Insensitive high explosives:IV.Nitroguanidine—Initiation&detonation
Ernst-Christian Koch
Lutradyn—Energetic Materials,D-67661,Kaiserslautern,Burgherrenstrasse 132,Germany
Keywords:Cook-off Detonation Insensitive munitions Nitroguanidine Shock sensitvity
A B S T R A C T This paper reviews the detonative properties of low bulk density(LBD),high bulk density(HBD)Nitroguanidine(NGu)(1),CAS-No:[556-88-7]and 82 explosive formulations based on NGu reported in the public domain.To rank the performance of those formulations they are compared with 15 reference compositions containing both standard high explosives such as octogen(HMX)(2),hexogen(RDX)(3),pentaerythritol tetranitrate(PETN)(4),2,4,6-trinitrotoluene(TNT)(5)as well as insensitive high explosives such as 3-nitro-1,2,4-triazolone (NTO)(6), 1,3,5-triamino-2,4,6-trinitrobenzene (TATB)(7), 1,1-diamino-2,2-dinitroethylene(FOX-7)(8)and N-Guanylurea dinitramide(FOX 12)(9).NGu based formulations are superior to those based on FOX-12 or TATB and are a close match with FOX-7 based explosives,the latter just having higher Gurney Energies(~10%)and slightly higher detonation pressure(+2%).NGu based explosives even reach up to 78%of the detonation pressure,82%Gurney energy and up to 95%of detonation velocity of HMX.LBD-NGu dissolves in many melt cast eutectics forming dense charges thereby eliminating the need for costly High Bulk Density NGu.Nitroguanidine based formulations are at the rock bottom of sensitiveness among all the above-mentioned explosives which contributes to the safety of these formulations.The review gives 132 references to the public domain.For a review on the synthesis spectroscopy and sensitiveness of Nitroguanidine see Ref.[1].
1. Introduction
Nitroguanidine is an important ingredient in triple base and insensitive,low erosion gun propellants,rocket propellants,gas generators for automobile restraint systems,smoke free pyrotechnics and shock insensitive high explosives[2].Though its use in high explosives is referred to in the literature[3—5]there lacks a comprehensive and contemporary overview of the detonative performance of nitroguanidine and its formulations and an assessment of the sensitiveness of these formulations and the response of munitions containing those formulations to insensitive munitions tests in accordance with NATO AOP-39[6].Fig.1 displays the valence bond structures of nitroguanidine(1)and the reference explosives octogen(HMX)(2),hexogen(RDX)(3),pentaerythritol tetranitrate(PETN)(4),2,4,6-trinitrotoluene(TNT)(5)as well as insensitive high explosives such as 3-nitro-1,2,4-triazolone(NTO)(6), 1,3,5-triamino-2,4,6-trinitrobenzene (TATB)(7), 1,1-diamino-2,2-dinitroethylene(FOX-7)(8)and N-Guanylurea dinitramide(FOX 12)(9).Table 1 list the basic properties of NGu and the main reference explosives.
2. Thermochemistry
2.1. Enthalpy of formation and enthalpy of vaporisation
The solid-state enthalpy of formation of NGu(ΔfH°)has been determined several times by combustion calorimetry[7—10].There is considerable scatter of data(ΔfH=-92 kJ/mol to-100 kJ/mol)and in Ref.8 some variation of ΔfH is attributed to different grain sizes with larger grains leading to lower combustion enthalpy.The gas phase enthalpy of formation has been estimated and calculated[11,12].The calculated value(ΔfH(g)=+44,77 kJ/mol)[12]fits the experimental data for the condensed state with the experimentally determined vaporisation enthalpy (ΔvapH=142.7 kJ/mol) [13]adding up nicely according to
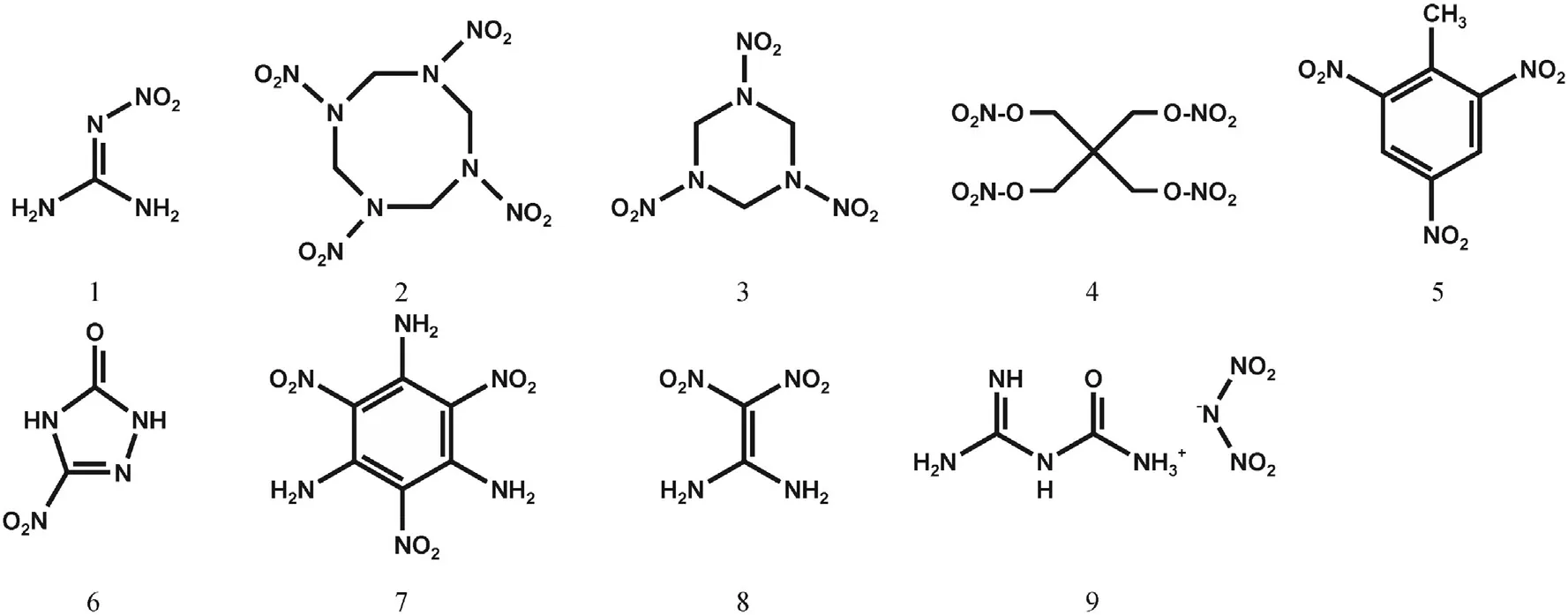
Fig.1.Structures of Nitroguanidine(1)and the reference explosives,HMX(2),RDX(3),PETN(4),TNT(5),NTO(6),TATB(7),FOX-7(8),FOX-12(9)dealt with in this review.
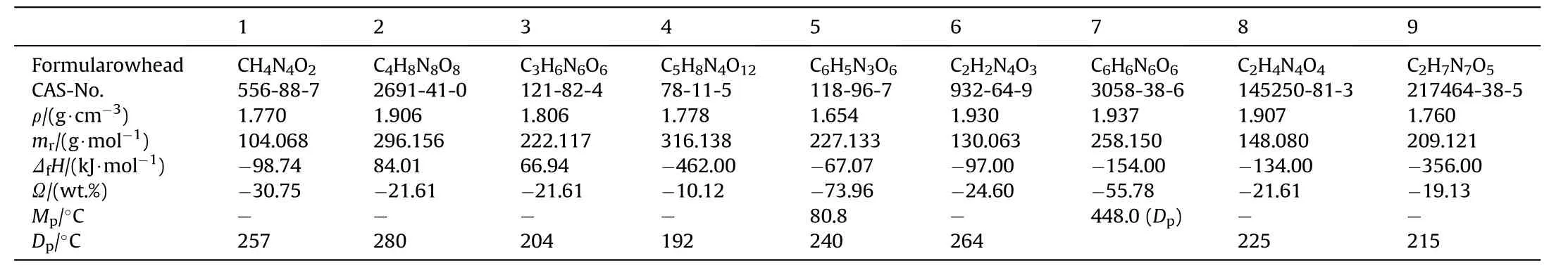
Table 1 Basic thermochemical properties of the reference explosives dealt with in this report after Ref.[59].
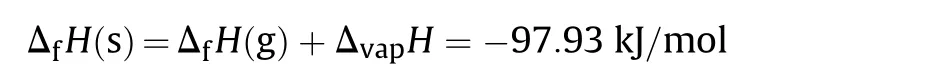
which is within the range of ΔfH(s)determined experimentally above(Table 2).
2.2. Enthalpy of detonation
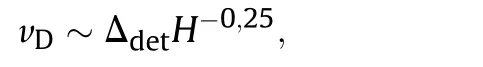
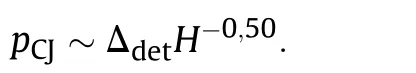
Precise knowledge of ΔdetH is therefore essential to assess the detonative performance of a high explosive. However, this isdifficult as the enthalpy of detonation is the heat released in the CJpoint and there is no way in experimentally determining this.Experimental determinations from detonation calorimeters using heavily confined charges(e.g.gold)hence rather correspond to the freeze-out region of the expansion isentrope and are correspondingly yield higher values than would be found exactly at the CJ point.ΔdetH can be calculated either based on semiempirical methods or based on the chemical composition of the post detonation residues from closed vessel detonations under an inert gas.In addition,ΔdetH can be determined in a detonation calorimeter from firing heavily confined(e.g.gold)charges[19].
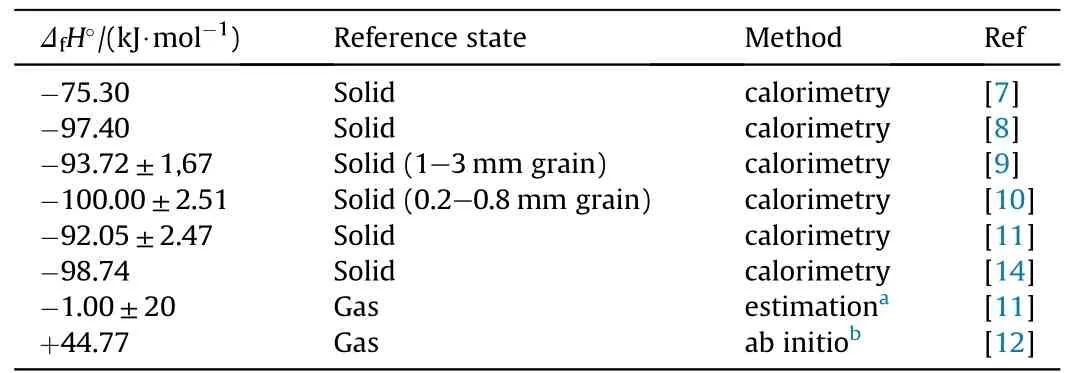
Table 2 Enthalpy of formation of nitroguanidine at 298.15 K for both condensed and gas phase.
2.2.1. Semiempirical calculation of enthalpy of detonation
The enthalpy of detonation can be estimated[20]or calculated based on the rules presented by Cooper[21].
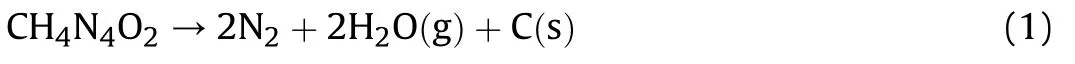
Based on Krien's value[10]for the enthalpy of formation using Cooper's method yields
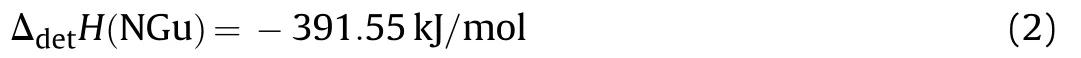
Taking into account the molar mass of NGu(mr:104.068 g/mol)this equals

2.2.2. Calculation of enthalpy of detonation based on detonation products
Pure NGu with low porosity is relatively hard to initiate and small charges(φ <40 mm)do not detonate ideally due to having a large critical diameter and quite a long run to detonation distance[22].Hence closed chamber(V=1.5 m3)detonation experiments in Ar-atmosphere have been conducted with NGu/TNT-based melt cast charges(hereafter designated Nigutol)with NGu-contents ranging from 40 wt%~60 wt%[23,24].Although the formal detonation according to Eq.(1)yields N2,H2O and C it is however observed upon analysis of the post detonation gases that significant amounts of both ammonia and hydrogen cyanide are formed.Table 3 shows the product composition for the detonation of both Comp B and various Nigutol charges in argon(0.1 MPa)highlighting the aforementioned.
Based on the above compositions the detonation enthalpy has been determined and is reproduced in Table 4.
In first approximation the enthalpy of detonation of a composition of two immiscible high explosives with both negative oxygen balance А and B the weight fractions n and m respectively is the sum of the enthalpy of detonation of its components.
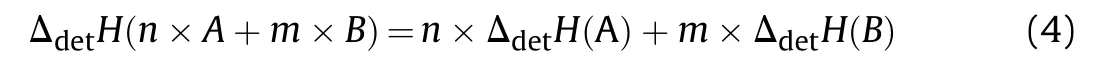
This assumes any chemical interaction of the individual explosive particles and their initial decomposition products does not occur untilreaching the CJ point.Table 5 compares the measured detonation enthalpy for RDX,HMX,TNT,Comp B and Octol with those values calculated detonation enthalpy for Comp B and Octol from Ref.[19]based on Eq.(4).Evidently the measured and calculated values for both compositions are within 1%of error.Rearrangement of Eq.(4)to resolve the enthalpy of detonation of NGu based from the detonation enthalpy of its composite Nigutol(TNT+NGu)with its weight fraction n yields Eq.(5):
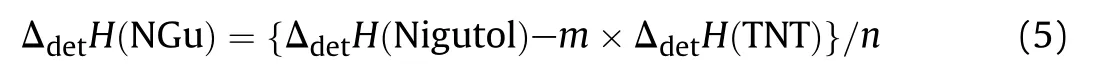
Inserting the individual figures from Table 3 and the value for TNT from Table 4 yields the ΔdetH(NGu)values depicted in Table 6.
The obtained value for ΔdetH(NGu)=-2,991 kJ/g is very close(-1%)to a value cited in Fedoroffs Encyclopedia of Explosives ΔdetH(NGu)=-3,016 kJ/g[3]giving some support for the latter.
3. Detonation
3.1. Detonation of neat NGu
3.1.1. High velocity detonation(HVD)of neat NGu
Gogyula et al.optically determined the detonation temperature for NGu(ρ=1.649 g/cm3)to 2 562 K[27]which is in the same ball park as the temperature calculated for a charge with the same density 2 830 K.
3.1.1.1. Detonation velocity.Price et al.have investigated the detonation velocity and critical diameter for neat unconfined NGucharges[28,29].The infinite diameter law for charges with densities ranging from ρ0=1.00—1.78 g/cm3accordingly reads
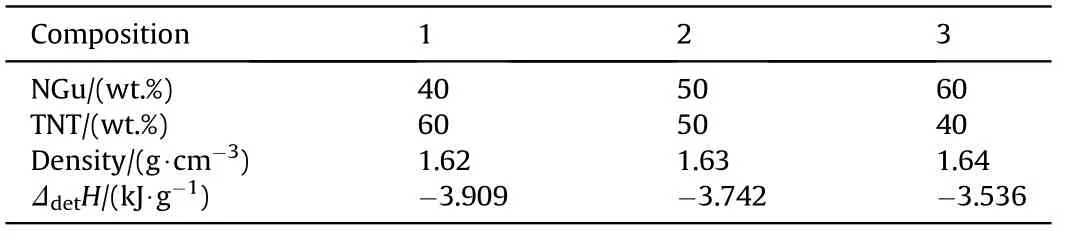
Table 4 Detonation enthalpy,H2O(g),of various Nigutol composites.
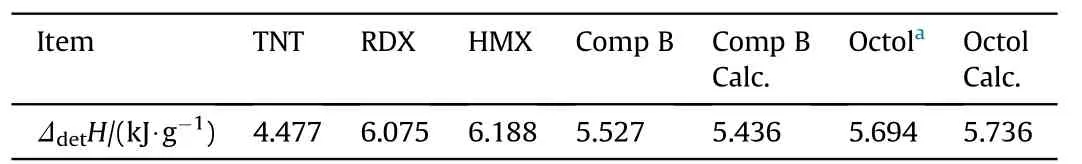
Table 5 Enthalpy of detonation of TNT,RDX and Comp B.
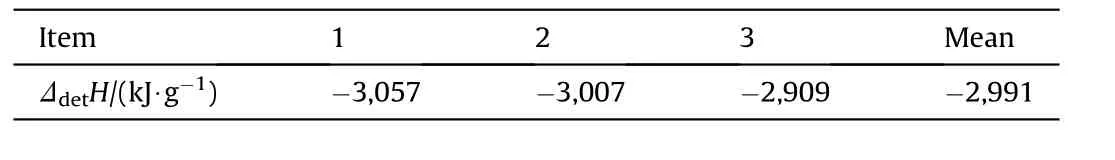
Table 6 Enthalpy of detonation of NGu from various Nigutol-composites.
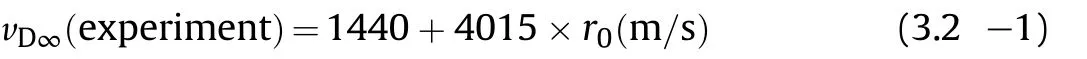
Predictions with Cheetah 7.0[30]based on an enthalpy of formation of NGu of ΔfH=-98.74 kJ/mol call for a significant steeper slope
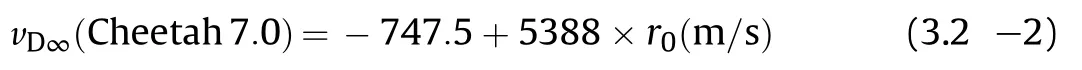
and overshoot the actual performance at ρ0 >1.6 g/cm3,while predictions with Cheetah 2.0[25].
Using the same enthalpy of formation show a slope more alike the experimentally determined one but undershoot the actual performance nearly constantly by 3%~4%in the range between ρ0=1.55—1.78 g/cm3(Fig.1).
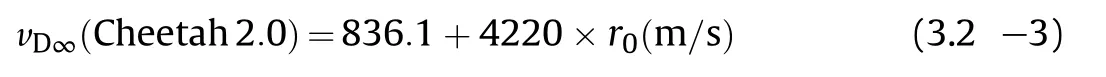
Experimental and calculated data on neat FOX-12[31]shown in Fig.2 indicate that FOX-12 has a lower detonation velocity than NGu at given density.
Fig.3 shows the influence of density on fixed diameter charges.With decreasing density,the detonation velocity of the individual diameter charges fans away from the infinite diameter line(Fig.3)as is also observed with many group 1 high explosives[32].
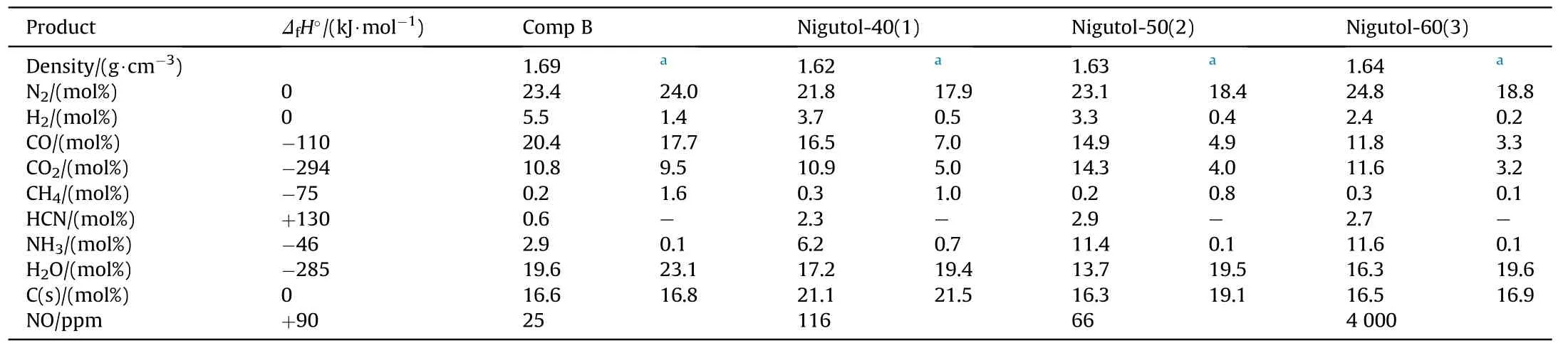
Table 3 Composition and enthalpy of formation of experimentally measured and calculated[25]post-detonation products from Comp B and Nigutol-50[26].
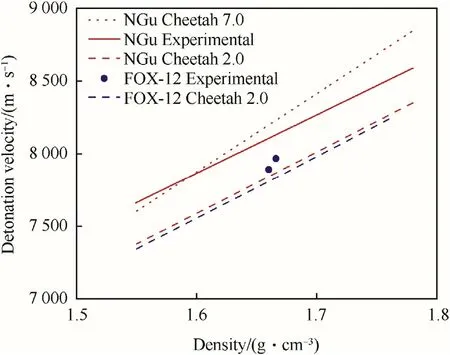
Fig.2.Experimental and calculated infinite diameter detonation velocity of NGu and FOX-12.
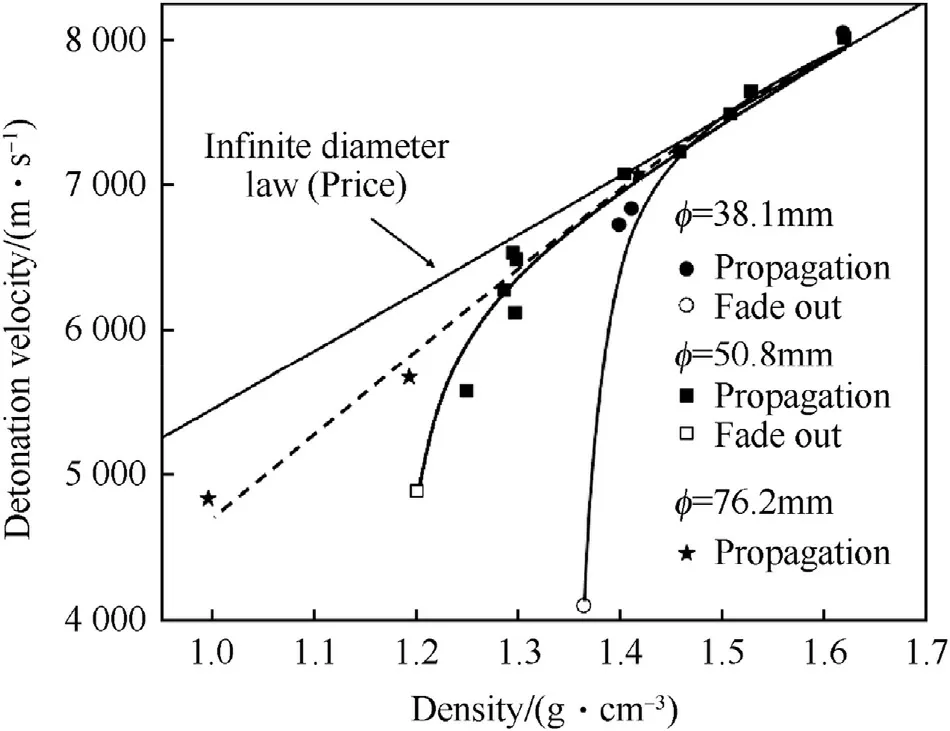
Fig.3.Effect of Density on Detonation Velocity at two fixed diameters.

Fig.4.Effect of Density on Detonation Velocity of confined charges at low ρ.
Even at densities,much lower than ρ <0.6 g/cm3,the detonation velocity of NGu about follows Price's law(Fig.4)but can be fitted more appropriately with the expression
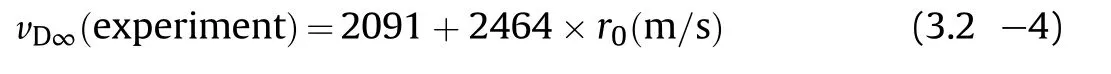
The inverse diameter detonation velocity relationship for unconfined ρ0=1.51 g/cm3is depicted in Fig.5.Below charge diameters of φ=14 mm the detonation fades out.Depending on the particle type of NGu LBD or HBD[1]the fade-out diameter for charges of varying density appears to differ as is depicted in Fig.6.Thus,in the considered density range LBD can be assigned a group 1 HE whereas HBD behaves like a group 2 material[29].
In comparison the critical diameter for FOX-12 with densities 1.60 ≤ρ ≤1.67 ranges from 24—54 mm[31].
3.1.1.2. Detonation pressure.Mader reasoned that the plate dent test typically applied to probe the pCJ-pressure is an inadequate tool for Nitroguanidine and its formulation as NGu fails to correlate with its PCJpressure due to its low energy and the resulting steep isentrope compared to most other explosives[33].Poor plate dent results for NGu in turn have fed the unsubstantiated“reputation”that NGu is an inferior explosive.Hence the data referred to in this review exclusively stem from copper cylinder tests unlike otherwise stated.
The experimentally determined detonation pressure for charges with densities ranging from 0.19 g/cm3to 1.7 g/cm3are given in Table 7[27,34—38]and depicted in Fig.7 together with the detonation pressure of neat FOX-12 [39] (pCJ(ρ=1.666 g/cm3)=26.11 GPa)and the calculated pCJfor both NGu and FOX-12.Mader also reasoned that though NGu has only half the detonation enthalpy of Comp B(see Table 5 and Table 6)it still performs comparable due to its favourable particle density of the detonation products due to the high hydrogen content in the explosive and consequently the water content in the final products[33].

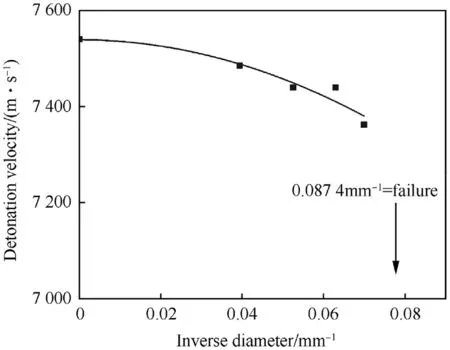
Fig.5.Diameter Effect on Detonation Velocity at ρ0=1.514 g/cm3.

Fig.6.Diameter effect on detonation velocity of LBD and HBD.
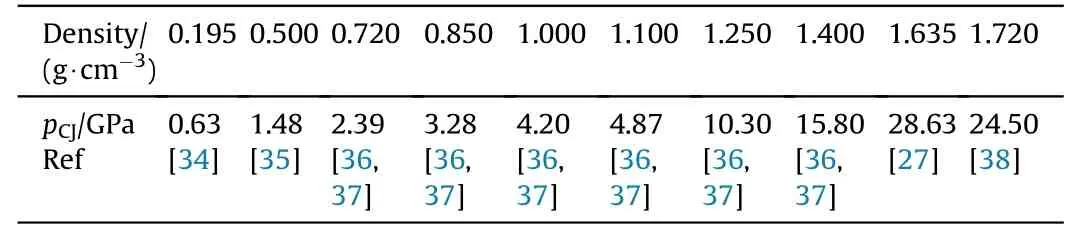
Table 7 Experimental pCJ of NGu at different densities.
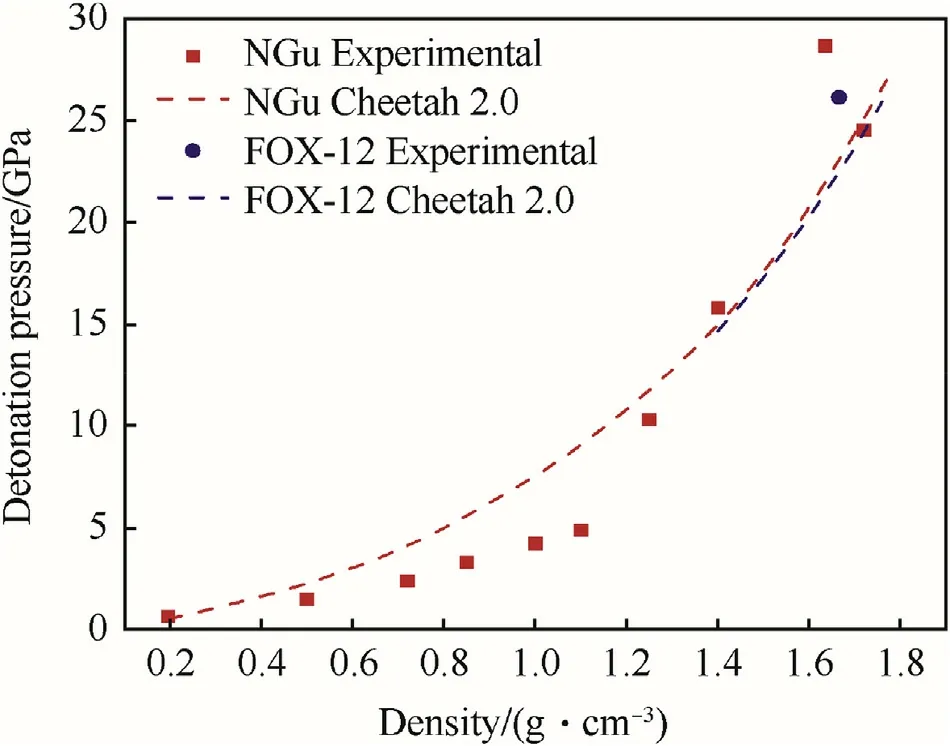
Fig.7.Experimental and calculated pCJ for NGu and FOX-12.
3.1.2. Low velocity detonation(LVD)of neat NGu
At charge densities below ρ0=1.2 g/cm3,HBD shows a stable low velocity detonation(LVD).Fig.8 depicts the observed velocities and Fig.6 shows the critical diameter for LVD with charges based on HBD after Price[32].
The effect of density on LVD has been tested by Montesi in the context of investigations on the water arm air safe detonator(WARAS)[45,46].
In low density charges(ρ=0.5 g/cm3)of NGu the gas pressure of the pockets has a distinct influence on the propagation of LVD and high pressures diminish propagation velocity and eventually inhibit propagation(Fig.9)[47].
3.1.3. Shock wave Hugoniot data on neat NGu
Hugoniot curve data for neat NGu of different particle density are presented as Us—upand p—V diagram in Figs.10 and 11[4,48].
3.2. Detonation of NGu-based formulations
3.2.1. Melt-castable formulations
3.2.1.1. NGu-TNT (Nigutol).By far the most thoroughly studied NGu-based high explosives mixtures are those based on TNT as melt cast binder. NGu/TNT mixtures (Nigutol), were initially developed as high explosives in wartime Germany[49,50]and were then used as an insensitive filler for armour piercing naval artillery shells.Research into Nigutol was resumed in Germany in the 1980s and the US in the early 1990s when new cheap insensitive high explosives were sought.This research was also motivated by new crystallisation processes developed then which allowed to produce NGu with high spherical high bulk density >1.0 g/cm3[1].Also,the first nanodiamonds formed by detonation were found by Volk et al.in the detonation soot of Nigutol and TATB/TNT mixtures[51].
The detonation enthalpy of various Nigutol formulations has been determined by Volk and Schedlbauer[23,24]and is already given above in Table 6.Fig.12 compares the experimental and calculated detonation enthalpy at given experimental density for Nigutol.The free-standing charges(φ=50 mm)yield about 88%of the calculated enthalpy whereas the charge confined in 9 mm glass yields 92%of the calculated enthalpy.
The critical diameter has been determined for Nigutol-50 with different particle types and size distributions as is indicated in Table 9[52,53].The general observation is that small particle sizes yield smaller critical diameters.
Schedlbauer[54],and Lungenstraβ[55]investigated a largearray of Nigutol formulations(Table 10).Fig.13 depicts the experimental detonation velocity,the calculated detonation velocity at TMD the calculated detonation velocity at the experimental density for Nigutol and Guntol(FOX-12/TNT)[56]and the baseline experimental detonation velocity of Comp B at ρ=1.71 g/cm3for comparison.In general,the experimental detonation velocities for Nigutol with ξ(NGu)<80 wt% undershoot the calculations in average by 2%whereas at ξ(NGu)=80 wt%and beyond the experimental detonation velocities are higher than calculated at given experimental density and supersede the Comp B baseline performance.The few Guntol(FOX-12/TNT)formulations investigated exhibit lower experimental detonation velocities at corresponding stoichiometries.
Table 8 Gurney Velocity for various neat high explosives.
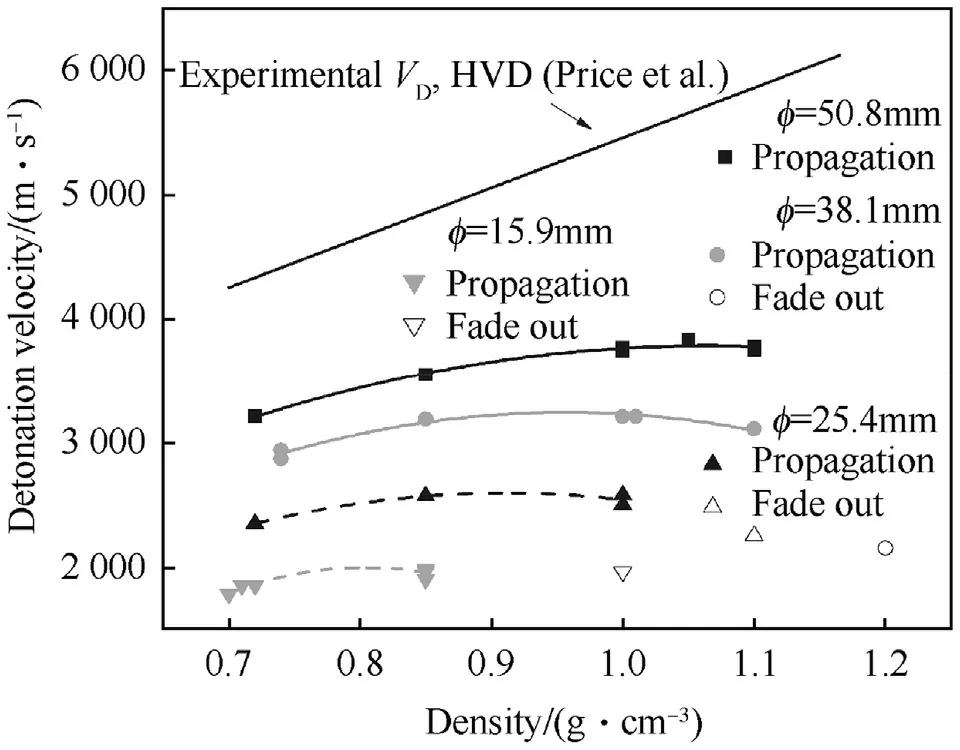
Fig.8.Diameter Effect on LVD HBD at different diameters.
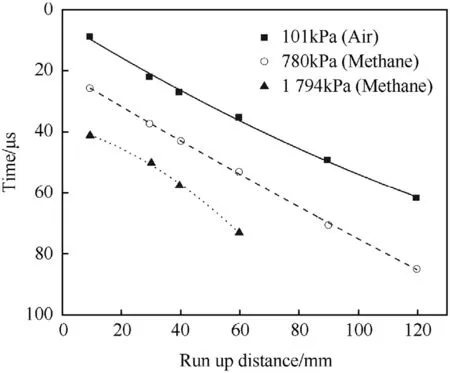
Fig.9.Influence of gas pressure on propagation of 11,11 mm diameter NGu-charges at ρ=0.5 g/cm3.
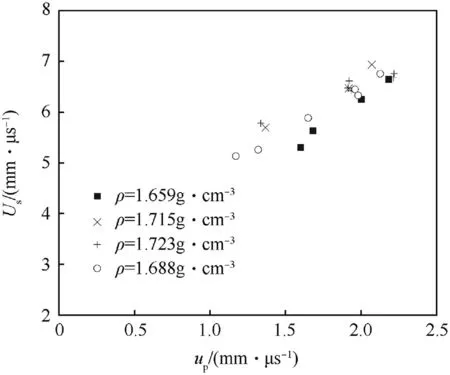
Fig.10.Us—up plane for pure NGu.
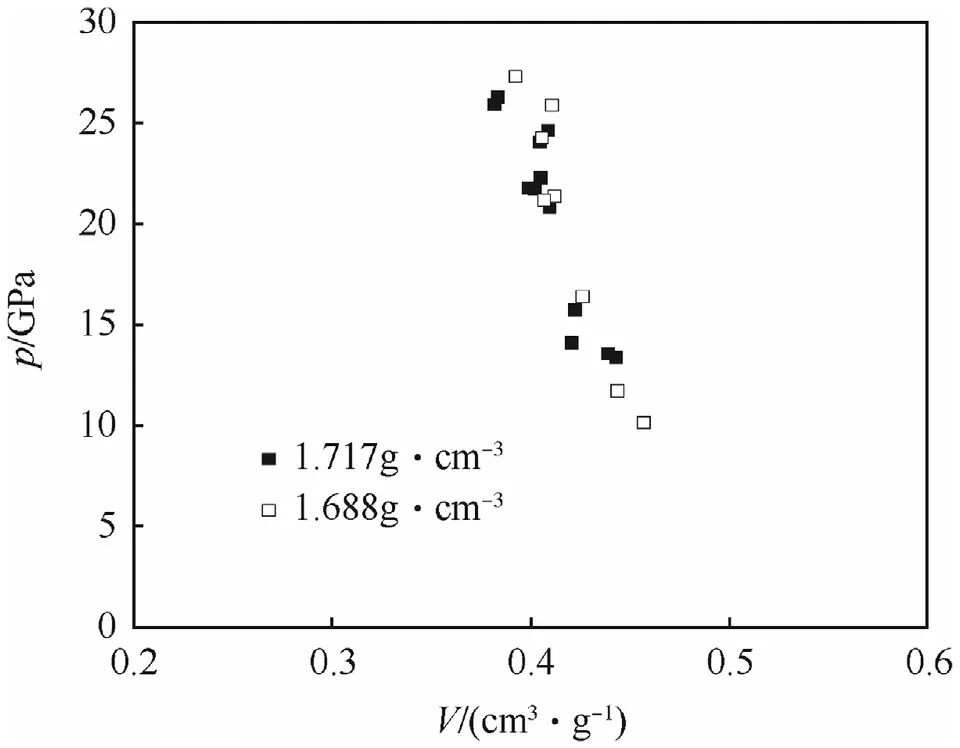
Fig.11.p—V plane for pure NGu.
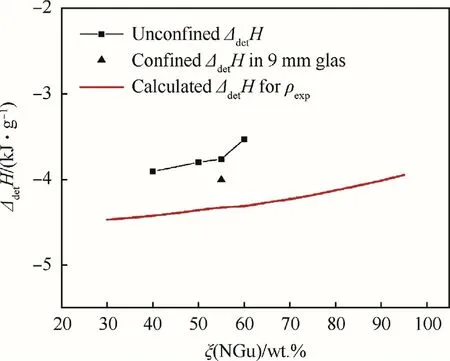
Fig.12.Detonation enthalpy of Nigutol.
The experimental detonation velocity of aluminized Nigutol and one single aluminized Guntol(Guntonal)[56].Is shown in Table 11[54,57].
The detonation pressure determined by cylinder tests has been reported by Hornberg for Nigutol-35,-50(Fig.14)and aluminized Nigutol[58].Table 12 displays those values together with formulations based on FOX-12.
The Gurney-Velocities of Nigutol and Guntol modified with either or both nitramine and aluminium are presented in Table 13.In essence Gurney-Energy for Nigutol is between 10%~17%higherthan for Guntol. Remarkable is that Nigutol-50 is equally powerfulas Guntol (35/40) modified with 25 wt% HMX (sic). While adding aluminium has no pronounced effect on Nigutol-35 the Gurney velocities of Guntol apparently decreases.

Table 9 Critical diameter of Nigutol- 50 with SHBD and HBD [52,53].
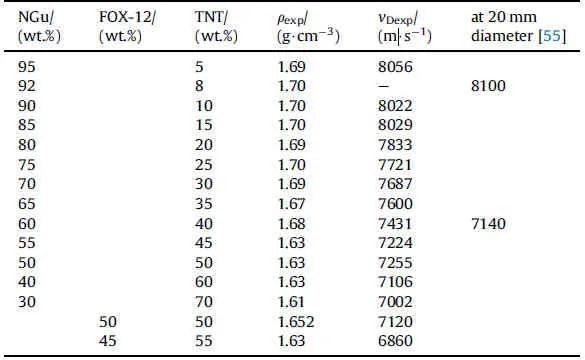
Table 10 Detonation velocity of various Nigutol (unconfined φ = 50 mm) and two Guntol (Cuconfined,φ = 60 mm) formulations.
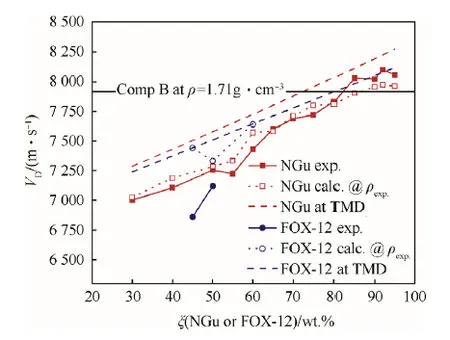
Fig. 13. Detonation velocity of Nigutol and Guntol as function of respective NGu andFOX-12 content.
3.2.1.2. IMX-101 and ALIMX-101.Two important NGu-based melt cast formulations comprising NTO as an additional insensitive filler are IMX-101 [63] (formerly known as OSX-CAN) and its aluminisedderivative ALIMX-101 [64]. Table 14 displays the disclosed composition for IMX-101 and the alleged formulation for ALIMX- 101, Table 15 shows the performance. Due to the large critical diameter of IMX-101 neither plate dent nor aquarium test have been conducted so far. The values used in Refs. [66,68] are based on a Cheetah 4.0 calculation at ρ = 1.63 g/cm3.
The unreacted Hugoniot data for IMXwas obtained by Roth et al. [68] and is displayed in Fig. 15.
3.2.1.3. PrNGu-NGu-HMX.n-Propylnitroguanidine (PrNGu) (mp:98.5 °C) is a substance currently investigated as potential melt-cast base for high explosives [69]. As a crystal density is unknown its density has been estimated using Ammon's procedure [70] to ρ = 1.35 g/cm3. A ternary formulation comprising about equal amounts PrNGu, NGu and HMX has been investigated by Samuels et al. (Tables 16 and 17) [71].
3.2.1.4. Eutectic systems based on NGu.NGu forms a series of eutectic systems with other explosive materials and dissolves nicely in many energetic ionic liquids. Hence highly dense charges can be obtained entirely without using costly SHBD.
Manuelli and Bernadini were the first to claim eutectic meltcastable formulations named Albite, based on NGu, ammonium nitrate and guanidinium nitrate with melting points below 130 °C[72]. Urbanski and Skrzynecki found that a formulation.
3.2.1.4.1. NGA
· Nitroguanidine 17.5 wt%
· Guanidinium nitrate 22.5 wt%
· Ammonium nitrate 60.0 wt%
Would melt as low as 113.2 °C [73]. In addition, they found two other binary eutectic mixtures.
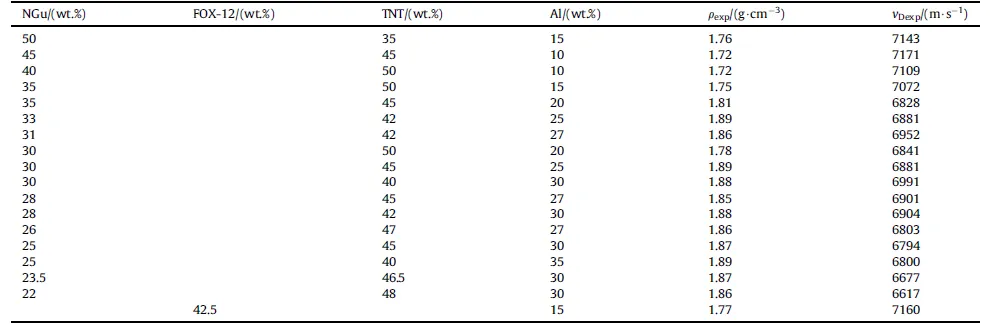
Table 11 Detonation velocity of various aluminized Nigutol (unconfined φ= 50 mm) and one aluminized Guntol (Guntonal) (Cu-confined, φ = 60 mm) formulations.
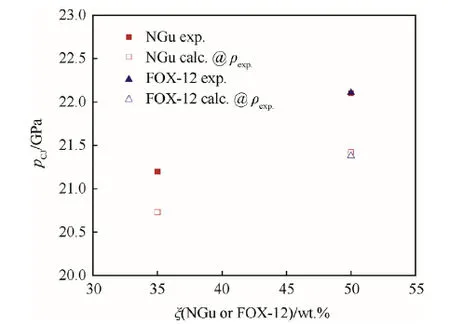
Fig. 14. Detonation pressure of TNT/NGu and TNT/FOX-12 as a function ofstoichiometry.
· Nitroguanidine 20 wt%
· Ammonium nitrate 80 wt% mp: 131.5°C
· Nitroguanidine 41 wt%
· Guanidinium nitrate 59 wt% mp: 166.5 °C
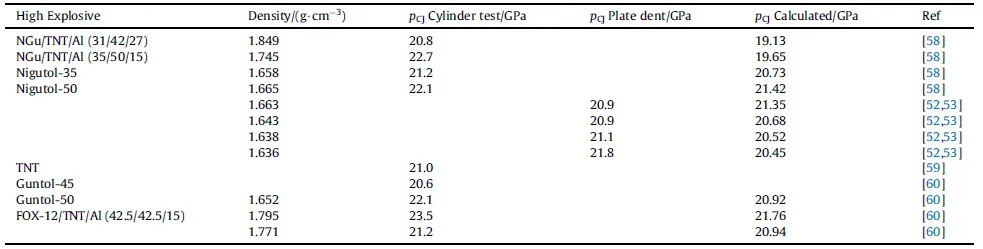
Table 12 Detonation pressure of Nigutol and related formulations.

Table 13 Gurney Velocity for various melt cast NGu-based explosives.
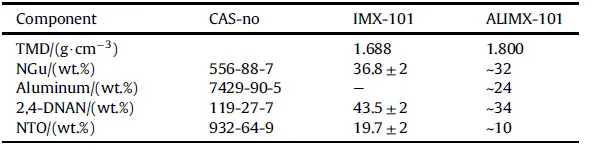
Table 14 Composition of NGu-based melt cast insensitive high explosives.
While neither Manuelli nor Urbanski have reported any data on the performance of NGA or any of the other formulations, Akts &Herskovitz have tested blends of NGA with other HE (Table 18 andTable 19) [74]. The critical diameter in steel confinement is well below 9.65mm for NGA/AN/RX while NGA/AN has a limiting diameter well above 9.65 mm. Though the detonation pressure nicely correlates with calculations for NGA/AN/RDX the detonation velocity falls dramatically short by 16% against predictions with Cheetah 2.0.
3.2.1.4.2. NGu-AN-ADNT.Ammonium 3,5-dinitro-1,2,4-triazolate, ADNT (Fig. 16) (ρ = 1.75 g/cm3, mp: 168°C, ΔfH: +4 kJ/ mol) forms a eutectic mixture with AN melting at 112 °C [75] which dissolves up to 12 wt% [76] of LBD-NGu. Two formulations with 33 and about 40% NGu (dissolved content plus HBD-NGu) have been formulated and tested (see Table 20 and Table 21). The experimental CJ-pressures exceed the predicted values by 6%~8%.
3.2.1.4.3. NGu-AN-EDDN.Ethylenediammonium dinitrate, EDDN (Fig. 17) (ρ = 1.603 g/cm3, mp: 186 °C, ΔfH: 653 kJ/mol) forms a eutectic mixture with AN melting at 98 °C and freezing at 81 °C [77]and dissolves LBD-NGu(Table 22 and Table 23).
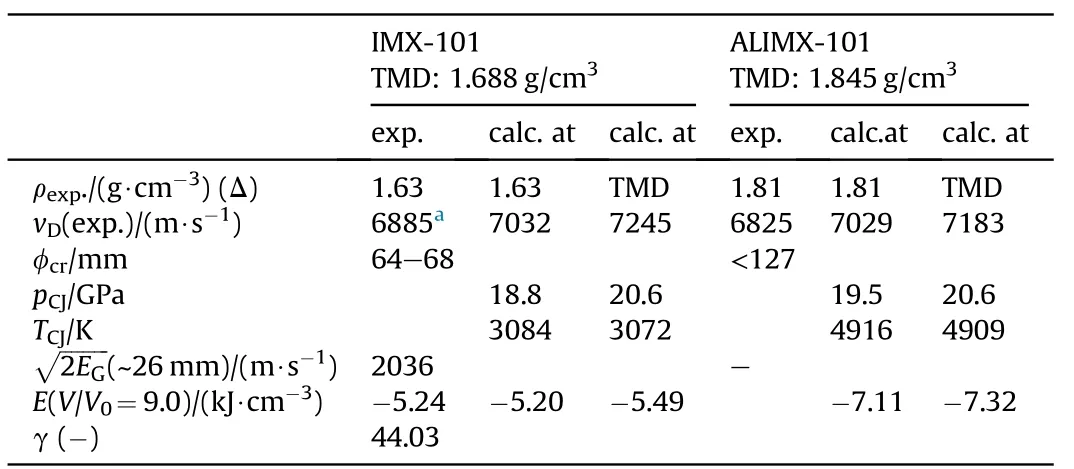
Table 15 Performance of IMX-101 and ALIMX-101[65—67].
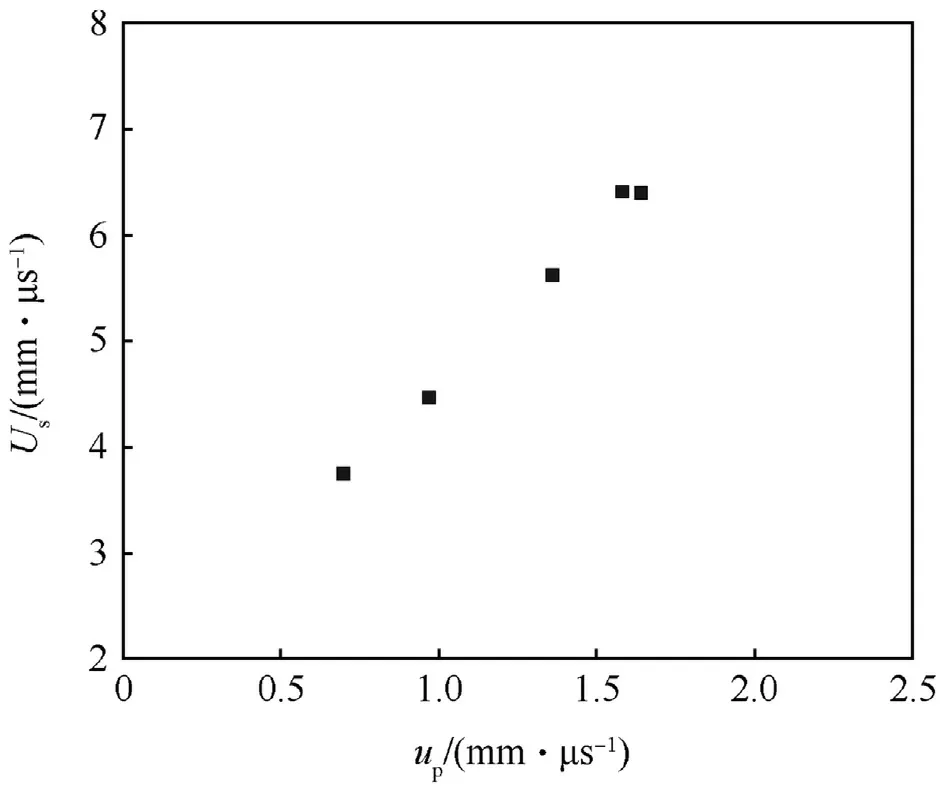
Fig.15.Us—up plane for IMX-101 at ρ=1.63 g/cm3.
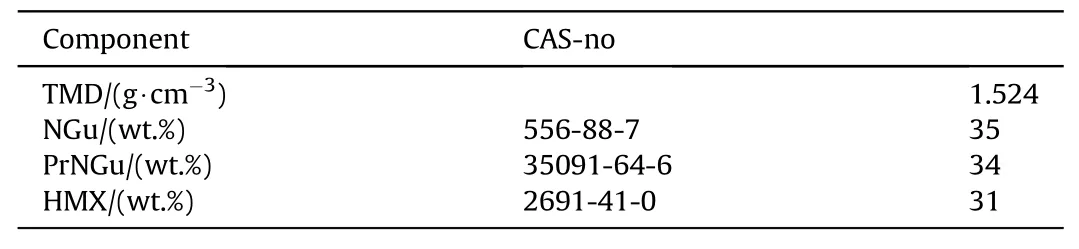
Table 16 Composition of NGu-based melt cast insensitive high explosives.
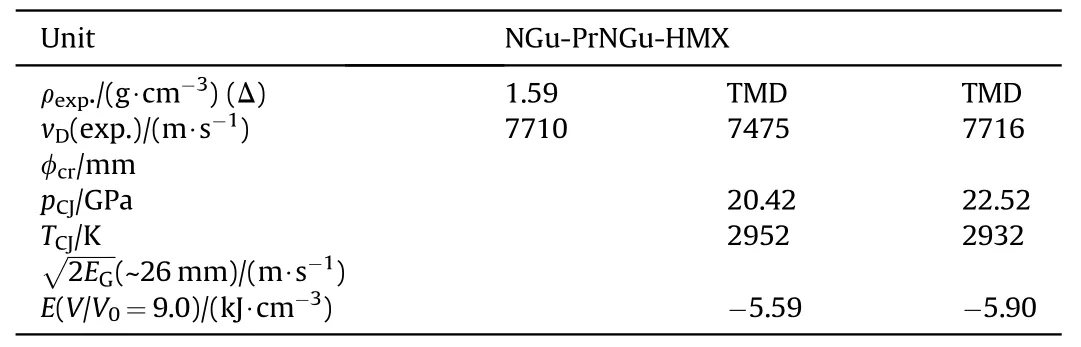
Table 17 Performance of NGu-PrNGu-HMX[71].
3.2.1.4.4. NGu-AN-MeNGu.NGu forms a eutectic with its methylated derivative MeNGu melting at 128°C[80].Likewise,AN forms two eutectics with MeNGu melting at 117 and 118°C[81].Three formulations have been reported(Table 24 and Table 25).
AFX-453 has been developed at Eglin Air Force Base as meltcastable blast explosive in the 1980s for use with the Mk82 bombs[82].AFX-453 is a modification of composition III given above in Table 18.There are two slightly different formulations reported in the literature(Table 26 and Table 27).AFX-453 has been reported to melt at 103°C which demonstrates the beneficial effect of NGu on the binary eutectic system AN/MeNGu.The reported performance of AFX-453 is for an unknown density.Fig.18 shows the variation of vDwith charge diameter of unconfined AFX-453.
Yet another eutectic melting at 104°C named DEMN is formed by the quaternary composition given in Table 28[85].
While the density of DEMN is too low to qualify for any application its mixtures with other high explosives such as additional NGu and RDX has been qualified as IMX-103(Table 29)[63].
3.2.1.4.5. NGu-CE-ECE.Tetryl and ethyltetryl in a mass ratio 70/30 form a eutectic melting at 85—88°C[59].This eutectic has been proposed as melt cast base for NGu by Schlüter and Hermann(Tables 30 and 31)[86].
3.2.2. Cure-castable formulations
Due to the low shock sensitivity of NGu,both hexogen and octogen have been applied as sensitizer in binary and ternary formulations with aluminium.Table 32 depicts the formulations while the performance is displayed in Table 33.
3.2.3. Pressable formulations
Several pressable formulations containing either NGu as the sole explosive component(AFX-902,X0228)[98—100]or in binary formulations with HMX(X0118,X0183)[102]as an additional explosive filler have been reported.These formulations are compared with formulations based entirely on 1,3,5-triamino-2,4,6-trinitrobenzene (TATB) (PBX9502), 1,1-diamino-2,2-dinitroethylene(FOX-7)(QRX080)[101]and octogen(HMX)(LX-14)(Tables 34,35a and 35b).
Fried&Souers describe and rank AFX-902 as an“ideal explosive”comparable to LX-14[121].This is not surprising as the detonation pressure,Gurney energy and detonation velocity of AFX-902 reach 77.5%, 82.0% and 94.8% respectively of LX-14.Though both TATB and FOX-7 possess higher densities than NGu(+10;+8%)and have both higher detonation enthalpies than NGu(+13;+25%)the detonation velocity of AFX-902 is equivalent if not superior to both PBX-9502 and QRX080.The detonation pressure of AFX-902 is comparable to PBX-9502 and just 94%of QRX080.The Gurney Energy of AFX-902 is about the same as for PBX9502 and just 92%that of QRX080.The critical diameter for both AFX-902 and PBX-9502 appears to be in the same range.No data on FOX-7 based critical diameter is available.
The shock Hugoniot data for X0228 are depicted in Figs.19 and 20.
3.2.3.1. Miscellaneous formulations.Gogyula et al.have reported about pressable binary formulations of NGu and Al in a mass ratio(85/15)[27,44].Table 36 depicts the performance of various formulations containing different type aluminium powder against HMX/Al formulations as comparison.
Heat resistant explosive formulations based on NGu having high specific surface area(9000—16000 cm2/g)are the subject of aformerly classified Soviet Union patent released 46 years after its submission(Table 37)[103].
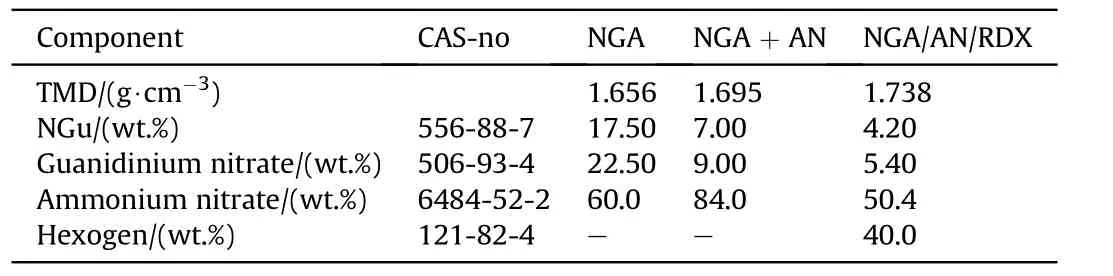
Table 18 Composition of NGu-based melt cast insensitive high explosives.
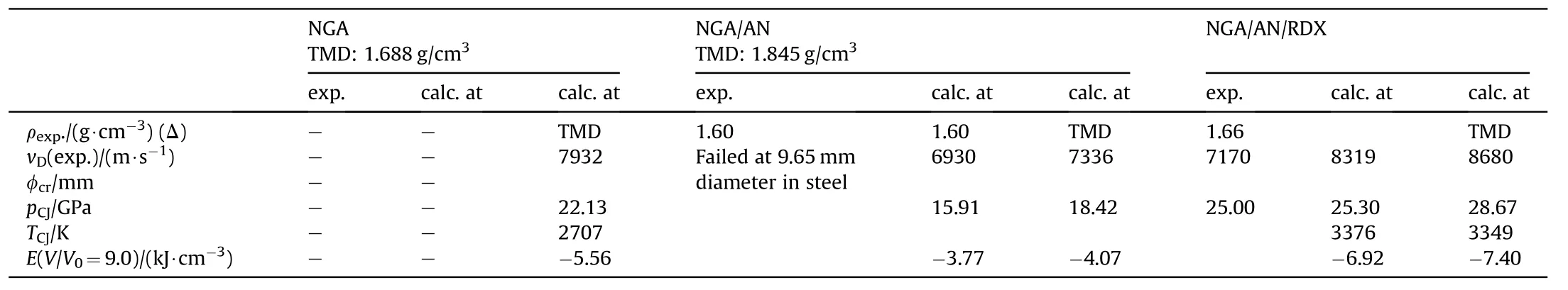
Table 19 Performance of NGA,NGA/AN and NGA/AN/RRDX[74].
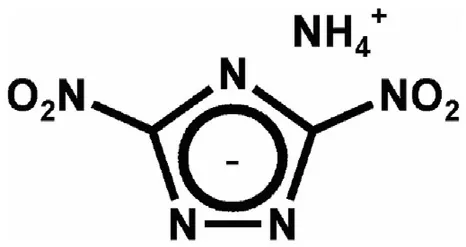
Fig.16.Structure of ADNT.
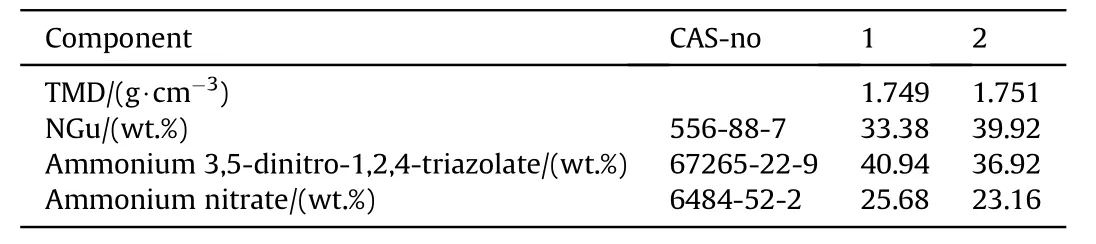
Table 20 Composition of NGu-based melt cast insensitive high explosives.
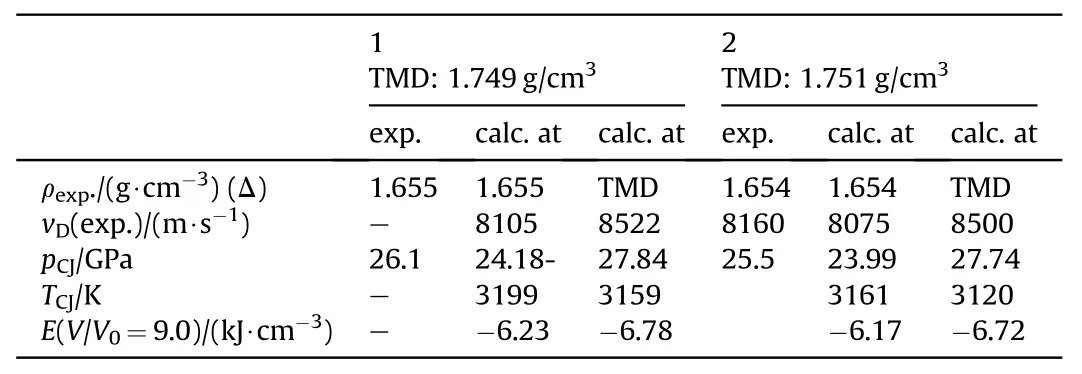
Table 21 Performance of NGA,NGA/AN and NGA/AN/RRDX[76].
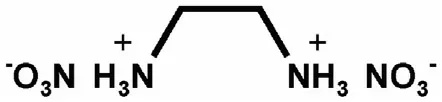
Fig.17.Structure of EDDN.
4. Sensitiveness
4.1. Friction and impact sensitivity
NGu and the formulations based on it are mostly not very friction or impact sensitive,however,more sensitive components may trigger sensitivity as indicated below in Table 38.
4.2. Shock sensitivity
Nitroguanidine and the formulations based thereon are very insensitive to shock.Hence and due to the comparatively large critical diameter shock sensitivity of NGu-based formulations are typically assessed with NOL-LSGT[28],the ELSGT[105]and the SLSGT[106].
4.2.1. Critical energy
Shock initiation of a high explosive occurs when its unit surface area is subjected by a specific minimum energy while shock pressure,p,and shock duration,t,may vary.The energy fluence,Ecrit,(J/cm2)in a specific volume is therefore a characteristic figure to describe the sensitivity of an energetic material towards shock initiation[107].
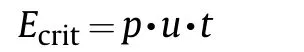
Lungenstraβ has determined Ecritfor NGu and formulations based thereon as well as reference high explosives(see Table 39)[55].
For the hot-spot model Mader calculated adiabatic explosion times for shock initiation of high explosives with spherical holes[108,109].Table 40 displays the variation of explosion time for different explosives and different temperatures(correlating with different shock sensitivity).Fig.21 shows the influence of spot size and shock pressure on the initiation of NGu,TATB and HMX.
4.2.2. LSGT
The influence of charge density on the shock initiation pressure of both LBD and HBD-NGu in LSGT is depicted in Fig.22[28].Itreflects the common observation that porosity is a prerequisite for successful shock ignition.
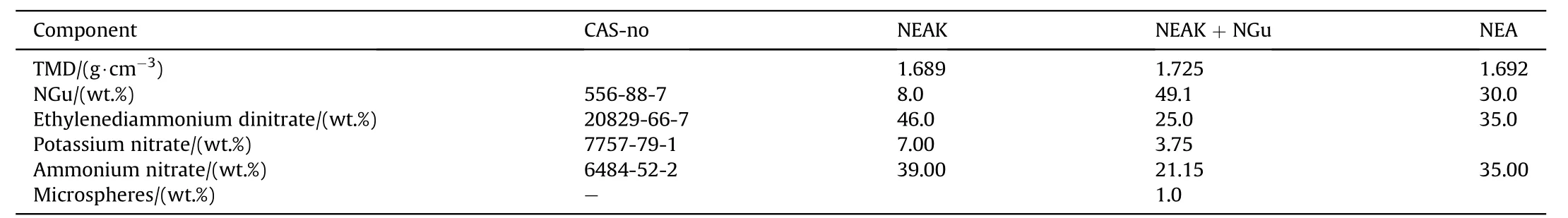
Table 22 Composition of NGu-based melt cast insensitive high explosives.
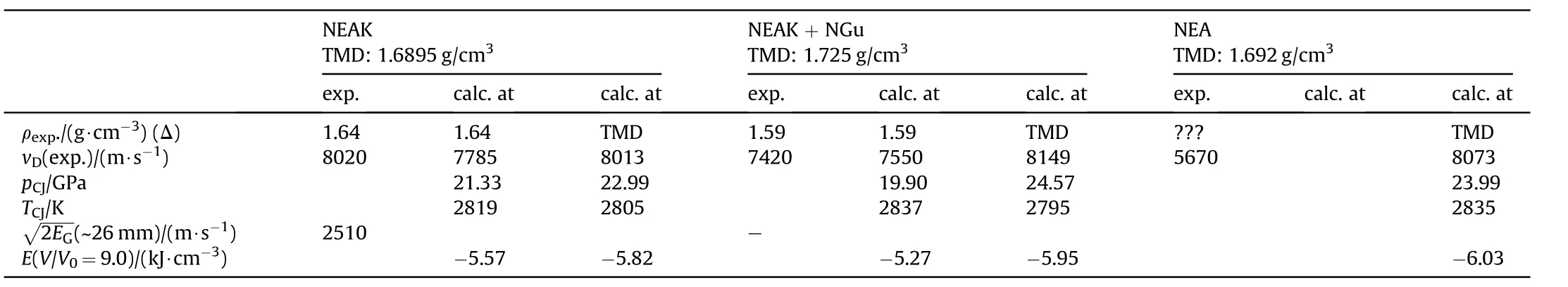
Table 23 Performance of NEAK[77—79].
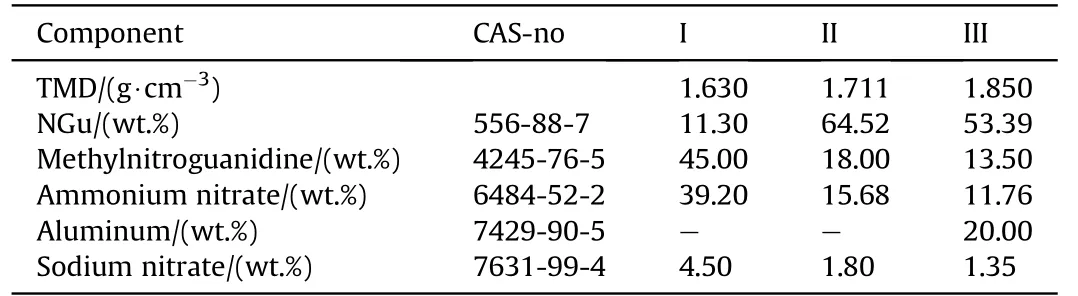
Table 24 NGu-AN-MeNGu.
LSGT-data on NGu-based formulations and reference materials are displayed in Table 41.
4.2.3. ELSGT
ELSGT data are displayed and compared in Table 42.
4.2.4. SLSGT
Data for the SLSGT have been reported in Ref.[65]and are compared with TNT and PBXN-109(Table 43).
4.2.5. BICT Gap test
Results of the BICT Gap test[114,115]on pressed Nigutol-40(having an unusual high porosity!)[54]and Guntol[60]have been published.However,both Nigutol and Guntol have critical diameters in the same ballpark as the test configuration(φ ~24 mm)which is why these data are of questionable quality and hence will not be discussed here.
4.2.6. Run-to-detonation distance for shock to-detonation transition(SDT)
The run-to-detonation distance for neat NGu has been determined by Popolato et al.[116]and is depicted in Fig.23.
The run-to-detonation distance for IMX-101 has been tested with different methods and is depicted in Fig.24 for a charge density of ρ=1.56 g/cm3[117].
The run-to-detonation distance of X0228 is depicted below in Fig.25.
The law for X0228 readslg(p)=1.42-0.19 lg(x)
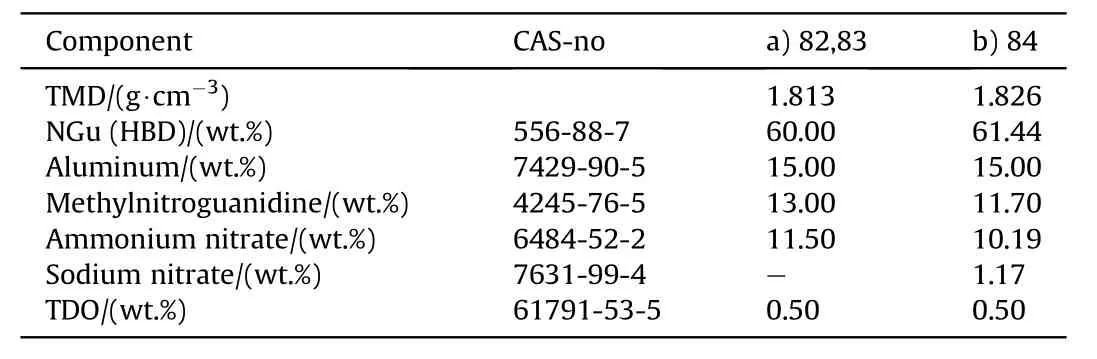
Table 26 Composition of AFX-453.
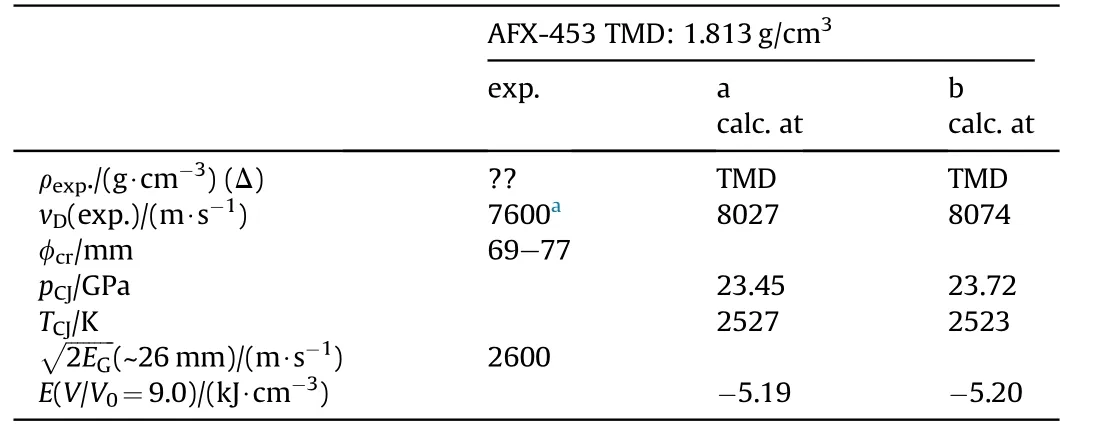
Table 27 Performance of AFX-453[82—84].
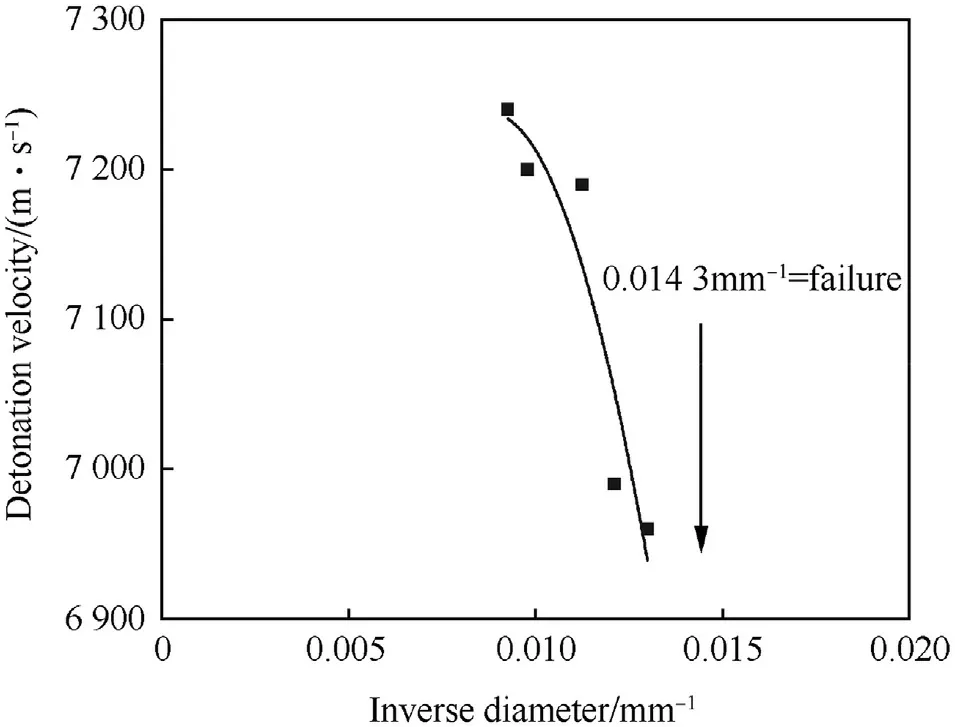
Fig.18.Inverse diameter detonation velocity relationship for unconfined AFX-453 charges at unknown density.
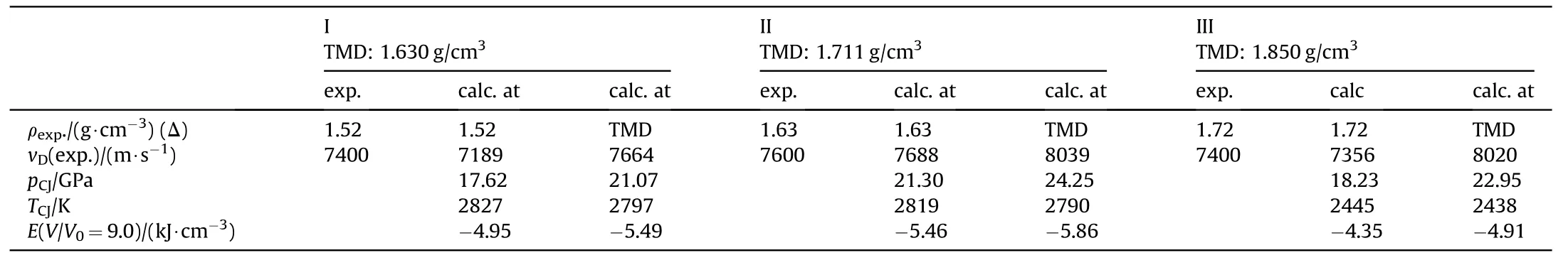
Table 25 Performance of NGu-AN-MeNGu[81].
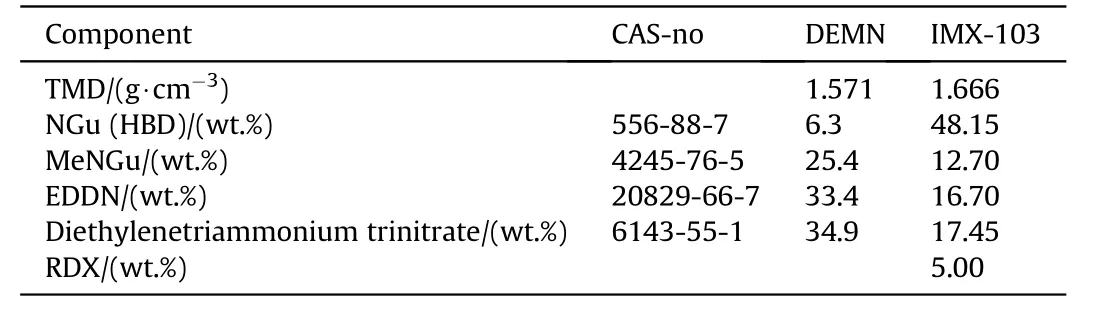
Table 28 Composition DEMN and IMX-103[86].
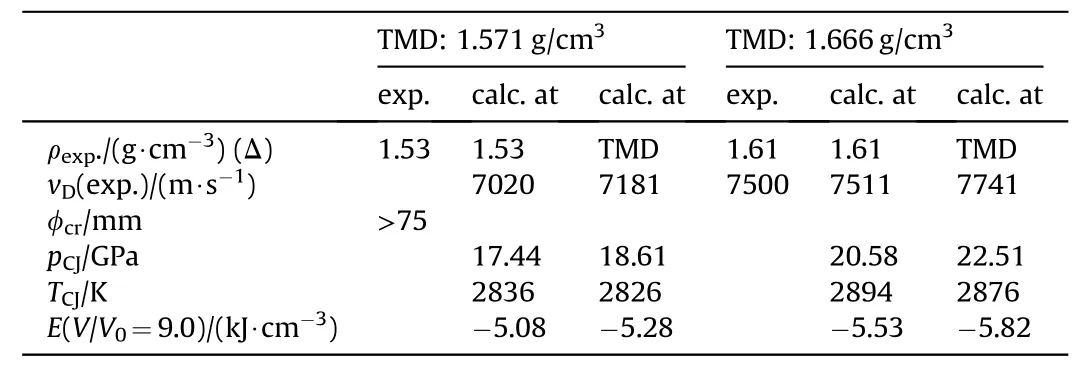
Table 29Performance of DEMN and IMX-103[63,85]
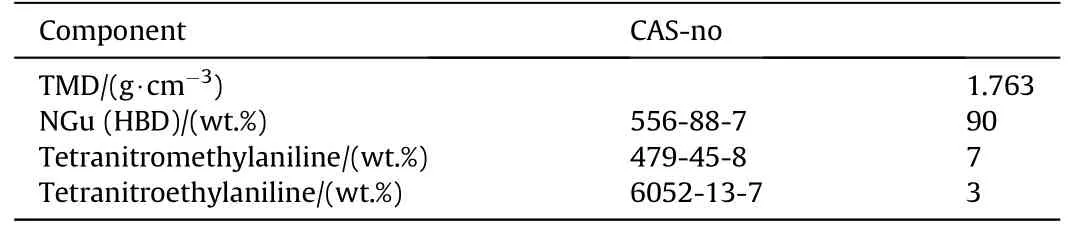
Table 30 Composition NGu-Tetryl-Tetryl-E.
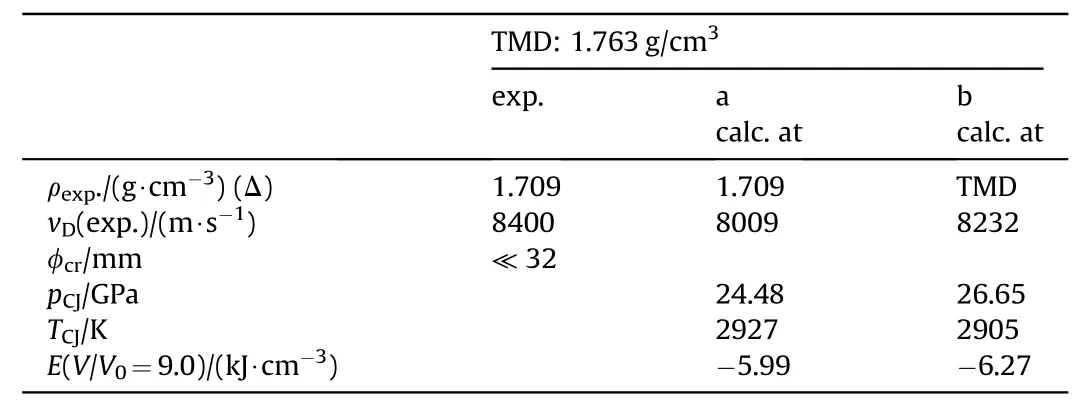
Table 31 Performance of NGu-Tetryl-Tetryl-E[86].
4.3. Projectile impact
Lee has calculated the critical projectile impact velocity versus projectile diameter relationship for bare X0228 from pop plot data.The results and comparative data for more sensitive high explosives Comp B and TNT are depicted in Fig.26[118,119].Though“initiations”for both X0228 and TNT can be expected in the full range of projectile diameters it must be remembered that stable detonations will probably only develop when the projectile diameter is in the same range as the critical diameter of the corresponding explosive which is about 15—20 mm for both X0228 and TNT.
5. Insensitive munitions tests of NGu based formulations
Insensitive Munitions Tests as defined in AOP-39 serve the evaluation of the response of a particular store or a test vehicle towards threats typically encountered in the life cycle of an ammunition[6].Table 44 displays those tests and the underlying scenario and the desired response of an article to be considered insensitive.
The corresponding responses are depicted in Table 45.The IM Signature Color code requires green if the response is met,yellow if the response is not more than one type higher,red if the response is more than one type higher and white if a test has not been conducted.
The IMX-101,AFX-770 and AFX-900 have been tested in full scale ammunitions(Table 46 and Table 47)and are compared against baseline vulnerable high explosive and blast formulations Comp B,TNT and H-6.
6. EI(D)S—Extremely Insensitive(Detonable)substances
Explosives that pass the full-scale UN-Test series 7 for formulations 7(a)~7(f)and the article 7(g)~7(k)are designated Extremely Insensitive(Detonating)Substances EIS(formerly EIDS).The corresponding articles(munitions containing those explosives)then are categorized as Hazard Division 1.6[123].Qualified EIS containing NGu are the aforementioned formulations AFX-760,AFX-770,AFX-920,and AFX-930[124].
7. Summary
Swiss chemist Alfred Stettbacher—considered an authority in the field of explosives in his time—in 1936 tried to detonate 2.5 g Nitroguanidine stemmed in a rifle(8×57)cartridge with a common(lead azide,mercury fulminate,PETN)cap on a mild steelplate.However,the result of his attempt was only a small dent in the steel plate.Stettbacher with his experimental setup overlooked the low shock sensitivity of NGu and the large critical diameter of it.However,this one single failed experiment led him to drew an ill conclusion “(…). Zufolge seiner betr¨achtlichen Sauerstoffunterbilanz von 30,75%bei gleichzeitg 53,85%Stickstoffgehalt ist dieser Nitrok¨orper kein Sprengstoff.Seine Wirkung ist selbst bei kr¨aftiger Zündung gering.(…)”which translates into“(…)Due to its considerable oxygen deficiency of 30.75%(sic!)combined with a high nitrogen content of 53.85%this nitro compound is no high explosive.Its performance even with fiercest initiation is feable(…)”[125].
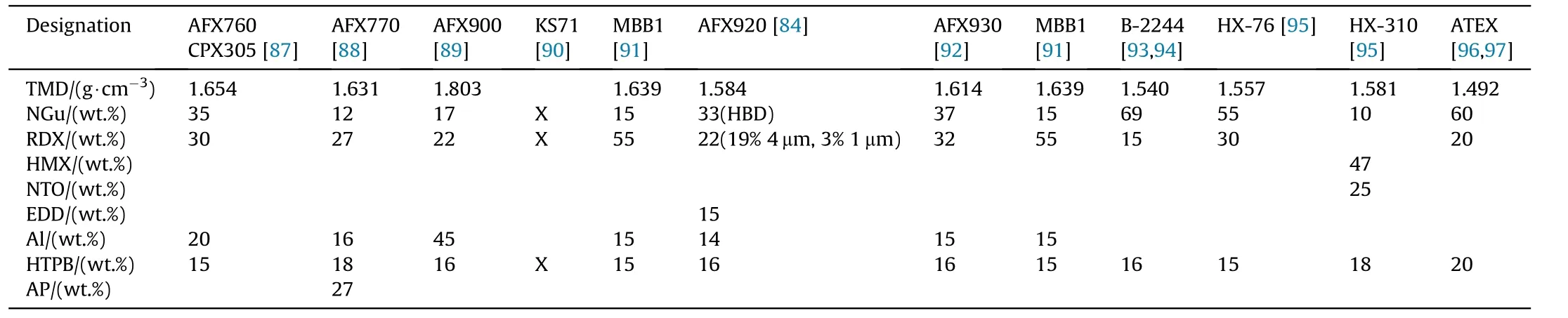
Table 32 Composition of various NGu-nitramine formulations.
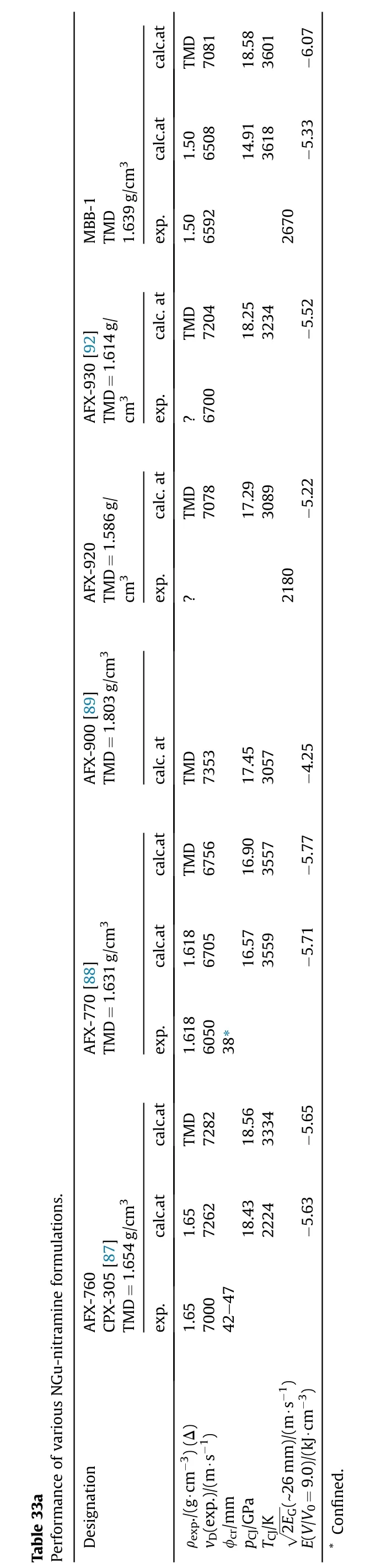
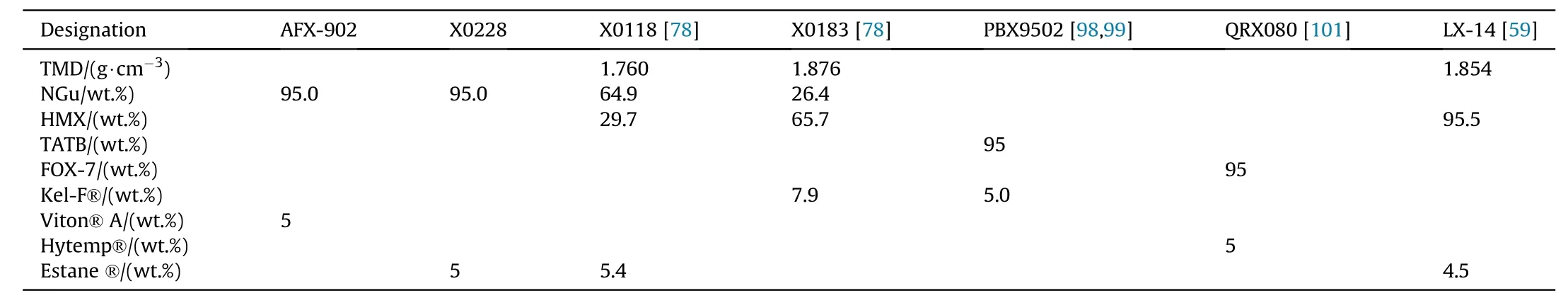
Table 34 Composition of various NGu-nitramine formulations.
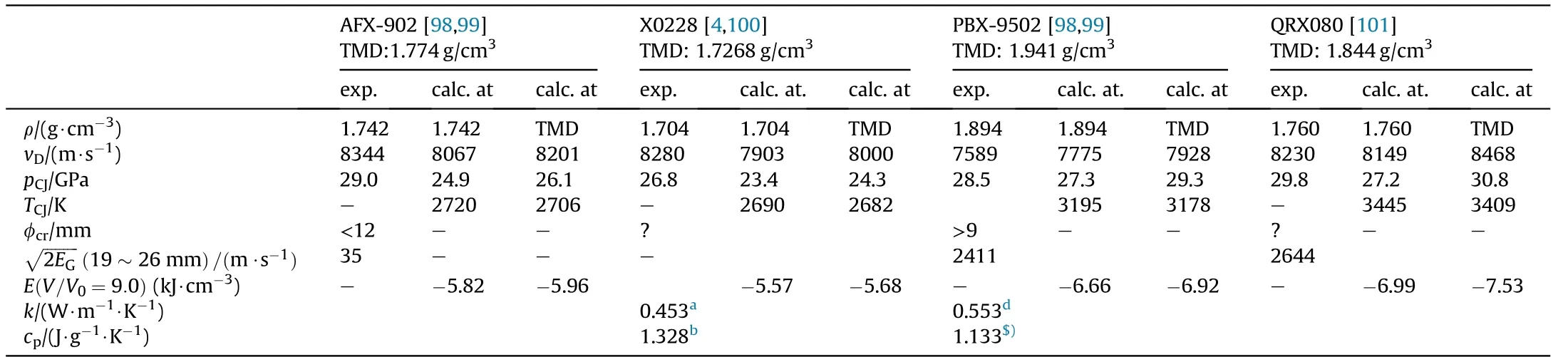
Table 35a Comparison of NGu based Explosives AFX-902 and X0228 with PBX9502(TATB)and QRX080(FOX-7).
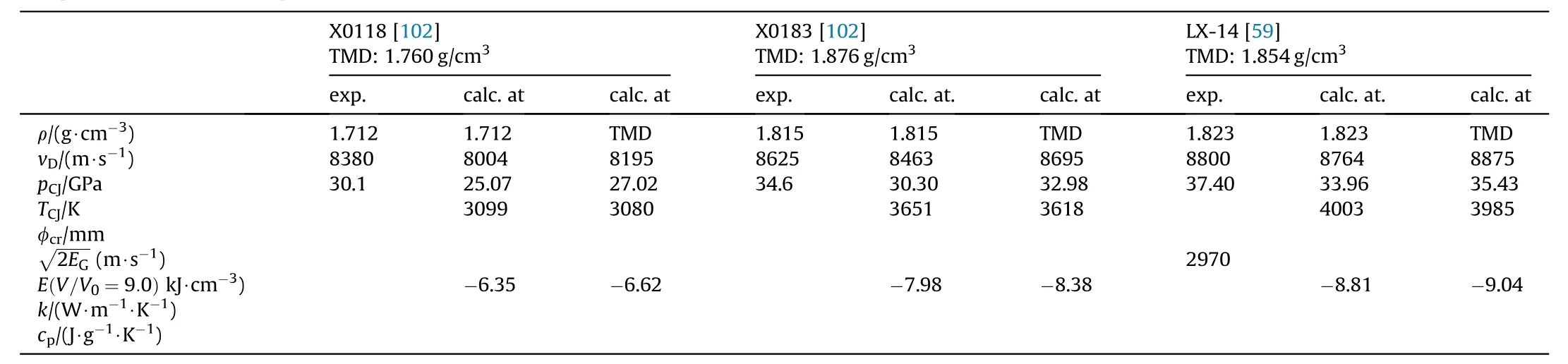
Table 35b Comparison of NGu based Explosives AFX-902 and X0228 with PBX9502(TATB)and QRX080(FOX-7).
In a popular review on insensitive high explosives in 1997 it was erroneously stated“NGu(…)does not meet the criterion of at least 75%HMX performance in detonation pressure and cylinder wall energy(…)”[126].The authors of said review must have picked wrong numbers from the literature.In addition,they overlooked the then recent work by Fried&Souers(1996)—the developers of Cheetah—which assessed AFX-902(95 wt%NGu)to perform like an ideal high explosive with the detonation pressure,Gurney energy and detonation velocity of it reaching 77.5%,82.0%and 94.8%respectively of LX-14 based on 95%HMX[121].
Table 48 displays a synoptic ranking of NGu experimental performance with FOX-12,TATB,NGu,FOX-7 and HMX and percentage of NGu performance.Green is NGu baseline performance,yellow is inferior and blue is superior.
In summary highly dense nitroguanidine clearly outperforms Nguanylurea dinitramide (GuDN or FOX-12) and 1,3,5-triamino-2,4,6-trinitroethylene (TATB) with regards to Gurney Energy,detonation pressure and velocity(See Table 48)it is a close match in performance with 1,1-diamino-2,2-dinitroethylene(FOX-7)(8)with which it is structurally related[1]and reaches even up to HMX delivering up to 78%detonation pressure,82%Gurney Energy and 95%detonation velocity.
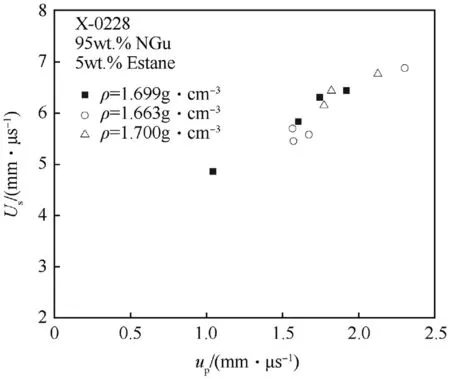
Fig.19.Us—up plane for X0228 at ρ=1.63 g/cm3.
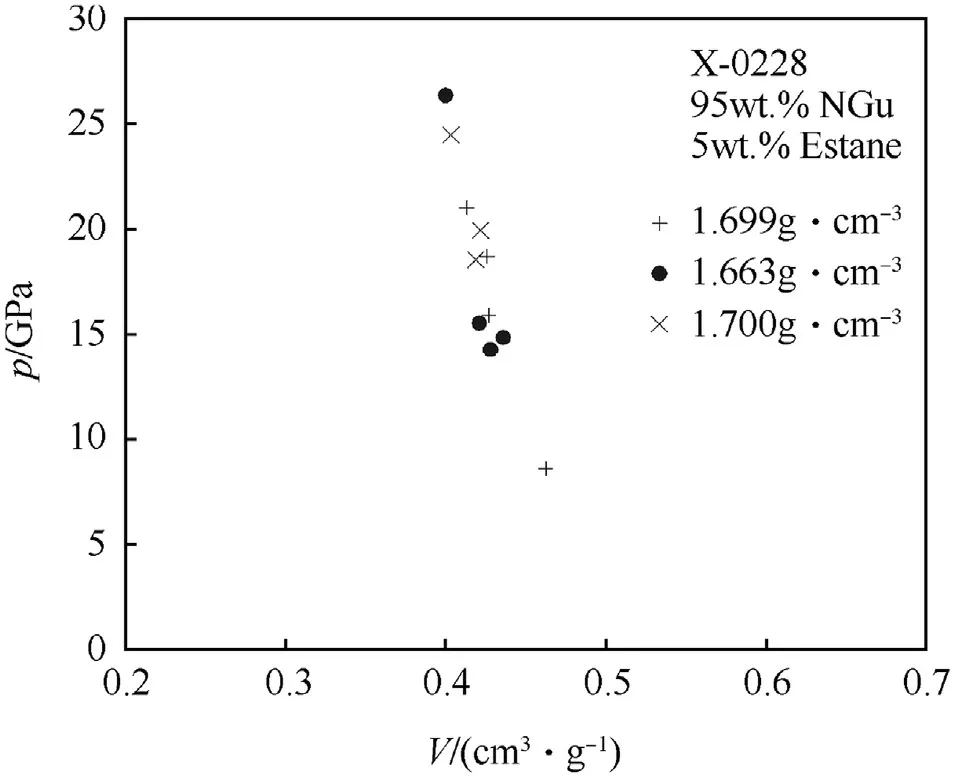
Fig.20.p—v plane for X0228 at various densities.
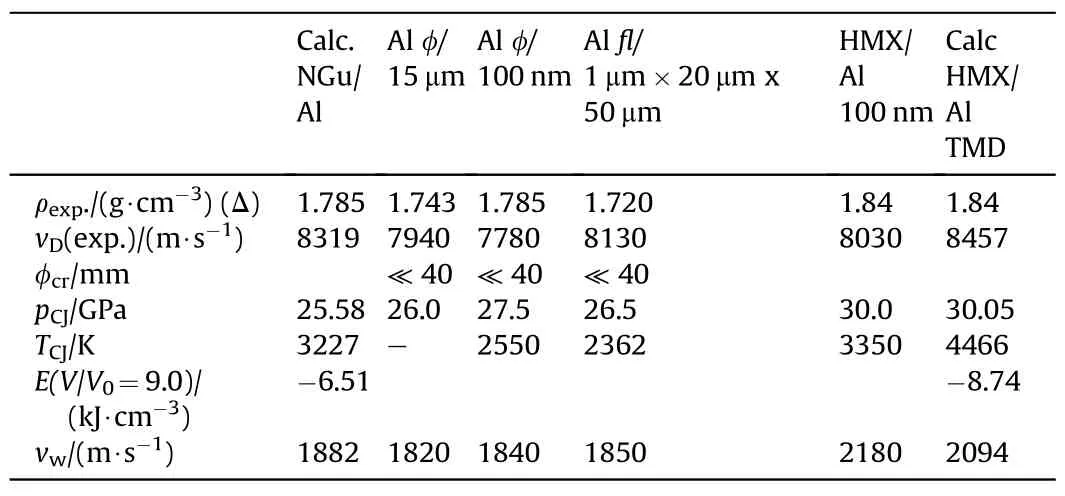
Table 36 Performance of NGu-Al and HMX-Al(85/15)as reference.
On top NGu and its formulations are the least sensitive dealt with regards to shock sensitivity.
8. Outlook
While costly spherical high bulk density(SHBD-)NGu has been used in the past to achieve dense charges this review shows that dense charges can be obtained too by dissolving common LBD-NGuin molten energetic ionic liquids(see§3.2.1.4).In view of the immense current international interest and research efforts in the field of new energetic ionic liquids for melt cast applications[127—131] and given the availability, good performance and extreme low sensitiveness of nitroguanidine,NGu is a naturalcandidate for future highly dense,high performance low sensitivity melt cast formulations.Groven et al. have just observed that Resonant Acoustic Milling(RAM)of LBD-Nitroguanidine yields agglomerates of dense NGu-bundles resembling HBD-NGu[133].
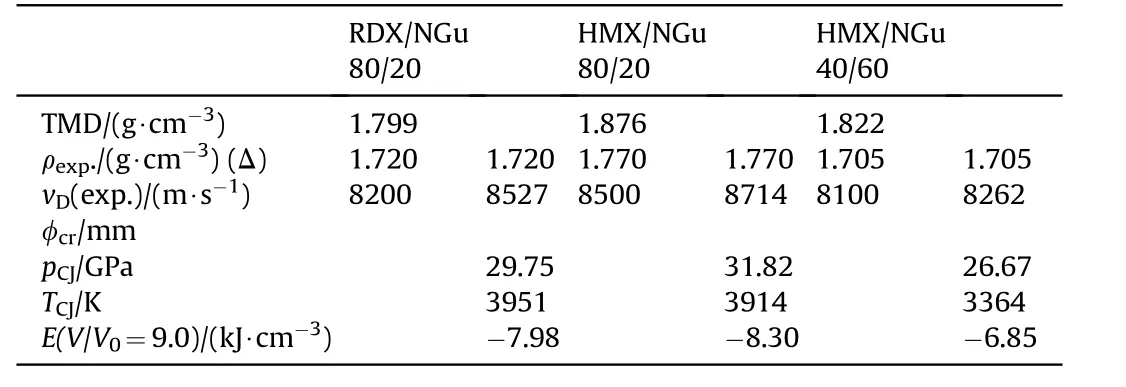
Table 37 Composition,experimental and calculated performance of NGu-nitramine explosive formulations[103].
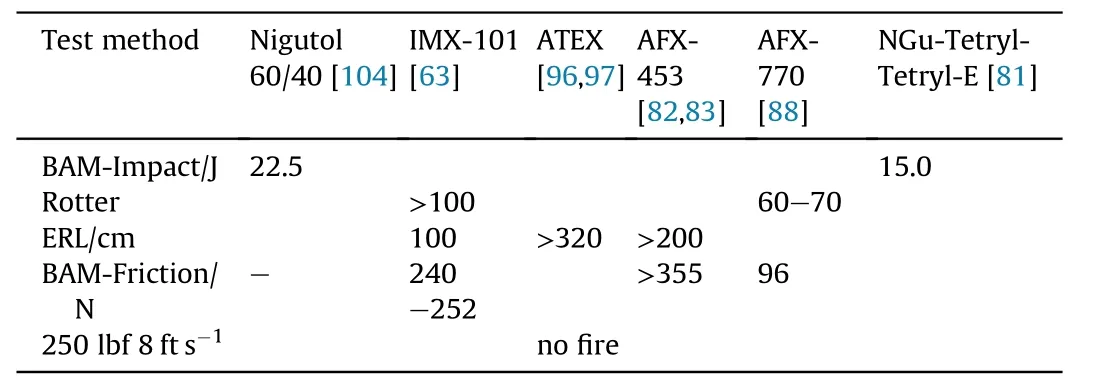
Table 38 50%-Friction,Impact,values of selected formulations.
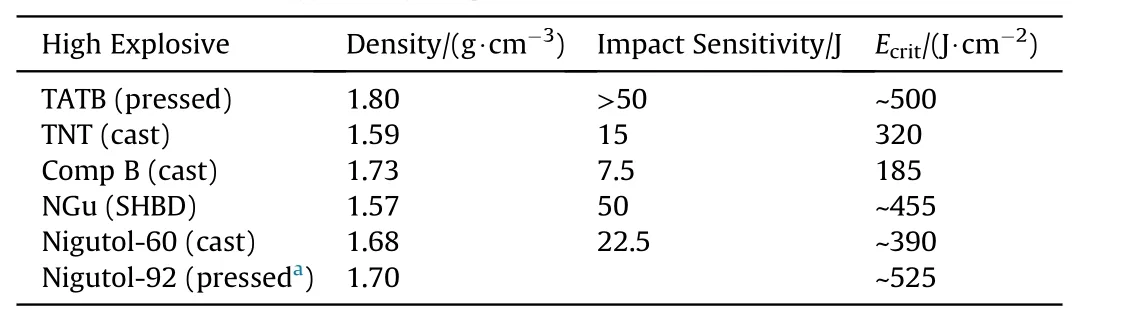
Table 39 Critical Initiation energy for high explosives.
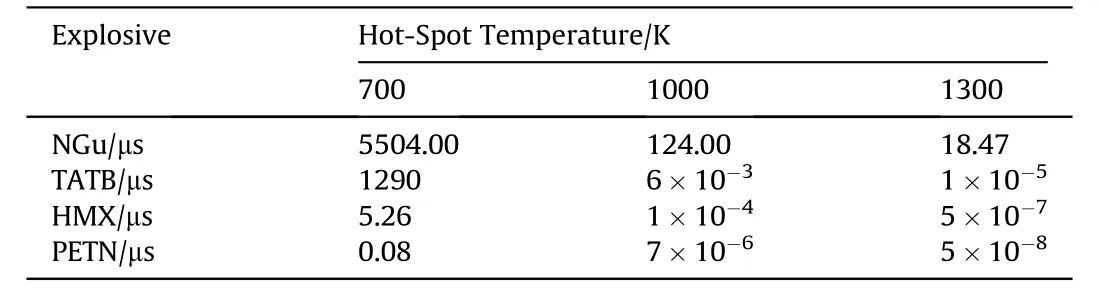
Table 40 Adiabatic explosion times for different explosives after Ref.[108,109].
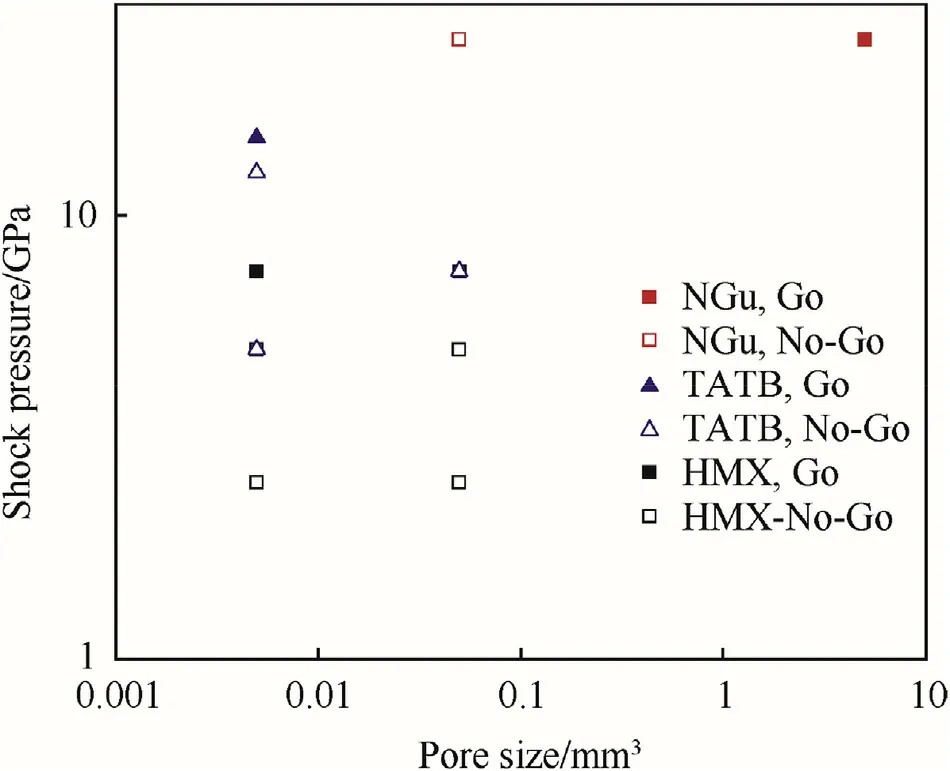
Fig.21.Influence of Pore size and shock pressure on Initiation of NGu and other high explosives after Ref.[108,109].
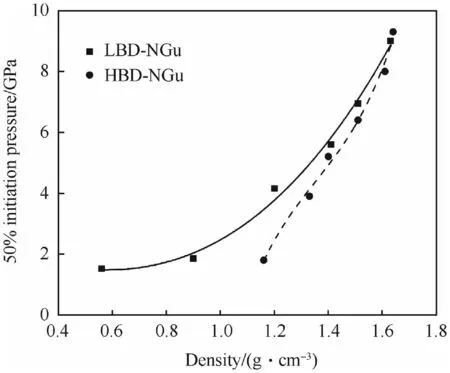
Fig.22.Influence of Density of HBD and LBD on Shock initiation pressure after[28].
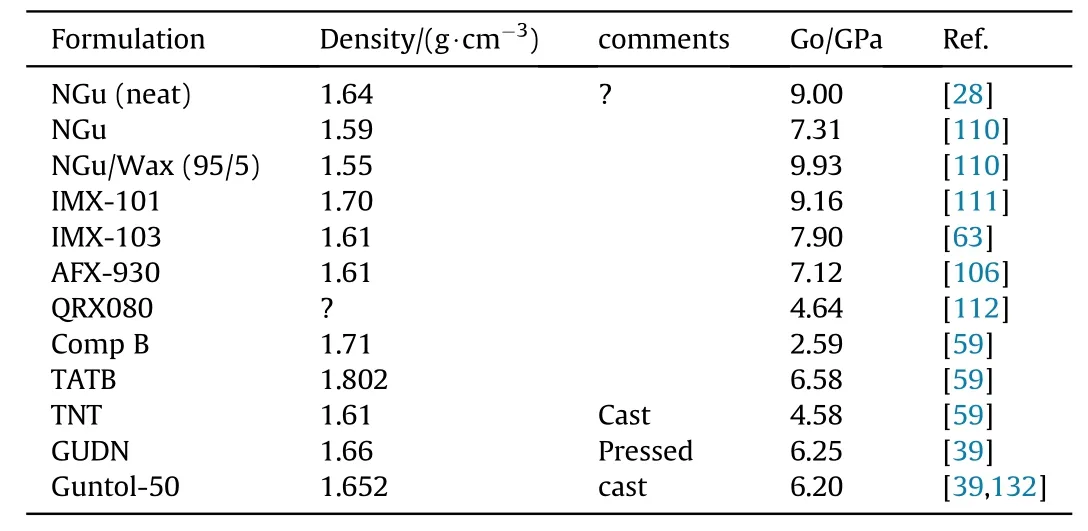
Table 41 LSGT data for NGu,its formulations and reference compositions.
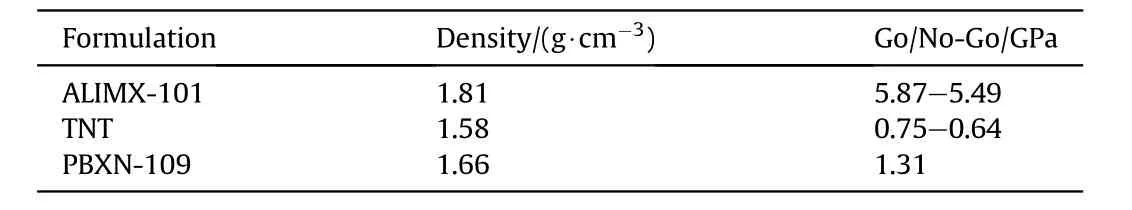
Table 43 SLSGT data for ALIMX-101 and two reference materials.
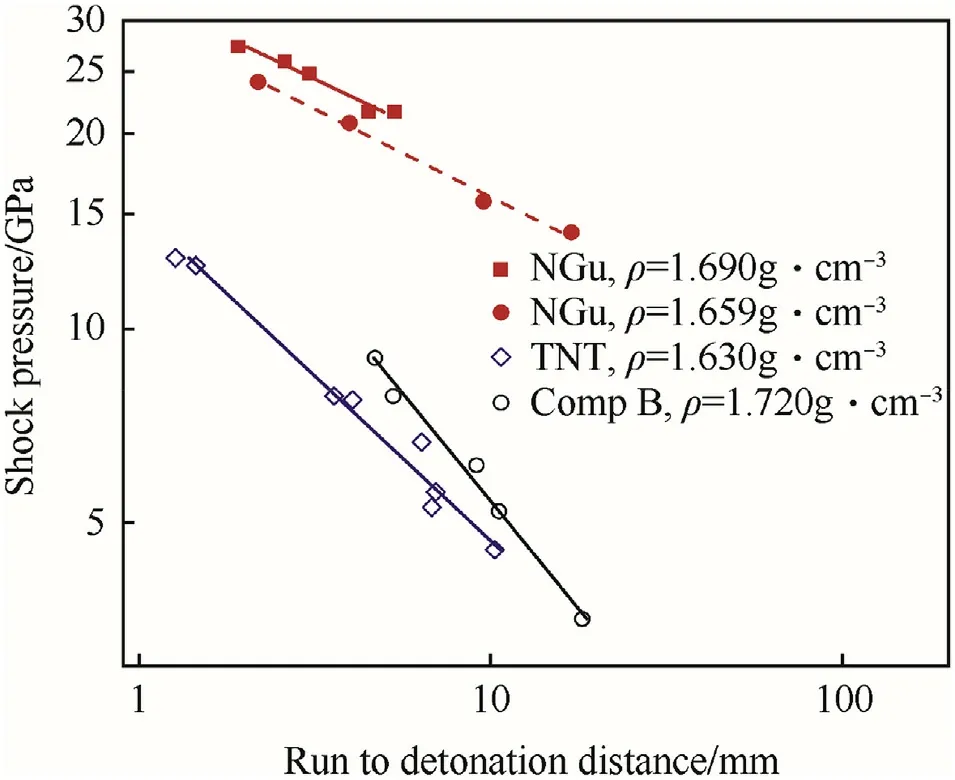
Fig.23.Pop-plot for NGu,TNT and Comp B.
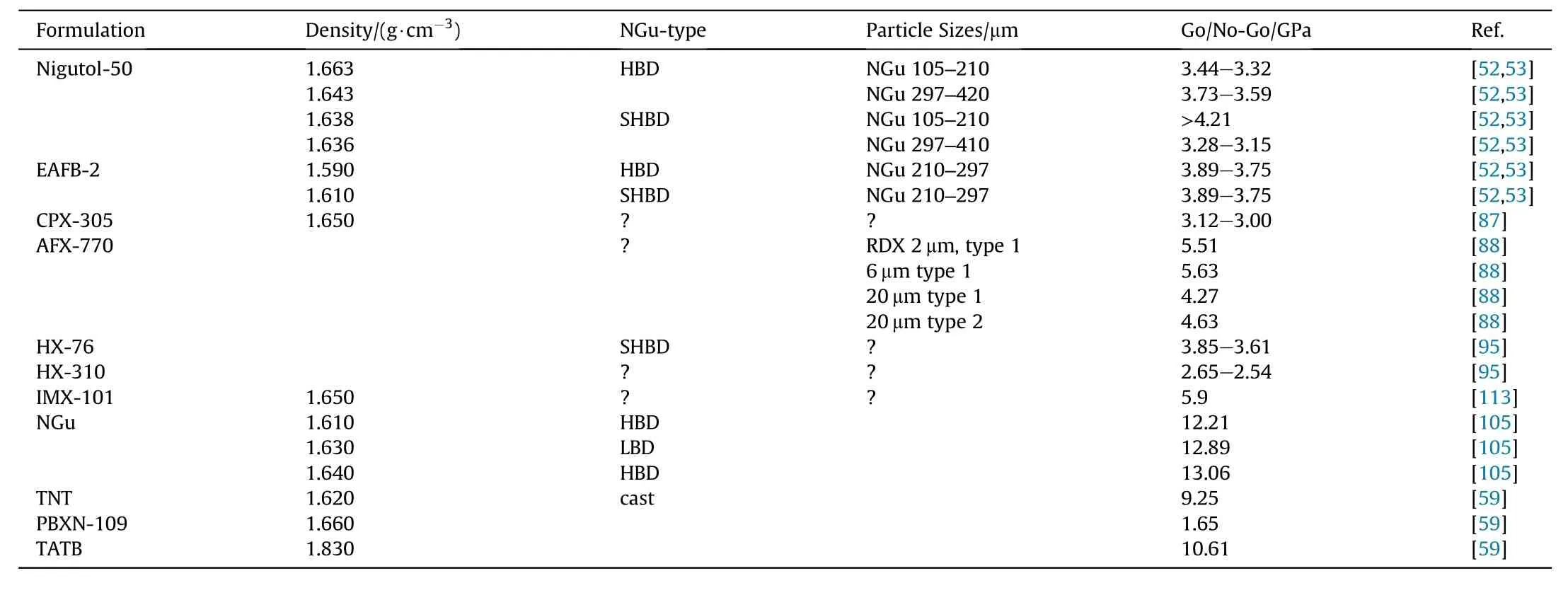
Table 42 ELSGT data for NGu,its formulations and reference compositions.

Fig.24.Pop-plot for IMX-101(ρ=1.56 g/cm3).
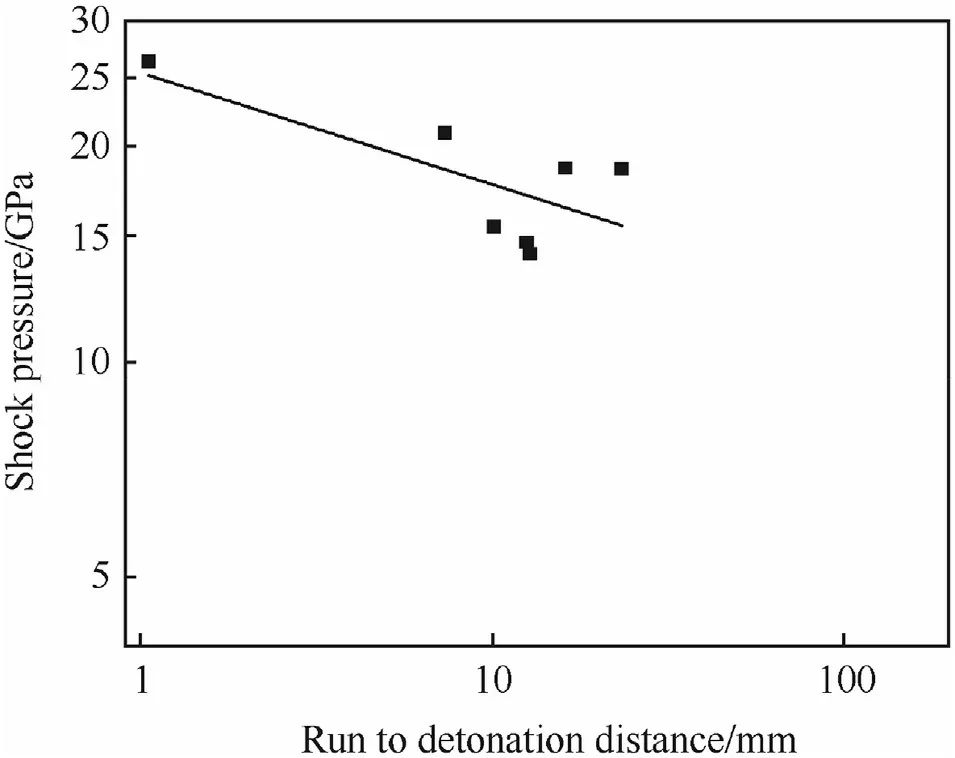
Fig.25.Pop-plot for X0228(ρ=1.699 g/cm3).
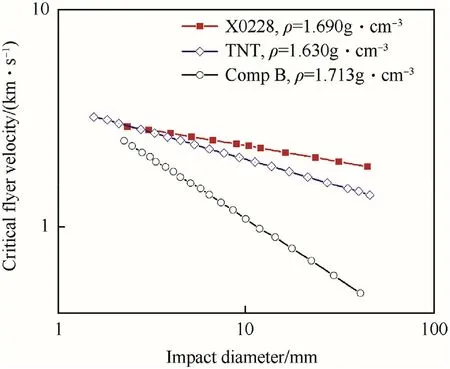
Fig.26.Critical flyer velocity for bare X0228 compared to TNT and Comp B.
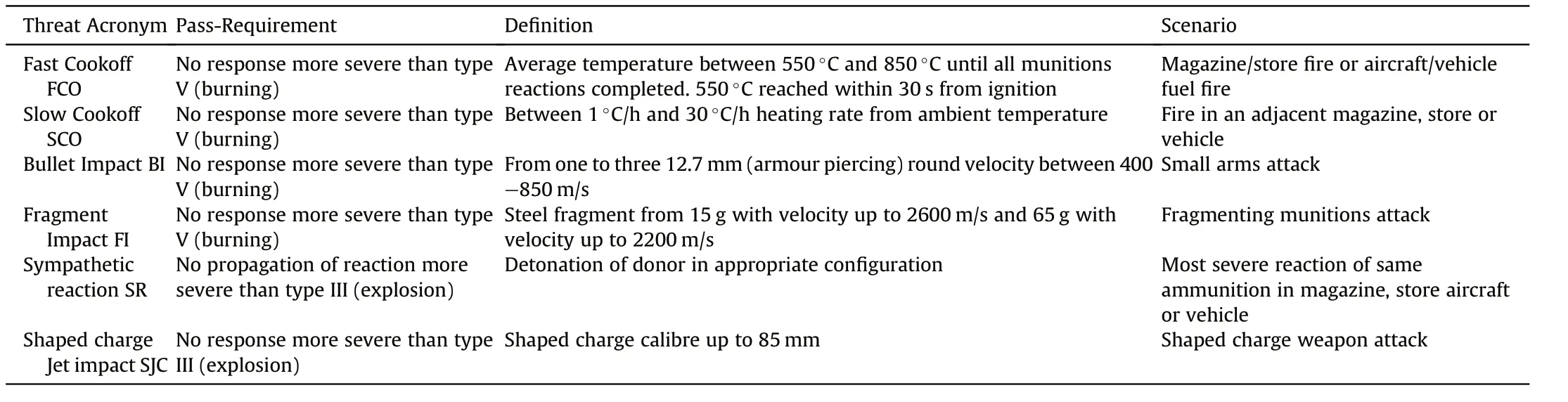
Table 44 Threat,definition and Minimum pass-requirement[120].
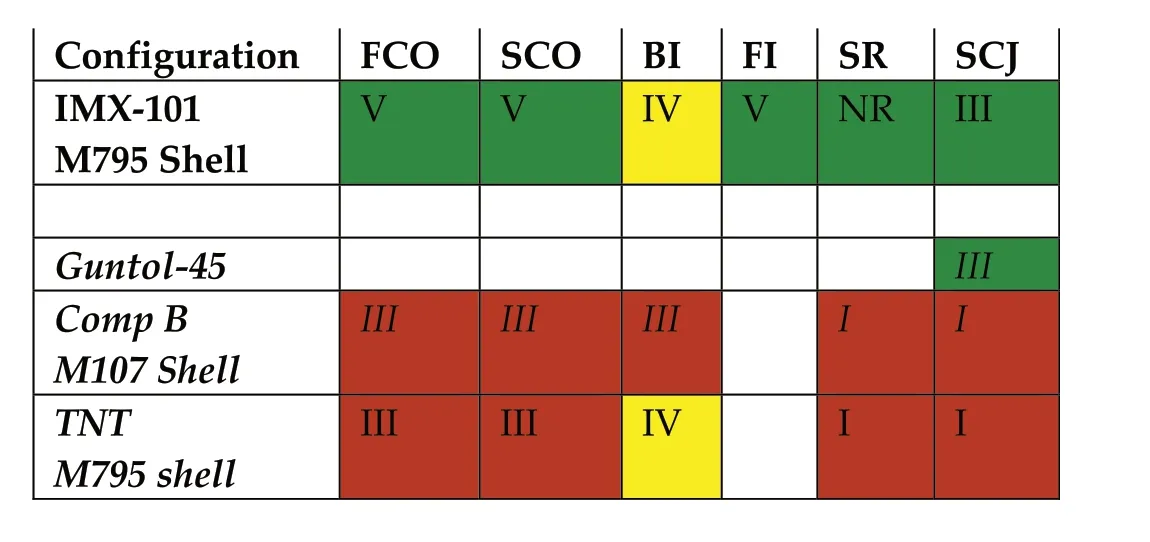
Table 45 Response descriptors for IM Tests i.a.w.STANAG 4439[120].
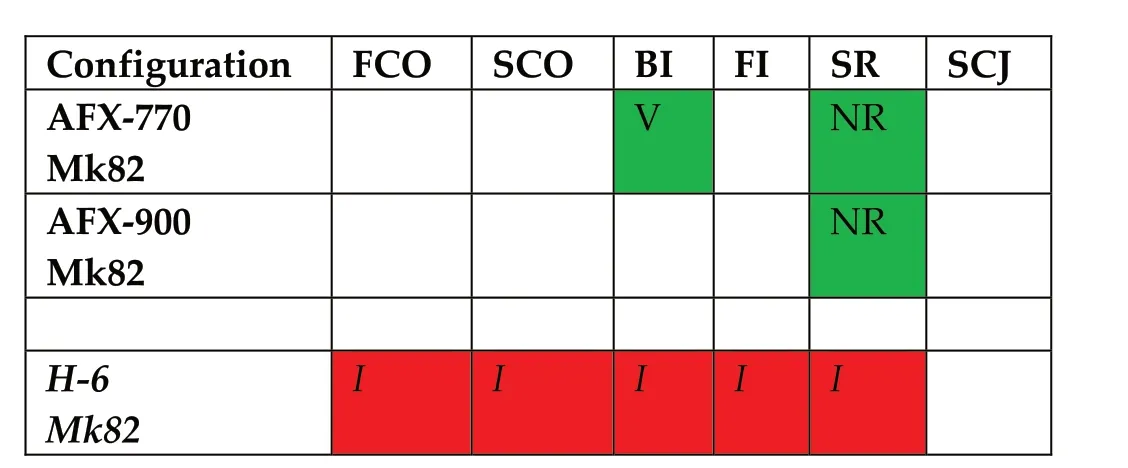
Table 46 IM-Test Signature for 155 mm Artillery Shell[122].
Table 47 IM-test signature for GP-bomb.
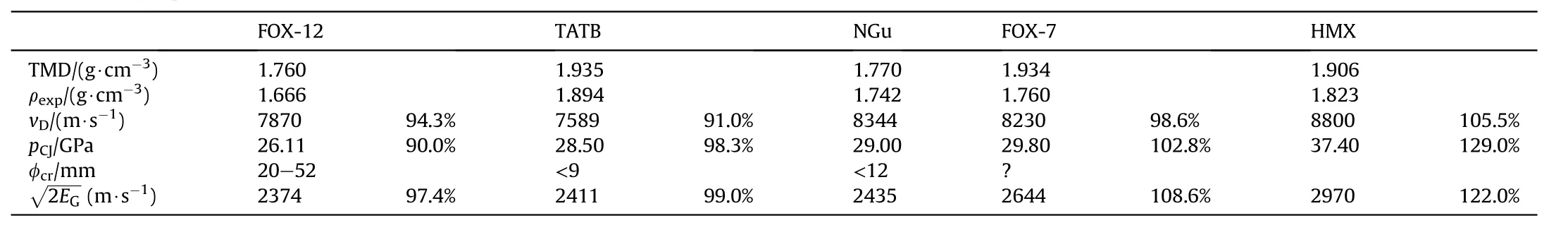
Table 48 Performance synopsis NGu-FOX-1-FOX-7-TATB-HMX.
Acknowledgement
The author is grateful for the support and help by te following individuals:
·Professor Dr.Muhamed Suceska,University of Zagreb,Croatia,for helpful discussions.
·Mr.Bruno Nouguez,France,for information on formulation B2244.
·Dr.Werner Arnold,Ingolstadt,Germany for proofreading an early version of this paper.
·Dr.Phil Samuels,USA,for information on IMX-101.
The author gratefully acknowledges AlzChem Trostberg GmbH,Trostberg,Germany for funding this work.
List of abbreviations
Øcr critical diameter,mm
ρ density,g⋅cm—3
ΔfH enthalpy of formation,kJ⋅mol—1
ΔdetH enthalpy of detonation,kJ⋅mol—1
ΔvapH enthalpy of vaporization,kJ⋅mol—1
μdpparticle diameter,mm
§ mass fraction,wt.%
Ω Oxygen balance,wt.%
AN Ammonium nitrate,NH4NO3
AOP NATO-Allied Ordnance Publication
BI Bullet Impact
CAS Chemical Abstracts Service
CE Tetryl,C7H5N5O8
dp decomposition point,°C
EI(D)S Extremely Insensitive(Detonating)Substance
ELSGT Extra Large Scale Gap Test
FCO Fast Cook Off
FI Fragment Impact
FOX-7 1,1-Diamino-2,2-dinitroethylene,C2H4N4O4
FOX-12 GUDN
GP General Purpose
GUDN N-Guanylurea dinitramide,C2H7N7O5
HBD high bulk density
HE high explosive
HMX Octogen,C4H8N8O8
IM Insensitive Munitions
IMX Insensitive Melt cast Explosive
LBD low bulk density
LSGT Large Scale Gap Test
LVD low velocity detonation
Mk Mark
mp melting point,°C
mrmolecular weight,g⋅mol—1
NATO North Atlantic Treaty Organization
NGu Nitroguanidine,CH4N4O2
NOL Naval Ordnance Laboratory
NTO 3-Nitro-1,2,4-triazolone,C2H2N4O3
P Pressure,GPa
PCJChapman Jouguet pressure,GPa
PETN Pentaerythritol tetranitrate,C5H8N4O12
RDX Hexogen,C3H6N6O6
SCJ Shaped Charge Jet Impact
SCO Slow Cook Off
SR Sympathetic Reaction
SHBD Spherical High Bulk Density
SLSGT Super Large Scale Gap Test
STANAGNATO Standardization Agreement
TATB 1,3,5-Triamino-2,4,6-trinitrobenzene,C6H6N6O6
TCJChapman Jouguet temperature,K
TDO N-Tallow-1,3-diaminopropane dioleate,CAS-No.[61791-53-5]
TMD Theoretical maximum density,g⋅cm—3
TNT 2,4,6-Trinitrotoluene,C7H5N3O6
Usshock velocity,m⋅s—1
Upparticle velocity,m⋅s—1
v specific volume,cm3⋅g—1
VD detonation velocity,m⋅s—1
V/V09.0 Cylinder Energy at expansion ration 1:9,kJ⋅cm—3
- Defence Technology的其它文章
- Modeling on the shock wave in spheres hypervelocity impact on flat plates
- Effect of energy content of the nitraminic plastic bonded explosives on their performance and sensitivity characteristics
- Effect of wave shaper on reactive materials jet formation and its penetration performance
- A comparative study for the impact performance of shaped charge JET on UHPC targets
- The role of crystal lattice free volume in nitramine detonation
- Optimizing the defensive characteristics of mild steel via the electrodeposition of Zn—Si3N4 reinforcing particles

