Genome-Wide Association Analysis and Allelic Mining of Grain Shape-Related Traits in Rice
Lv Yang, Wang Yueying, Noushin Jahan, Hu Haitao, Chen Ping, Shang Lianguang, Lin Haiyan, Dong Guojun, Hu Jiang, Gao Zhenyu, Qian Qian, Zhang Yu, 2, Guo Longbiao
Research Paper
Genome-Wide Association Analysis and Allelic Mining of Grain Shape-Related Traits in Rice
Lv Yang1, #, Wang Yueying1, #, Noushin Jahan1, Hu Haitao1, Chen Ping1, Shang Lianguang1, Lin Haiyan1, Dong Guojun1, Hu Jiang1, Gao Zhenyu1, Qian Qian1, Zhang Yu1, 2, Guo Longbiao1
(#)
Excavating single nucleotide polymorphisms (SNPs) significantly associated with rice grain shape and predicting candidate genes through genome-wide association study (GWAS) can provide a theoretical basis for discovery and utilization of excellent genetic resources in rice. Based on 16 352 SNPs, 161 naturalrice varieties with various grain sizes in southern China were used for GWAS of grain shape-related traits, referring to grain length (GL), grain width (GW), 1000-grain weight (TGW), and grain length/width (GLW). Phenotypic statistics showed that coefficient of variation values for these four traits GL, GW, TGW and GLW were 9.92%, 9.09%, 20.20% and 16.38%, respectively. Each trait showed a normal distribution, and there was a certain correlation between these traits. Through general linear model correlation analysis, a total of 38 significant loci were identified, and a range of 100 kb upstream and downstream of the significant loci was identified as the candidate interval. On chromosome 3,andwere found to regulate GL. On chromosome 6,andwere found to regulate TGW. Also, some QTLs related to grain shape were found on chromosomes 5 and 9. Besides that, using sequenced 3K-germplasm resources, we found that there are 22 overlapped varieties between these two natural populations. Twenty-six SNPs and fourteen haplotypes were identified in five regions ofgenes. The detection of multiple candidate genes/QTLs within the candidate interval is beneficial for further excavation of superior rice genetic resources.
candidate gene; grain shape; genome-wide association study; haplotype;rice; single nucleotide polymorphism
Rice (L.) is one of the most important food crops in the world. More than 65% population in China treats rice as their staple food (Zhu, 2007). Rice Grain shape is an important component of rice yield, which determines the level of yield and quality (Li et al, 2008). It is a complex comprehensive trait that is directly affected by multiple factors, referring to grain length (GL), grain width (GW), grain length/width (GLW) and 1000-grain weight (TGW) (Yang et al, 2001; Wu et al, 2002; Huang and Qian, 2017; Liu et al, 2018). Grain weight is an important determinant of rice silage capacity and yield. It is a comprehensive index related to grain length and grain width, and is mainly controlled by the additive effects of multiple genes (Shi and Shen, 1995). Grain length/width is also an important index to measure rice grain shape (Fu and Wang, 1994). So far, through a large number of genetic analyses and map-based cloning methods, a batch of genes/QTLs related to rice grain shape have been cloned, explaining their molecular mechanisms successfully (Rabiei et al, 2004). With the development of DNA molecular marker technology, genes(Hu et al, 2015),(Song et al, 2007),(Fan et al, 2006),(Gao et al, 2015),/(Prasad et al, 2010),/(Wan et al, 2008),(Li et al, 2011),(Ishimaru et al, 2013),(Wang et al, 2015),(Wang et al, 2012),(Zhao et al, 2018) controlling grain shape have been identified or cloned.
Risch and Merikangas (1996) found that association analysis is more advantageous than linkage analysis in the study of complex human diseases, and thus proposed the concept of genome-wide association study (GWAS). Recent years, with the application of GWAS in the field of plants, re-sequencing technology and single nucleotide polymorphism (SNP) chip technology have become more and more mature, and GWAS has also begun to be widely used in excellent rice gene mining gradually (Huang and Han, 2014). GWAS analysis to elucidate the mechanism of genetic control of rice grain shape has important implications for the excavation of excellent genetic resources and the improvement of rice yield. Huang et al (2010) used 373rice varieties to perform association analysis of 14 rice agronomic traits, and identified a total of 80 related sites. Then, Huang et al (2012) identified a total of 32 heading date sites and 20 grain type sites using 950 rice varieties. Zhao et al (2011) used 413 rice varieties from 82 countries to perform GWAS on 34 traits, and totally 234 associated sites were detected.(Fan et al, 2006) and(Wan et al, 2008), which regulate grain shape, and(Yano et al, 2016), which regulates heading date, and(Sasaki et al, 2002), which regulates plant height, were found by GWAS. Zuo et al (2014) used a total of 315 rice varieties from the International Core Rice Germplasm Bank to perform GWAS analysis on five panicle traits, and a total of 36 candidate associated regions were detected. Si et al (2016) used the GWAS method to clonegene controlling rice grain size. The cloning of this gene further confirms the reliability of the GWAS method.
Rice grain shapes are closely related to the yield and quality. GWAS can be applied more efficiently and reliably to the excavation of superior rice alleles and improve the breeding level. The objective of this study was to detect the SNP loci and determine related candidate genes affecting the rice grain shape significantly to reveal its genetic basis and molecular mechanism, and lay a foundation for the marker- assisted selection in high-yielding breeding of rice (Guo et al, 2014).
MATERIALS AND METHODS
Rice materials
A total of 161 naturalrice varieties with various grain sizes collected from 11 provinces in southern China were used (Supplemental Table 1). All varieties were stored at the China National Rice Research Institute (CNRRI), Hangzhou City, Zhejiang Province, China.
Field trials and phenotypic data collection
All experiments were conducted in the experimental field of CNRRI, Hangzhou, China, and a randomized complete block design was applied. Seeds with uniform germination were directly sown under a spacing of 20 cm × 20 cm (6 line × 6 rows for each variety). When ripening, 10 plants were selected in each variety. After threshing, 30 spikelets were randomly selected from each plant. The grain length and grain width were measured with a ruler, and grain length/width was calculated. Finally, 1000-grain weight was weighed and recorded by an electronic balance.
Statistical analysis
Excel 2014 and SAS 9.4 were employed for data compilation, and the mean, standard deviation and coefficient of variation of each trait were calculated. Correlation analysis was performed for the four grain traits (GL, GW, GLW and TGW).
DNA extraction and SNP genotyping
Genomic DNA from the samples was isolated from three to five leaves of 21-day-old plants per line using the CTAB method and finally diluted to 50 ng/μL. The 60 K SNP chip of Illumina (Wright et al, 2010) was applied in SNP genotyping, and markers with a minimum allele frequency (MAF) less than 0.03 were deleted.
Population structure
Genetic diversity and distance measures were estimated using the PowerMarker (Liu and Muse, 2005). The model-based program Popgene (Glaubitz, 2010) was used to infer population structure and to assign individual varieties into subpopulation. SAS 9.4 was used to perform statistical analysis on the relative kinship between the combinations.
Genome-wide association analysis and allele mining
Association analyses were performed with and without correcting for population structure. General linear model (GLM) approach implemented in TASSEL was used to correlate the grain shape and the corresponding SNP loci, and a Manhattan map was generated by using the R language. Significant marker trait associations were determined based on a threshold of -lg() as 4. Adjacent significant SNP associated with the same trait within a physical distance of 200 kb were regarded as a candidate region. Candidate genes were screened through the Rice Genome Annotation Project Database (http://www.ricedata.cn/gene/), and haplotype analyses of candidate genes were performed in combination with the rice 3K Resource Sequencing Library data (http://www.rmbreeding.cn/Index/).
RESULTS
Statistical analysis of four phenotypes related to grain shape
A total of 161rice varieties were evaluated for GL, GW, TGW and GLW. The mean values of GL, GW, TGW and GLW were 8.37 mm, 2.97 mm, 26.05 g and 2.87, respectively (Table 1). CV value ranged from 9.09% to 20.20%, indicating that the grain shape was rich in genetic variation. The distribution pattern of each trait showed a significant normal distribution, and the correlation result revealed that there were a positive correlation between GL with GLW and TGW, and a weak negative correlation with GW. GW was positively correlated with TGW and negatively correlated with GLW (Fig. 1).

Table 1. Statistical analysis of grain shape related traits.
GL, Grain length; GW, Grain width; TGW, 1000-grain weight; GLW, Grain length/width; SD, Standard deviation (= 3); Max, Maximum value; Min, Minimum value; CV, Coefficient variance.
Basic statistics of SNP markers
Based on the genomic sequencing results, the set of marker available for GWAS after filtering the minor allele frequency consisted of 16 352 SNPs (4.2 SNP sites per 100 kb on average). Sites are distributed on all 12 chromosomes of rice, with a number of SNP markers per chromosome ranging from 708 to 2 120, PIC values of different chromosome markers ranging from 0.11 to 0.70. The results showed that the selected SNP markers were polymorphic and can perform GWAS analysis covering the entire rice genome.
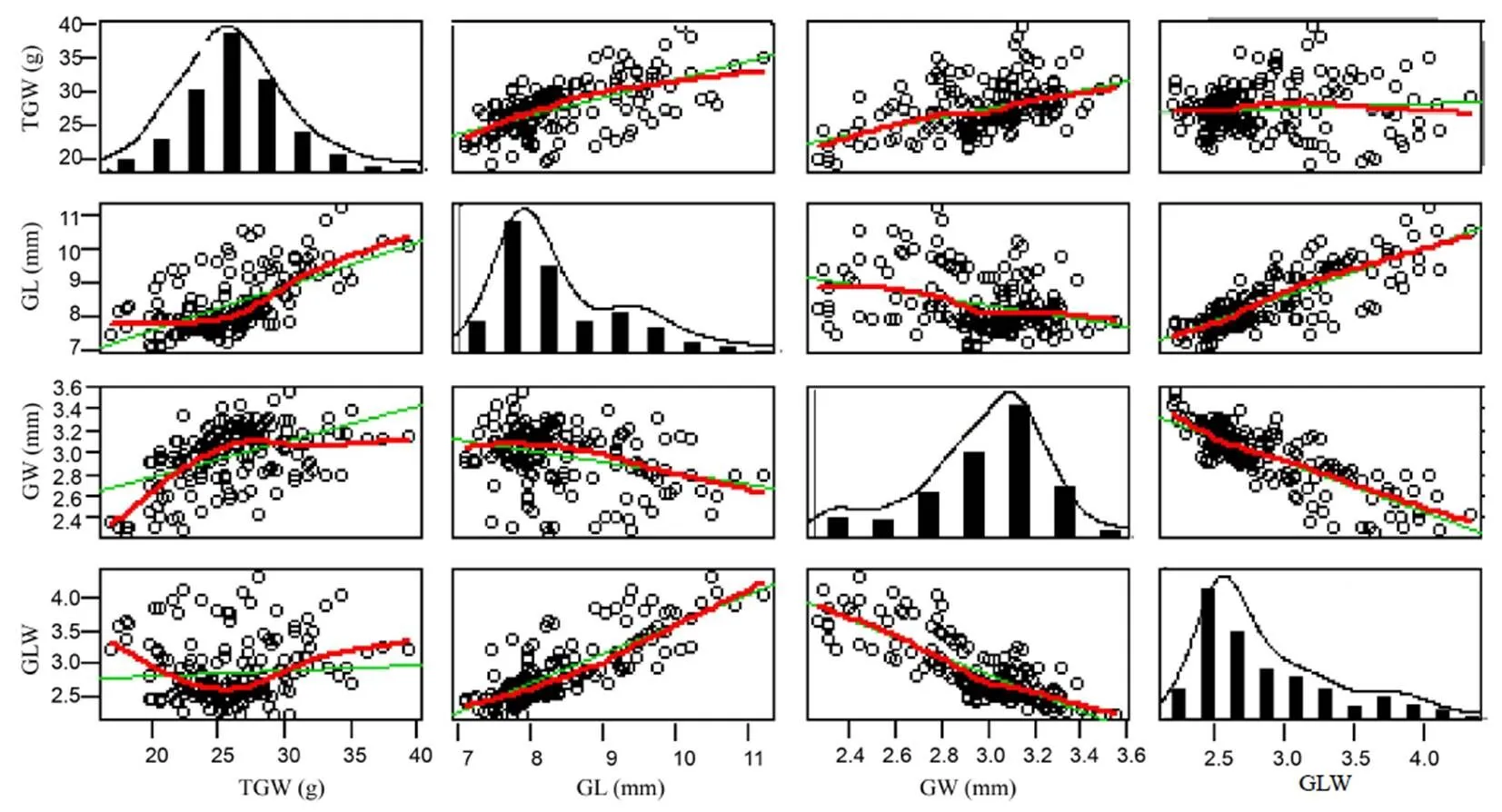
Fig. 1. Statistical analysis of four grain shape traits.
TGW, 1000-grain weight; GL, Grain length; GW, Grain width; GLW, Grain length/width.
The green lines represent the correlation coefficient, and the red lines represent the correlation trend.
Analysis of population structure
According to the genetic distance analysis, the neighbor-joining tree identified these varieties into two major groups (cluster I, 45 varieties; cluster II, 116 varieties) (Fig. 2-A). The phylogenetic value ranged from 0 to 0.5311 with the mean of 0.2720 and the data fluctuation value was small. The results showed that only 0.3% of the phylogenetic value was greater than 0.50, and 0.2% of the phylogenetic value was smaller than 0.05 (Fig. 2-B). Therefore, the relationship between varieties was relatively long and the varieties were suitable for GWAS analysis.
Genome-wide association analysis
The GLM approach was used to analyze the grain shape of rice materials. The results showed that with -lg() > 4 as the screening threshold, 38 significantly associated loci for the four traits were identified and distributed on 12 rice chromosomes (Fig. 3). The highest number (9) was distributed on chromosome 3, and the maximum number of -lg() was of 13.38, suggesting that the target candidate genes/QTLs are likely to be present on chromosome 3 (Table 2). There were 8, 9, 20 and 1 SNP loci that were significantly associated with GL, GW, TGW and GLW, respectively, and 11 sites were associated with two or more grain shape traits at the same time, suggesting that there may be a trait correlation or a pleiotropic effect.
Allelic mining and haplotype analysis
Six candidate genes/QTLs were screened out in association with 92 genes/QTLs. The results showed that two grain shape-related QTLs were detected on chromosomes 5 and 9 asand, respectively (Table 2). Two candidate regions, chr03_17302647 and chr03_19320570 on chromosome 3, were associated withandgenes.negatively regulates grain size and encodes a cysteine-rich domain protein of the TNFR/NGFR family.encodes a protein phosphatase that contains a Kelch repeat domain and positively regulates rice grain length. Two candidate regions of chromosome 6, chr06_24170016 and chr06_26025122, were associated withandgenes, respectively, which code for indole-3-acetic acid (IAA)-glucohydrolase and histone acetyltransferase, respectively.
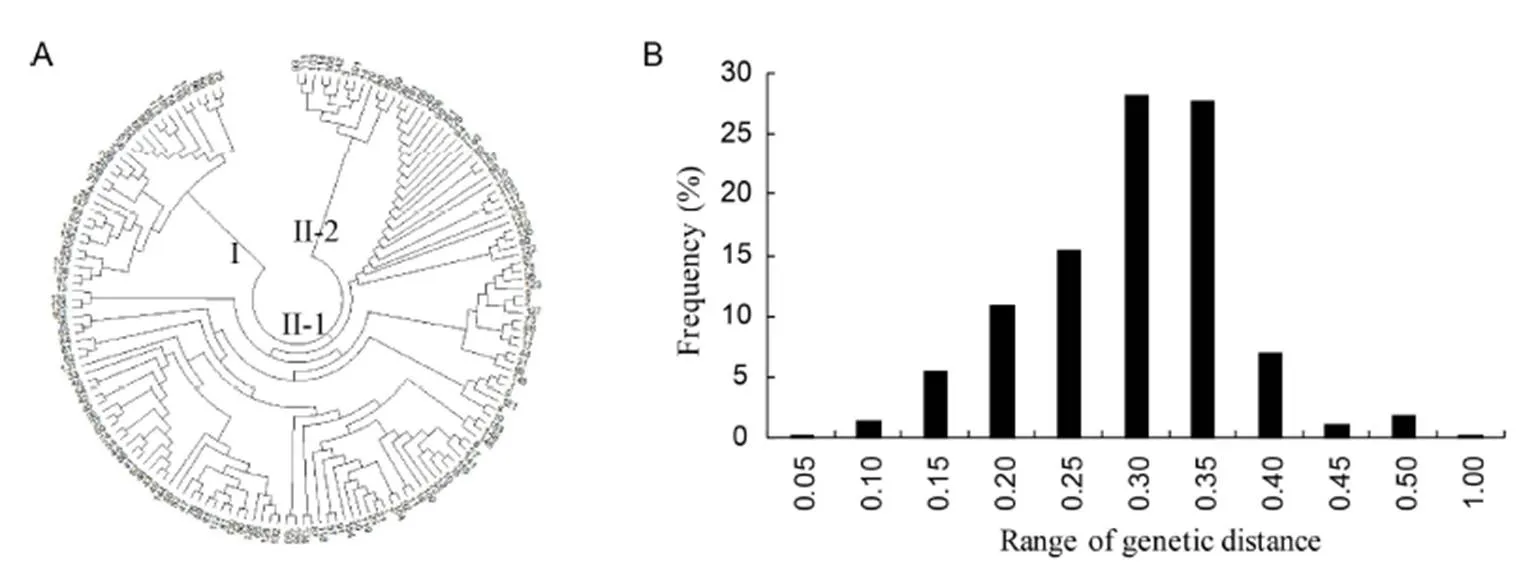
Fig. 2. Population genetic relationship evolution analysis.
A, Population structure based on neighbor-joining method. B, Frequency distribution of kinship between varieties.
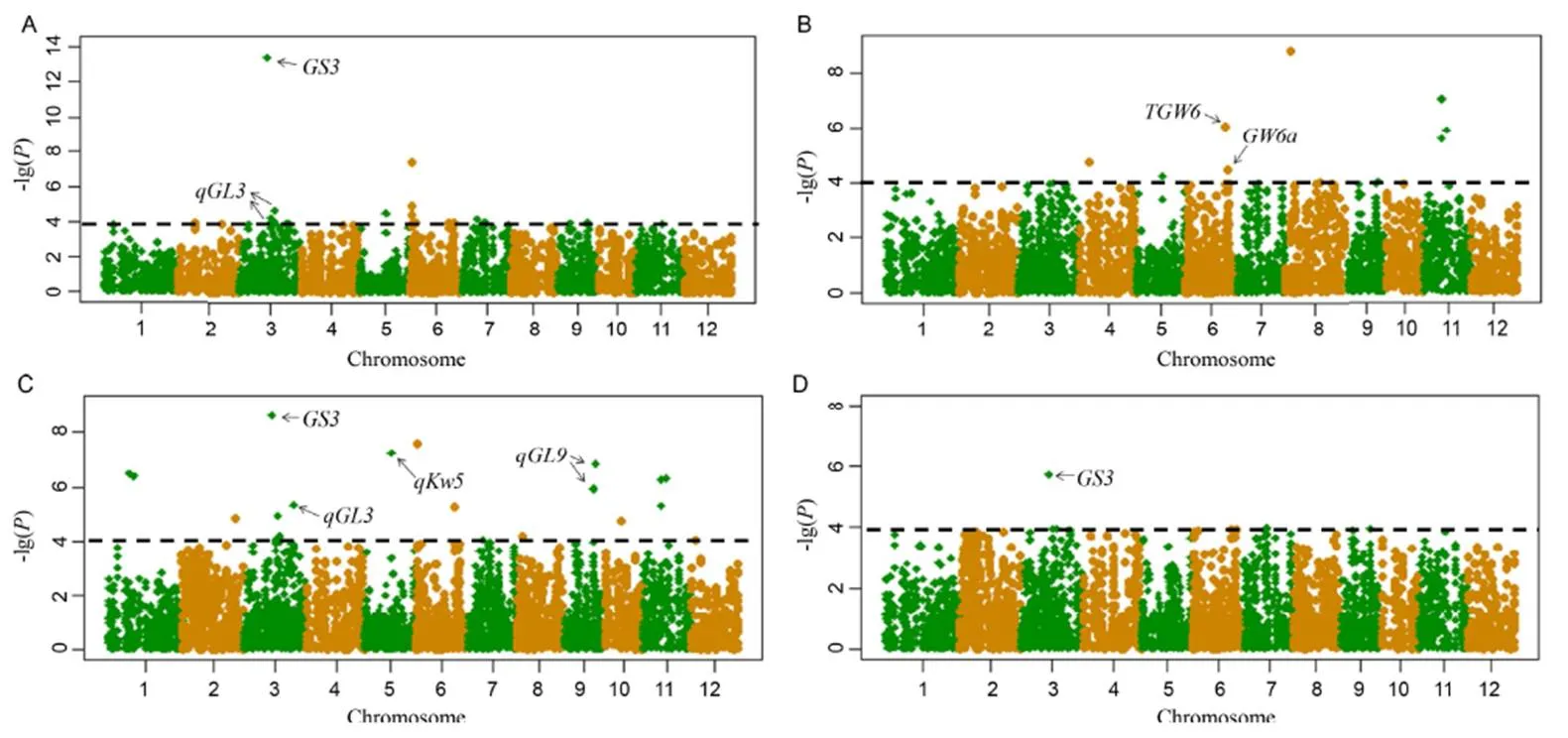
Fig. 3. Manhattan plots for grain length (A), grain width (B), grain length/width (C), and 1000-grain weight (D) by genome-wide association study.
The significance threshold -log10() is 4.
A total of 22 rice varieties (overlapped varieties between these two natural populations, Supplemental Table 2) in this study were consistent with the 3K rice resource sequencing database (http://www.rmbreeding.cn/Index/). The haplotype analysis of() and() genes was performed using the Halpoview software combined with 3K resource sequencing library (Fig. 4). Twenty-six SNP loci were detected in five exons of, and 22 varieties were divided into 14 haplotypes. There were differences in the grain traits of different haplotypes. CX145 (Supplemental Table 1) with grain length longer than 9.6 mm belongs to the dominant haplotype of GS3-11, and the grain size of the haplotype CX79 is also longer than 9 mm. Based on exon differences, 22 varieties ofwere divided into five haplotypes. A total of 11 varieties had excellent haplotype T-G-G, and the mean grain width of these is 3.19 mm. Allelic effect analysis revealed that when the upstream SNP site (chr06_25094225, upstream 0.98 kb) changed from T to G, the TGW6-1 dominant haplotype varieties increased by 23%, and the increase in grain width exceeded 0.4 mm (Supplemental Table 2). There was a significant correlation between this locus and grain width traits.

Table 2. Thirty-eight single nucleotide polymorphisms (SNPs) significantly associated with grain size by genome-wide association study.
Position of all SNPs is based on Rice Rap2.
Chr, Chromosome; MAF, Minimum allele frequency; QTL, Quantitative trait locus; GL, Grain length; GW, Grain width; GLW, Grain length/width; TGW, 1000-grain weight.
DISCUSSION
Strengthening genetic research on rice grain shape has important implications for improving the level of yield and quality (Qiu, 2013). At present, the genetic research of rice grain shape has made great progress (Wei et al, 2009; Xuan et al, 2010). The extensive application of GWAS in the plants field also provides an efficient and reliable method for the excavation of excellent genetic resources (Ishimaru et al, 2013).
GWAS analysis showed that 38 significant SNP sites were detected for four rice grain shape traits. In the range of 100 kb upstream and downstream, a number of related genes/QTLs are associated within the candidate interval, includingand, which regulate grain length, andand, which regulate grain weight, indicating the results of this experiment are very reliable. Moreover, in this study, chr3_17302647 locus on chromosome 3 showed different correlations for different grain shape traits, which may be caused by a pleiotropic effect or closely linked genes. The highest -lg() value was 13.38, which is associated with the. The gene controls grain length and grain weight of rice by regulating the cysteine domain and VWFC module proteins of the TNFR/NGFR family (Zhang et al, 2004). Two QTLs associated with grain shape on chromosomes 5 and 9 wereand, respectively. Clonal QTL regulation is an indispensable process in breeding. In addition, some new sites with significant significance were also detected, such as chr01_12869918and chr01_15765545 on chromosome 1, chr06_1764762on chromosome 6, chr08_3681009 and chr08_12028646on chromosome 8, chr11_12028646 and chr11_14891499 on chromosome 11. The -lg() values of these sites are relatively large, and they should be further fine mapped.
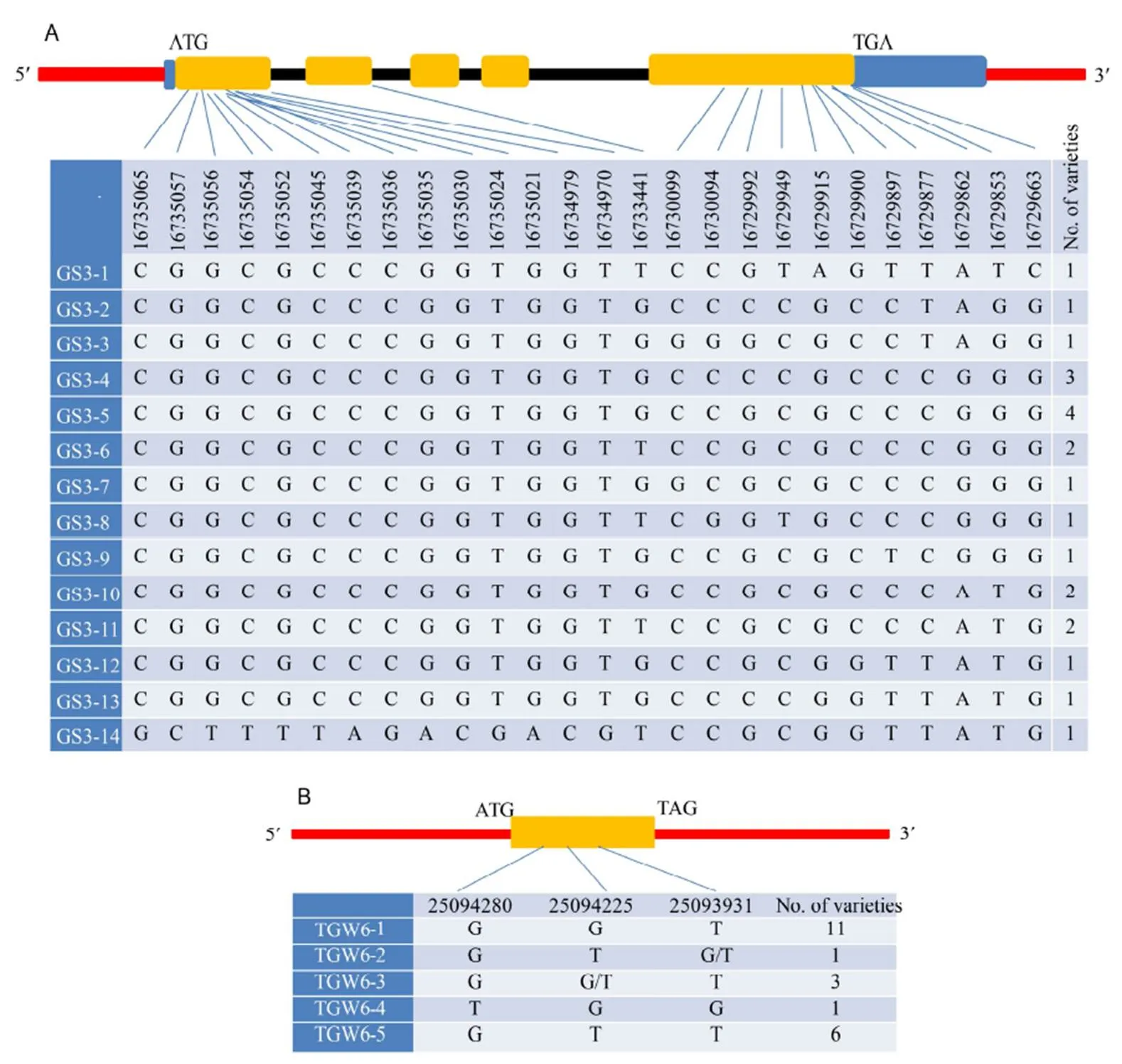
Fig. 4. Gene structure andsingle nucleotide polymorphism analysis of(A) and(B) haplotype.
Rice is rich in genetic resources and can produce a series of allelic variations. Different allelic variations have different adaptability and functions. Some alleles have been widely used in production., a major gene with a wide grain size, encodes an E3 ubiquitin ligase that negatively regulates cell division. A 1 bp deletion causes mutations in the large-grainedgene, accelerating cell division and increasing grain width and grain weight (Song et al, 2007).gene on chromosome 7 encodes a TRM protein and contains two tandem repeats of GL7-S1/S2, which can significantly increase grain length and decrease grain width (Wang et al, 2015). The A/C variation of exon 2 ofgene has a significant effect on rice grain length and grain quality, and Fan et al (2009) developed a functional marker SF28 based on this mutation. In this study, we detected 14 haplotypes of thegene by combining the sequencing information of different cultivars. The grain length of the GS3-11 haplotype was significantly greater than that of the other haplotypes G-G is the dominant haplotype of thegene. In general, compared with previous studies (Redoña and Mackill, 1998; Lin and Wu, 2003; Mao et al, 2010), there are few numbers of SNP site were obtained in this study. However, using the GWAS analysis method to correlate multiple reported genes/QTLs within the candidate interval, the correlation results are more reliable, the accuracy of the GWAS association was verified. Based on the significant association SNP loci, the sequence differences ofandgene with Minghui 63 as a recurrent parent, Yang et al (2010) used thegene and other superior genes for polymerization and breeding to achieve rice quality improvement, Lang (2015) found that deletion of the G base in position 313 of the nucleic acid did not result in increased production of thegene in Kasalath. Thus, by mining alleles and using the differences in these SNP sites, it is possible to effectively use the grain shape-related genes in crop breeding.
Acknowledgements
This study was supported by the Natural Science Foundation of China (Grant Nos. 31461143014, 31771778 and 31801336), the National Key Research and Development Program of China (Grant No. 2016YFD0100902-07), the China Postdoctoral Science Foundation (Grant No. 2018M641556) and the Zhejiang Provincial Natural Science Foundation of China (Grant No. LGN19C130006).
supplemental data
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Table 1. One hundred and sixty-onerice varieties from 11 provinces in China.
Supplemental Table 2. Phenotype and genotype classification of 22 rice varieties.
Fan C C, Xing Y Z, Mao H L, Lu T T, Han B, Xu C G, Li X H, Zhang Q F. 2006., a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein., 112(6): 1164–1171.
Fan C C, Yu S B, Wang C R, Xing Y Z. 2009. A causal C-A mutation in the second exon ofhighly associated with rice grain length and validated as a functional marker., 118(3): 465–472.
Fu F H, Wang F. 1994. Genetic analysis of grain characters in hybrid rice., 20(1): 39–45. (in Chinese with English abstract)
Gao X Y, Zhang X J, Lan H X, Huang J, Wang J F, Zhang H S. 2015. The additive effects ofandon rice grain length regulation revealed by genetic and transcriptome comparisons., 15(1): 156.
Gao Z Q, Zhan X D, Liang Y S, Cheng S H, Cao L Y. 2011. Research advances on the inheritance of rice grain shape traits and related gene mapping and cloning., 33(4): 314–321. (in Chinese with English abstract)
Glaubitz J C. 2010. CONVERT: A user-friendly program to reformat diploid genotypic data for commonly used population genetic software packages., 4(2): 309–310.
Gong L H, Gao Z Y, Ma B J, Qian Q. 2011. Advances in research on rice grain size genetics., 46(6): 597–605. (in Chinese with English abstract)
Guo L B, Ye G Y. 2014.Use of major quantitative trait loci to improve grain yield of rice.,21(2): 65–82.
Hu J, Wang Y X, Fang Y X, Zeng L J, Xu J, Yu H P, Shi Z Y, Pan J J, Zhang D, Kang S J, Zhu L, Dong D J, Guo L B, Zeng D L, Zhang G H, Xie L H, Xiong G S, Li J Y, Qian Q. 2015. A rare allele ofenhances grain size and grain yield in rice., 8(10): 1455–1465.
Huang H X, Qian Q. 2017. Progress in genetic research of rice grain shape and breeding achievements of long-grain shape and good qualityrice., 31(6): 665–672. (in Chinese with English abstract)
Huang X H, Wei X H, Sang T, Zhao Q, Feng Q, Zhao Y, Li C Y, Zhu C R, Lu T T, Zhang Z W, Li M, Fan D L, Guo Y L, Wang A H, Wang L, Deng L W, Li W J, Lu Y Q, Weng Q J, Liu K Y, Huang T, Zhou T Y, Jing Y F, Li W, Lin Z, Buckler E S, Qian Q, Zhang Q F, Li J Y, Han B.2010. Genome-wide association studies of 14 agronomic traits in rice landraces., 42: 961–967.
Huang X H, Wei X H, Sang T, Zhao Q, Feng Q, Zhao Y, Li C Y, Zhu C R, Lu T T, Zhang Z W, Li M, Fan D L, Guo Y L, Wang A H, Wang L, Deng L W, Li W J, Lu Y Q, Weng Q J, Liu K Y, Huang T, Zhou T Y, Jing Y F, Li W, Zhang L, Qian Q, Zhang Q F, Li J Y. 2012. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm., 44: 32–39.
Huang X H, Han B. 2014. Natural variations and genome-wide association studies in crop plants., 65(1): 531–551.
Ishimaru K, Hirotsu N, Madoka Y, Murakami N, Hara N, Onodera H, Kashiwagi T, Ujiie K, Shimizu B, Onishi A, Miyagawa H, Katoh E. 2013. Loss of function of the IAA-glucose hydrolase geneenhances rice grain weight and increases yield., 45(6): 707–711.
Lang Y L. 2015. A preliminary analysis of genetic effects of seven rice genotypes [MS Thesis]. Nanjing: Nanjing Agricultural University. (in Chinese with English abstract)
Li M M, Xu L, Liu C W, Cao G L, He H H, Han L Z. 2008. Advances in rice grain shape inheritance and QTLs mapping., 10(1): 34–42. (in Chinese with English abstract)
Li Y B, Fan C C, Xing Y Z, Jiang Y H, Luo L J, Sun L, Shao D, Xu L J, Li X H, Xiao J H, He Y Q, Zhang Q F. 2011. Natural variation inplays an important role in regulating grain size and yield in rice., 43(12): 1266–1269.
Lin L H, Wu W R. 2003. QTL mapping of grain shape and grain weight in rice., 1(3): 337–342. (in Chinese with English abstract)
Liu K, Muse S V. 2005. PowerMarker: An integrated analysis environment for genetic marker analysis., 21(9): 2128–2129.
Liu X, Mou C L, Zhou C L, Cheng Z J, Jiang L, Wan J M. 2018. Research progress on cloning and regulation mechanism of rice grain shape genes., 32(1): 1–11. (in Chinese with English abstract)
Mao H L, Sun S Y, Yao J L, Wang C R, Yu S B, Xu C Q, Li X H, Zhang Q F. 2010. Linking differential domain functions of theprotein to natural variation of grain size in rice., 107: 19579–19584.
Prasad K, Parameswaran S, Vijayraghavan U. 2010., a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an earlyacting regulator of inner floral organs., 43(6): 915–928.
Qi P, Lin Y S, Song X J, Shen J B, Huang W, Shan J X, Zhu M Z, Jiang L, Gao J P, Lin H X. 2012. The novel quantitative trait locuscontrols rice grain size and yield by regulating Cyclin-T1: 3., 22(12): 1666–1680.
Qiu X J. 2013. Identification and identification of a major gene
Rabiei B, Valizadeh M, Ghareyazie B, Moghaddam M, Ali A J. 2004. Identification of QTLs for rice grain size and shape of Iranian cultivars using SSR markers., 137(3): 325–332.
Redoña E D, Mackill D J. 1998. Quantitative trait locus analysis for rice panicle and grain characteristics., 96: 957–963.
Risch N, Merikangas K. 1996. The future of genetic studies of complex human diseases., 273: 1516–1517.
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K Saito T, Kobayashi M, Khush G S, Kitano H, Matsuoka M. 2002. Green revolution: A mutant gibberellin-synthesis gene in rice., 416: 701–702.
Shi C H, Shen Z T. 1995. Genetics and improvement of early grain shape., 9(1): 27–32. (in Chinese with English abstract)
Si L Z, Chen J Y, Huang X H, Gong H, Luo J H, Hou Q Q, Zhou T Y, Lu T T, Zhu J J, Shang G Y, Chen E W, Gong C X, Zhao Q, Jing Y F, Zhao Y, Li Y, Cui L L, Fan D L, Lu Y Q, Weng Q J, Wang Y C, Zhan Q L, Liu K Y, Wei X H, An K, An G, Han B. 2016.controls grain size in cultivated rice., 48: 447–456.
Song X J, Huang W, Shi M, Zhu M Z, Lin H X. 2007. A QTL for rice grain width and weight encodes a previously unknown RING-typeubiquitin ligase., 39(5): 623–630.
Wan X Y, Weng J F, Zhai H Q, Wang J K, Lei C L, Liu X L, Guo T, Jiang L, Su N, Wan J M. 2008. Quantitative trait loci (QTL) analysis for rice grain width and fine mapping of an identified QTL allele, 179(4): 2239–2252.
Wang S K, Wu K, Yuan Q B, Liu X Y, Liu Z B, Lin X Y, Zeng R Z, Zhu H T, Dong G J, Qian Q, Zhang G Q, Fu X D. 2012. Control of grain size, shape and quality by, 44(8): 950–954.
Wang Y X, Xiong G S, Hu J, Jiang L, Yu H, Xu J, Fang Y X, Zeng L J, Xu E B, Xu J, Ye W J, Meng X B, Liu R F, Chen H Q, Jing Y H, Wang Y H, Zhu X D, Li J Y, Qian Q. 2015. Copy number variation at thelocus contributes to grain size diversity in rice., 47(8): 944–948.
Wei X H, Yuan X P, Yu H Y, Wang Y P, Xu Q, Tang S X. 2009. Analysis of genetic variation of main rice varieties in China., 23(3): 237–244. (in Chinese with English abstract)
Wright M H, Tung C W, Zhao K, ReynoldsA,McCouchS R, Bustamante C D. 2010. ALCHEMY: A reliable method for automated SNP genotype calling for small batch sizes and highly homozygous populations., 26(23): 2952–2960.
Wu C M, Sun C Q, Chen L, Li Z C, Wang X K. 2002. Analysis QTL of grain shape by using of RFLP map in rice., 27(5): 3–7. (in Chinese with English abstract)
Xuan Y S, Jiang W S, Liu X H, Cheng H Z, Hee-Jong Koh, Yuan D L. 2010. Genetic diversity of main rice cultivars in northeast China., 11(2): 206–212. (in Chinese with English abstract)
Yang L S, Bai Y S, Xu C W, Hu X M, Wang W M, She D H, Chen G Z. 2001. Advances in research on rice grain shape and its inheritance., 29(2): 164–167. (in Chinese with English abstract)
Yang T F, Zeng R Z, Zhu H T. 2010. Effect of rice grain length geneon polymerization breeding., 8(1): 59–66.
Yano K, Yamamoto E, Aya K, Takeuchi H, Lo P C, Hu L, Yamasaki M, Yoshida S, Kitano H, Hirano K, Matsuoka M. 2016. Genome- wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice., 48(8): 927–934.
Zhang G H, Zhang G P, Qian Q, Xu L P, Zeng D L, Teng S, Bao J S. 2004. Analysis of QTLs for quantitative traits of rice grain under different environmental conditions., 18(1): 16–22. (in Chinese with English abstract)
Zhao D S, Li Q F, Zhang C Q, Zhang C, Yang Q Q, Pan L X, Ren X Y, Lu J, Gu M H, Liu Q Q. 2018.acts as a transcriptional activator to regulate rice grain shape and appearance quality., 9(1): 1240.
Zhao K Y, TungC W, Eizenga G C, Wright M H, Ali L M, Price A H, Norton G J, Islam M R, Reynolds A, Mezey J, McClung A M, Bustamante C D, McCouch S R. 2011. Genome-wide association mapping reveals a rich genetic architecture of complex traits in., 2(1): 467.
Zhu L H. 2007. My opinion about high yield rice breeding in China., 30(1): 129–135. (in Chinese with English abstract)
Zuo S M, Kang H X, Li Q Q, Chen Z X, Zhang Y F, Liu W D, Wang G L, Chen H Q, Pan X B. 2014. Genome-wide association analysis and utilization of gene related to ear traits in introduced rice germplasm., 28(6): 649–658. (in Chinese with English abstract)
10 July 2018;
21 September 2018
GUO Longbiao (guolongbiao@caas.cn); ZHANG Yu (zhangyu08@caas.cn)
Copyright © 2019, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2018.09.002
(Managing Editor: Wang Caihong)
- Rice Science的其它文章
- Genetic Diversity and Allelic Frequency of Selected Thai and Exotic Rice Germplasm Using SSR Markers
- Impact of Rice-Catfish/Shrimp Co-culture on Nutrients Fluxes Across Sediment-Water Interface in Intensive Aquaculture Ponds
- Soil Nitrogen Distribution and Plant Nitrogen Utilization in Direct-Seeded Rice in Response to Deep Placement of Basal Fertilizer-Nitrogen
- Characterization of a Novel Gain-of-Function Spotted-Leaf Mutant with Enhanced Disease Resistance in Rice
- Application of Micronutrients in Rice-Wheat Cropping System of South Asia
- Sensing and Uptake of Nitrogen in Rice Plant: A Molecular View

