine pyrophosphorylase 1 (UAP1) induces early leaf senescence and defence responses in rice., 66(3): 973–987.
Wellburn A R. 1994. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution., 144(3): 307–313.
Williams B, Dickman M.2008. Plant programmed cell death: Can’t live with it; Can’t live without it., 9(4): 531–544.
Wu C J, Bordeos A, Madamba M R S, Baraoidan M, Ramos M, Wang G L, Leach J E, Leung H. 2008. Rice lesion mimic mutants with enhanced resistance to diseases., 279(6): 605–619.
Wu J L, Wu C J, Lei C L, Baraoidan M, Bordeos A, Madamba M R S, Ramos-Pamplona M, Mauleon R, Portugal A, Ulat V J, Bruskiewich R, Wang G L, Leach J, Khush G, Leung H. 2005. Chemical- and irradiation-induced mutants ofrice IR64 for forward and reverse genetics., 59(1): 85–97.
Xu X, Chen Z, Shi Y F, Wang H M, He Y, Shi L, Chen T, Wu J L, Zhang X B.2018. Functional inactivation ofinduces enhanced disease resistance topv.in rice., 18: 264.
Yamanouchi U, Yano M, Lin H X, Ashikari M, Yamada K. 2002. A rice spotted leaf gene,, encodes a heat stress transcription factor protein., 99(11): 7530–7535.
Yin Z C, Chen J, Zeng L R, Goh M, Leung H, Khush G S, Wang G L. 2000. Characterizing rice lesion mimic mutants and identifying a mutant with broad-spectrum resistance to rice blast and bacterial blight., 13(8): 869–876.
Yuan Y X, Zhong S H, Li Q, Zhu Z R, Lou Y G, Wang L Y, Wang J J, Wang M Y, Li Q L, Yang D L, He Z H. 2007. Functional analysis of rice-like genes reveals thatis the rice orthologue conferring disease resistance with enhanced herbivore susceptibility., 5(2): 313–324.
Zeng L R, Qu S H, Bordeos A, Yang C W, Baraoidan M, Yan H Y, Xie Q, Nahm B H, Leung H, Wang G L. 2004., a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity., 16(10): 2795–2808.
Zhang X B, Feng B H, Wang H M, Xu X, Shi Y F, He Y, Chen Z, Sathe A P, Shi L, Wu J L.2018. A substitution mutation inconfers bacterial blight resistance by activating the salicylic acid pathway., 60(2): 160–172.
Zhou Q Y, Yu Q, Wang Z Q, Pan Y F, Lv W T, Zhu L L, Chen R Z, He G C. 2013. Knockdown ofgene reveals reactive oxygen species-induced leaf senescence in rice., 36(8): 1476–1489.
27 March 2019;
31 May 2019
ZHANG Xiaobo (hello.xiaobo@163.com); WU Jianli (beishangd@163.com)
Copyright © 2019, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2019.03.001
(Managing Editor: Li Guan)
- Rice Science的其它文章
- Genetic Diversity and Allelic Frequency of Selected Thai and Exotic Rice Germplasm Using SSR Markers
- Genome-Wide Association Analysis and Allelic Mining of Grain Shape-Related Traits in Rice
- Impact of Rice-Catfish/Shrimp Co-culture on Nutrients Fluxes Across Sediment-Water Interface in Intensive Aquaculture Ponds
- Soil Nitrogen Distribution and Plant Nitrogen Utilization in Direct-Seeded Rice in Response to Deep Placement of Basal Fertilizer-Nitrogen
- Application of Micronutrients in Rice-Wheat Cropping System of South Asia
- Sensing and Uptake of Nitrogen in Rice Plant: A Molecular View

