The catalytic activity of transition metal oxide nanoparticles on thermal decomposition of ammonium perchlorate
Jlp A.Vr ,Prgnesh N.Dve ,b,*,Shlini Chturvedi
a Department of Chemistry,K S K V Kachchh University,Mundra Road,Bhuj,370 001,Gujarat,India
b Department of Chemistry,Sardar Patel University,Vallabh Vidyangar,388 120,Gujarat,India
c Samarpan Science and Commerce College Gandhinagar,Gujarat,India
Keywords:Metal oxide nanoparticles(MONs)Ammonium perchlorate(AP)Catalytic activity Activation energy
A B S T R A C T The catalytic proficiency of three MONs for AP thermal decomposition was studied in this work.A chemical co-precipitation method was used for synthesis of MONs(CuZnO,CoZnO,and NiZnO)and their characterization carried out by utilizing XRD,FTIR,and SEM.The TGA/DSC technique was employed for the investigation of the catalytic proficiency of MONs on the AP.The DSC data were used for measuring activation energy of catalyzed AP by using Ozawa,Kissinger,and Starink method.The MONs were much sensitive for AP decomposition,and the performance of AP decomposition was further improved.Among all the MONs,the CuZnO exhibits higher catalytic action than others and decomposition temperature of AP is descending around 117°C by CuZnO.The reduction in the activation energy was noticed after the incorporation of MONs in AP.
1. Introduction
The transition metals have important utilization account in vast fields caused by its exceptional characteristics like optical,magnetic,electronic and also the catalytic proficiency[1—3].The catalytic proficiency enhances sharply by nanometer size oxide particles than micrometer size oxide particles[4].Nano-size materials gained attention in extreme research activities, mainly because of size effect,the optical and electronic properties and the role played by surface phenomena.The catalytic applications of transition metal oxides in the composite solid propellants[5,6].The MONs with AP affect on the process of decomposition and the gasphase reaction of the AP reported in literature[7].Among propellants AP is the most vital and main oxidizing agents,it has a deciding and competitive part in the burning process[4,8—11].
AP is a stable chemical composite that gradually decompose at a low temperature. Therefore, it is important to get better or improved decomposition performance of AP to demand to generate high energy at low temperature.For that reason,the researchers are taking more interest in the thermal behavior and ignition of AP,because of its thermal behavior,AP is especially sensitive to the diminutive quantity existence of additives [4,12,13]. The investigators have described that metal oxides,such as MnO2[14],NiO[15],ZnO[16],CuO[17],Cu2O[18],Co3O4[19],CuFe2O4and MnFe2O4[9]exhibit better catalytic proficiency in the AP thermal decomposition.These minute amount additives are working like a ballistic catalyst to tailor the propellants ballistic properties.Nanosized particles have great catalytic actions due to the small size and vast surface areas.Therefore,researchers more interested in doing better combustion performance of composite solid propellants with these nanomaterials[20—22].
In the present studies,three types of MONs(CuZnO,CoZnO,and NiZnO) have synthesized through co-precipitation route. The comparison study of three MONs as catalyst was simultaneouly studied on the AP decomposition.The catalytic ability of MONs have been measured on thermal behavior of AP by TG-DSC techniques.The Starink,KAS and FWO techniques have been applied to calculate activation energies of catalyzed AP.
2. Experimental
2.1. Reagents and chemicals
All metal nitrate and NaOH were acquired from Merck.AP was acquired from National Chemicals and used with no additional purification.
2.2. Synthesis of nanoparticles
The synthesis of MONs(CuZnO,CoZnO,and NiZnO)were earlier reported through co-precipitation procedure[23].0.2 M solution of metal nitrate(Cu,Co,and Ni)and 0.4 M zinc nitrate solution prepared.Afterward,mixing both solutions and then dropwise adding of 0.5 N NaOH with continued stirring.Keep constant pH 11—12 of the reaction till metal hydroxides precipitates. Wash the precipitates with water to make them free from nitrate ions.The brown precipitation was dried at 60°C in the oven for 5 h and then calcined at 300°C for 5 h.
2.3. Characterization
The characterization of all nanoparticles was done by utilizing X-ray Diffraction(powder XRD,Rigaku mini flex 600),with CuKα radiation(λ=1.5418)and FTIR spectra were investigated by utilizing MB 3000 FTIR spectrometer(ABB Pvt.Ltd.,Germany)with ATR(horizontal attenuated total reflection).The morphology of nanoparticles is characterized by utilizing Scanning Electronic Microscopy(SEM,JEOL JSM-6510 LV)with 30 kV voltages.The crystallite size was estimated by Scherrer's equation[24].
2.4. Thermal analysis
The catalytic competency of MONs investigated after the addition of MONs in AP by utilizing TG-DSC(PerkinElmer STA-8000 instrument).The virgin AP is carried out in the TG-DSC for the comparative study.All samples were recorded ~10 mg of pure AP and AP with nano-catalyst in the proportion of 99:1 in N2atmosphere(20 ml min-1)at 10°C⋅min-1heating rate by using platinum crucible.
2.5. Kinetic studies
The DSC experiments carried out with three heating rate 5,10 and 15°C⋅min-1.The activation energy was calculated by three methods including Flynn wall Ozawa(FWO),Starink methods and Kissinger Akahira Sunose(KAS)[25—27].By using FWO,KAS and Starink techniques,activation energy was calculated based on Eqs.(1)—(3),respectively.The activation energy was estimated from the slope of a graph of lnβ for FWO,ln(β/T2)for KAS and ln(β/T1.92)for Starink against 1000/T by different(three)heating rates(β),where Tmis the peak temperature of the DSC thermogram.
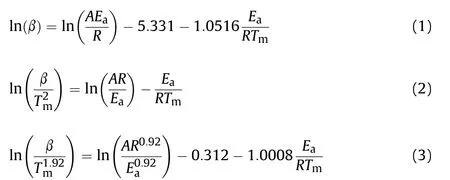
The slope value of the plot gives the activation energy(Ea).The value of the exponential factor(A)can be estimated from the intercept of the respective plots.
3. Results and discussion
3.1. Characterization of nanoparticles
The XRD graphs of three metal incorporated ZnO nanoparticles are unleashed in Fig.1.XRD of MONs shows sharp and high diffracted intensity of peaks,it indicates that all the particles display fine crystalline nature.The XRD diffractogram for three metal doped samples are in concurrence with the JCPDS file no 36—1451[28].It was simply indexed to hexagonal wurtzite phase with P63mc group.Incorporation of the metals in ZnO influences the lattice parameter of peaks and it diffuses to the crystal site,Cu,Co,and Ni are changed zinc site in the ZnO so they are enhanced the size of crystallite[29,30].In the case of CoZnO and NiZnO two additional peaks due to secondary phase formation have been observed in the XRD spectra.Nevertheless,the extra peaks at 59.01°and 64.90°in CoZnO nanoparticles are analyzed to Co3O4(JCPDS file No.42—1467)secondary phase.Whereas,NiZnO nanoparticles confirmed the presence of two extra peaks at 74.96°and 79.07°which are analyzed to be NiO(JCPDS Card 47—1049).These may be attributed due to accomplishment of the saturated state of doping level by Cu,Co and Ni-doped ZnO nanoparticles respectively[31,32].
Broad nature of diffraction peaks due to the microstrain also indicates the nanosized nature of the prepared MONs.The crystallite size D has been obtained from the highest diffraction peak along the plane by using the Scherrer formula[24]as follows:

where λ is the wavelength of the employed CuKα radiation(0.15418 nm),β is the full width at half-maximum(FWHM)of the peaks,and θ is the Bragg angle obtained from 2θ value corresponding to maximum intensity peak in XRD pattern.The strain can be calculated by the formula:
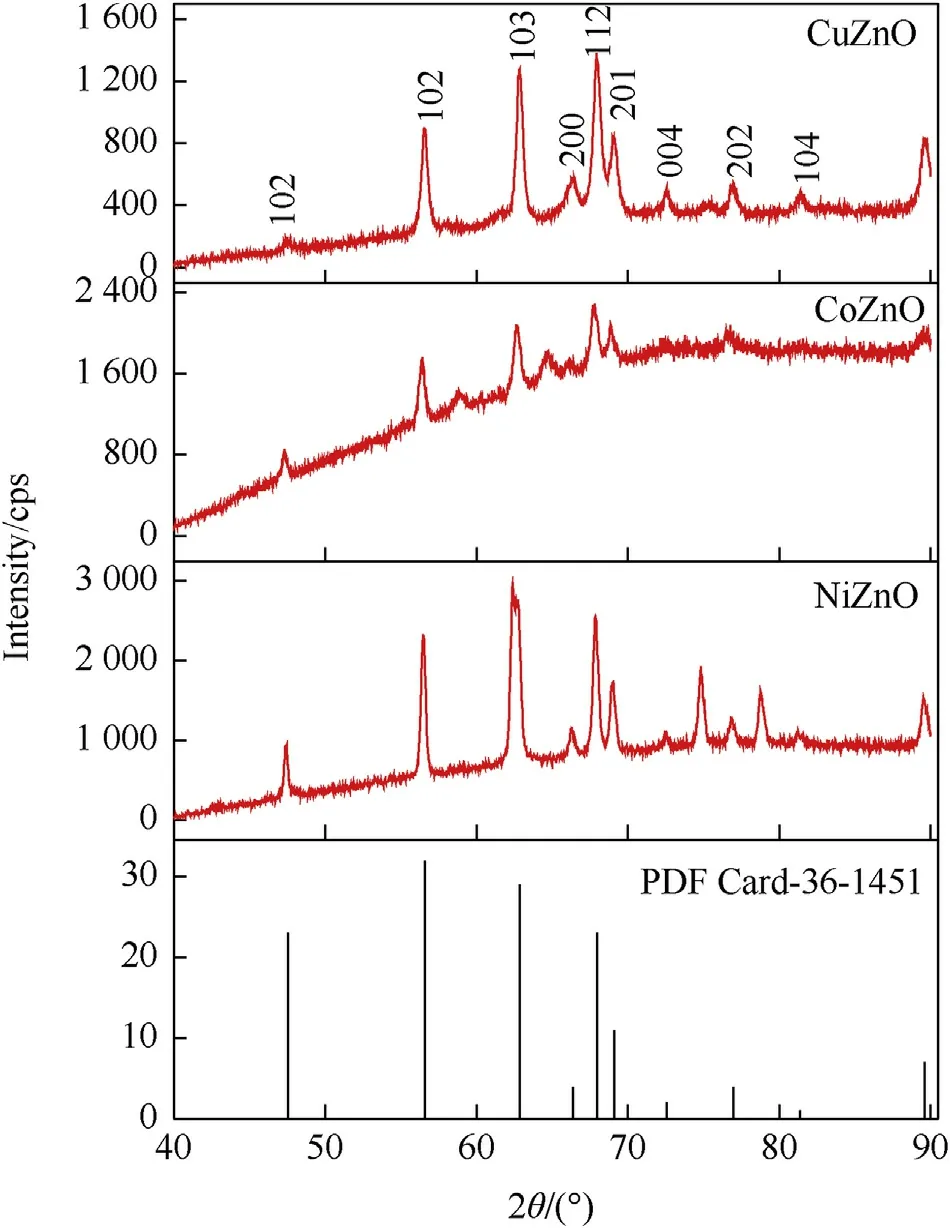
Fig.1.XRD Pattern of metal oxide nanoparticles.

Fig.2.FTIR graphof Metal oxide nanoparticles.

The crystallite size and microstrain of the synthesized MONs is reported in Supplementary Material Table 1.The microstrain follows the order:CoZnO >CuZnO >NiZnO.
The comparative FTIR graph of CuZnO,CoZnO,and NiZnO are shown in Fig.2,which gives complementary nature of metal oxides[33].All the samples show broad band around ~3400 cm-1in spectra which represents the O—H stretch of hydroxyl group attached on surface of metal oxide.It indicates that adsorbed H2O molecules on metal surface during synthesis process[34,35].The less intensive frequency band noticed at ~1640 cm-1explains bending vibration(H—O—H)of hydrated water[36].In all spectra,the intensive peaks of M-O bond of stretching vibration mode below 1000 cm-1frequency region noticed,which confirms the forming of metal oxide[37,38].The summary of all peaks are described in Supplementary Material Table 2.
The SEM images of CuZnO,CoZnO,and NiZnO nanoparticles are shown in Fig. 3. These images indicate that the shape and morphology of MONs.The images show that oxides are agglomerated.CuZnO was observed spherical in shape,and other two nanoparticles polygonal shape was observed.The image for MONs(Fig.3)manifests the synthesized nanoparticles with reasonably uniform size distribution with some unspecified reason larger than that of the grain size obtained from XRD analysis as depicted in Table 1.This could be indicant for the formation of secondary particles by aggregation of the primary particles.For the Cu,Co and Ni doped ZnO samples,the particles seem to be more and more agglomerated,and consequently it is hard to say with greater degree about the grain size obtained from the less-resolved SEM images(Fig.3)[39].
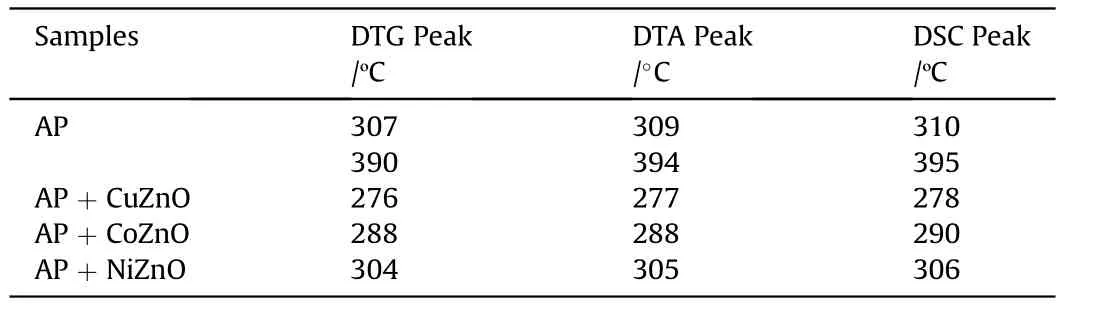
Table 1 Thermal analysis data of AP and AP with MONs(heating rate 10°C min-1).
3.2. Catalytic activity of MONs
The thermal performance of AP in the presence of 1%MONs was investigated with 10°C⋅min-1heating rates by utilizing PerkinElmer STA 8000 instrument.The weight loss in the TG thermograms presented in Fig.4 which has been coordinated with the derivative thermogravimetry(DTG)data revealed in Fig.5.Fig.4 offers the two-step weight loss of virgin AP at 285-425°C temperature range[40—42].In the initial step,16%weight loss of pure AP occurred within the temperature range 285—350°C coincides with the conversation of AP into intermediate products such as NH3and HClO4.In another step,80%of weight loss noticed to complete decomposition of AP with the formation of volatile products at higher temperature in range 350—425°C.While adding MONs in AP,the temperature of decomposition shifted at 285-330°C.Fig.4 clearly depicts that the MONs has a catalytic competency over the AP decomposition.Fig.4 is thermogram of AP with three MONs(CuZnO,CoZnO,and NiZnO)demonstrated weight loss of 98.96%,98.55%,and 98.19%in one-step respectively.The catalytic competency of MONs on the quick oxidation of the AP gives the first step decomposition at a lower temperature.
Fig.6 displays the DSC graph of virgin AP with three distinct peaks.The one endothermic peak is recognized at 243.12°C that expressed the crystal structural transition from orthorhombic to cubic[43]and another two exothermic peaks demonstrated at 310.30°C and 395.12°C respectively.The peak noticed at 310.30°C denotes the fractional decomposition of AP at lower temperature and another peak appeared at 395.12°C denotes the complete decay of AP at high temperature.The incorporation of MONs with AP leads to change in the thermal decay pattern of the AP as revealed in Fig.6.The DSC graph of MONs with AP have no variation in the endothermic peak but the exothermic peak appeared with one intense peak at 278,290,and 306°C for three different MONs at lower temperature and reported in Table 2.The DTA data also support to DSC data shown in Fig.7.After incorporation of MONs with AP that lower the decomposition temperature around 90-120°C as compared to pure AP.The rapid decomposition of AP occurred at lower temperature in the presence of MONs.CuZnO that exhibits good thermal catalytic competency on AP decomposition, which reduced the decomposition temperature around 117°C.

Fig.3.SEM images.
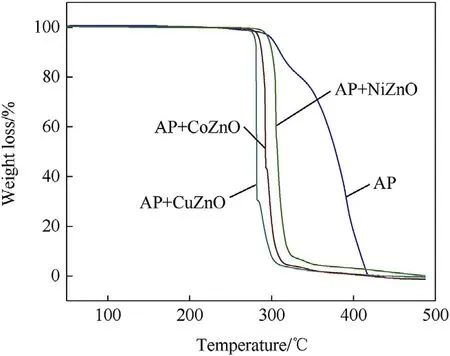
Fig.4.TG thermogram of AP and AP with MONs(heating rate 10°C·min-1).
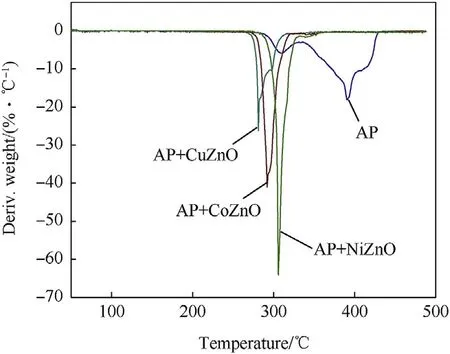
Fig.5.DTG thermogram of AP and AP with MONs(heating rate 10°C·min-1).
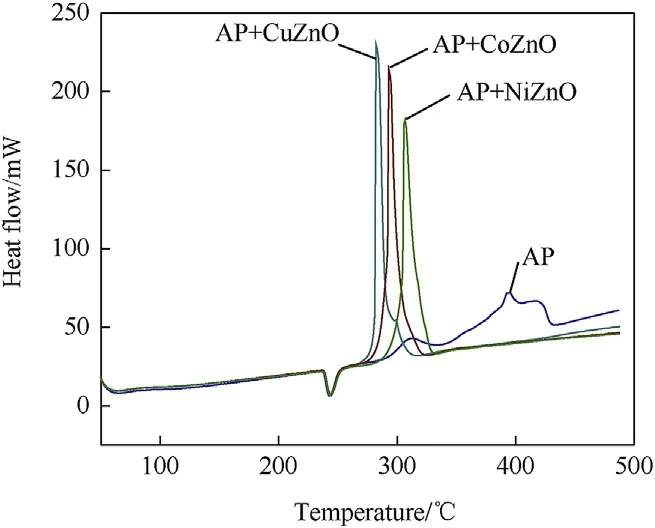
Fig.6.DSC thermogram of AP and AP with MONs(heating rate 10°C·min-1).

Fig.7.DTA thermogram of AP and AP with MONs(heating rate 10°C·min-1).
DTA curve of virgin AP observes three main events and presented in Fig.7.The endothermic peak is appeared at 244°C for AP and two exothermic peaks around 309 and 394°C.AP with MONs has obvious variation in the exothermic peak listed in Table 1 that also concord DSC results. Furthermore, our results also demonstrate that the effect of CuZnO, CoZnO and NiZnO nanoparticles on thermal decomposition of AP leads high temperature decomposition(HTD)shifts to low temperature decomposition(LTD).Also,it can be concluded that AP with CuZnO,CoZnO,and NiZnO nanoparticles have better catalytic proficiency on the reduction of temperature and enhancement in releasing heat than single MONs in the order of CuZnO ˃CoZnO ˃NiZnO illuminating the advantage of using MONs as catalyst in reaction process.
According to data of thermal analysis,these MONs catalysts have competency to reduce decomposition temperature of AP,presented in Table 1,CuZnO was found more proficient catalyst than the others and the decomposition temperature of AP sliding to 117°C.
3.3. Kinetic studies
The kinetic studies of catalytic proficiency of MONs onto AP have been scrutinized by three different methods viz FWO,KAS,and Starink methods with different heating rate (5, 10, and 15°C⋅min-1)[25—27].The plots of lnβ,ln(β/T-2)and ln(β/T-1.92)against 1000/T of all samples-virgin AP and AP with MONs are presented in Fig.8.From Table 2,the calculated values of activation energies of virgin AP are 277.58,276.23,and 276.47 kJ mol-1by FWO,KAS,and Starink respectively.After addition of MONs,activation energy reduces significantly.The results point out that CuZnO has excellent catalytic proficiency in order to increase AP decomposition rate.Table 2 displays the lower in activation energy for AP in compare to MONs.The correlation coefficient(r)is close to one.The activation energy decreases with decreasing the exponential factor inturn the catalytic proficiency increases.
In the DSC based thermokinetics,activation energy of KAS and Starink techniques are similar to each other and lesser than the FWO method.The equations used in the KAS and Starink techniques have almost same activation energy that is pointed out from the results.These parameters are achieved from the dependence of exothermic peak temperatures established in a role of heating velocity.The Kissinger correlation can be used to define the relationship concerning the decomposition temperature and heating rate[44].The MONs have broad surface area caused by their verysmall size and there are many reactive sites over the surface.The promotions of reactions endorsed by MONs with involvement absorbing the gaseous reactive molecules on their surface in the exothermic decomposition.
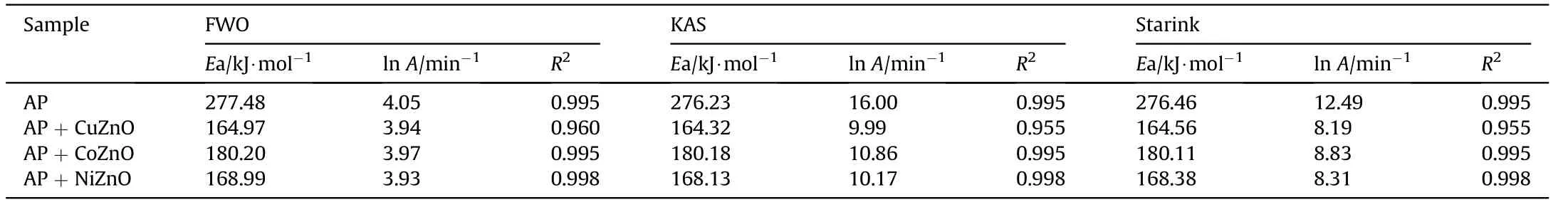
Table 2 Thermokinetics data of AP,AP with MONs by using Ozawa,Kissinger and Starink methods.

Fig.8.Plot of AP and APwith threeMONs by using FWO,KAS and Starink methods.
The results stated that the decomposition of AP from intermediate products to gaseous products in presence of CuZnO nanoparticle has a superior catalytic activity in comparison to CoZnO,and NiZnO.The activation energy of decomposition AP with CuZnO nanoparticles reduced,exhibiting distinguishable kinetic parameters of a self-increasing reaction.
3.4. Mechanism of thermal decomposition of AP
The thermal decomposition of AP studies by two most significant mechanisms.The first electron shift from perchlorate ion to ammonium ion and second proton shift from ammonium ion to perchlorate ion,but proton transfer is more acceptable as follows[7,45—50]:
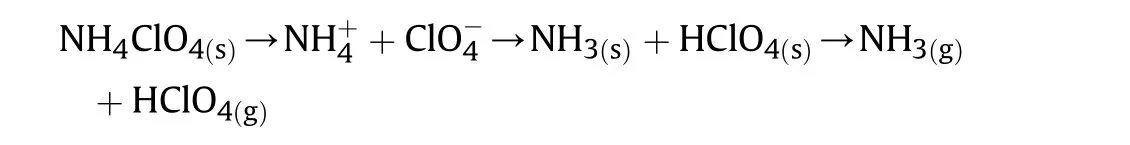
The AP decomposition gives two most important products NH3and HClO4identified in the experiments by the researcher[7,47—49].This postulates that the primary point of AP decomposition process is proton shift. This mechanism includes three important steps:The step-1,includes a pair of ions in AP lattice.The step-2,includes decay or sublimation step that begins with proton movement starting from the cationto the anionthen the molecular complex is formed and decomposes into NH3and HClO4in step-3.The molecules of NH3and HClO4also react in the adsorbed layer on the surface of perchlorate or desorbs and inspiring relating in the gas phase[45]:
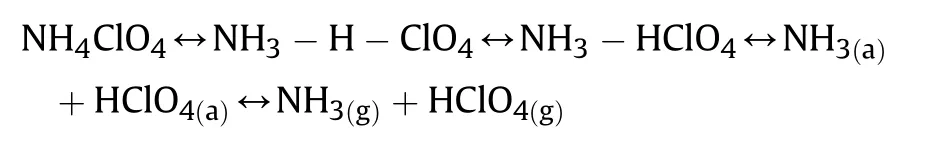
The absorption of gaseous reactive molecules on the surface MONs which enhances reaction rate by the proton transfer mechanism.The increment observed in the thermal decay rate of AP is by virtue of increasing the development of more holes within the ptype semiconducting area.The mechanism of catalytic efficiency of catalyst is in the interest of theion on the exterior part of the catalyst.Theformed throughout decomposition of AP and the surfaceof catalysts are likely the proton traps through the following reaction[50,51]:
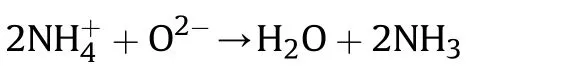
The involvement of catalyst information and gas absorption on its surface are main reasons in the completion of AP thermal decay.The CuZnO nanoparticles surface is able to produce morein comparison with other CoZnO and NiZnO NPs.Therefore,CuZnO nanoparticles are increasing in quick progression of AP thermal decay than others.
The perfect mechanism of thermal decay of AP has not understood totally yet.The mechanism of AP thermal decay through chain reaction has been proposed by YU Zongxue's[52].The NH3,H2O and a minor quantity of N2O and O2are forming during the low-temperature thermal decay of AP.The HCl,H2O,N2O,NH3,Cl2,NO,O2,NO2and a minor quantity of ClO2have been formed in the high-temperature point of AP decay.
At low temperature:
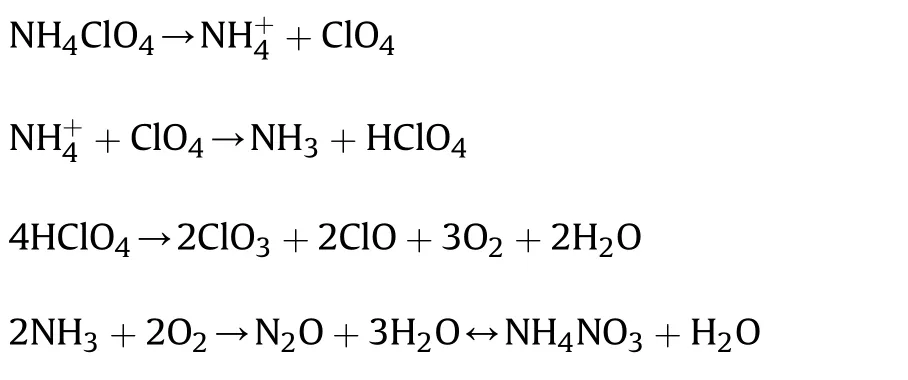
At high temperature:

The mechanism of AP combustion has been investigated by many research workers and role of condensed phase reaction at a pressure around 10—14 MPa is critical in many types of studies.The decomposition of AP occurs more than 70%in the condensed phase[53—55].
4. Conclusion
The three different types of CuZnO2, CoZnO2, and NiZnO2nanoparticles were synthesized via co-precipitation procedure.The study presents a new way to get better thermal decomposition of AP through synthesized MONs. Thermal analysis techniques including TGA-DSC,DTA and DTG were applied to study thermal responses. The CuZnO showed superior catalytic action than CoZnO2,and NiZnO2and shifting decomposition temperature at 117°C in downhill for AP.
The thermal decomposition temperature was found in the order CuZnO ˃CoZnO ˃NiZnO nanoparticles and all three MONs have good catalytic activity.The results prove that activation energies of AP with MONs are lower than virgin AP.So,AP with MONs can be promising candidates for solid propellants for energetic materials.
Acknowledgement
The authors are grateful to the Department of Chemistry,KSKV Kachch University,Bhuj for laboratory facility and for XRD,SEM and TGA-DSC analysis and also thankful to Chemistry Department,Sardar Patel University,Vallabh Vidyanagar for providing ATR-FTIR instrument facility.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2019.04.002.
- Defence Technology的其它文章
- Modeling on the shock wave in spheres hypervelocity impact on flat plates
- Insensitive high explosives:IV.Nitroguanidine—Initiation&detonation
- Effect of energy content of the nitraminic plastic bonded explosives on their performance and sensitivity characteristics
- Effect of wave shaper on reactive materials jet formation and its penetration performance
- A comparative study for the impact performance of shaped charge JET on UHPC targets
- The role of crystal lattice free volume in nitramine detonation

