Effect of energy content of the nitraminic plastic bonded explosives on their performance and sensitivity characteristics
Svtopluk Zemn ,Ahmed K.Hussein ,Mrcel Jungov ,Ahmed Eleih
a Institute of Enegetic Materials,Faculty of Chemical Technology,University of Pardubice,CZ-532 10,Pardubice,Czechia
b Military Technical College,Kobry Elkobbah,Cairo,Egypt
Keywords:Enthalpy Explosive strength Combustion Sensitivity Nitramines PBX Thermal stability
A B S T R A C T Information about the forty nine nitraminic plastic bonded explosives(PBXs)and different nitramines were collected.Fillers of these PBXs are nitramines 1,3,5-trinitro-1,3,5-triazinane(RDX)and β-1,3,5,7-tetranitro-1,3,5-tetrazocane (β-HMX), cis-1,3,4,6-tetranitro-octahydroimidazo-[4,5-d]imidazole (bicyclo-HMX, BCHMX) and ε-2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (ε-HNIW, CL-20)which are bonded by polyfluoro-elastomers, polydimethyl-siloxane, poly-glycidyl azide, polyisobutylene, polystyrene-butadiene, poly-acrylonitrile-butadiene and hydroxyl-terminated polybutadiene in addition to a melt cast compositions based on 2,4,6-trinitrotoluene.For thirty two of these PBXs the relationships are specified and analyzed between heats of their combustion and relative explosive strengths;by means of these relationships it might be possible to estimate,which groupings in the macromolecule of binder could be liable to their primary fission in the PBXs initiation.Similarly,for forty two of these explosives,the relationships are described and analyzed between their enthalpies of formation and impact sensitivities;here is especially attention paid to PBXs filled by BCHMX.Specific rate constants from Vacuum Stability Test(VST)of four nitramines and twenty PBXs are introduced into relationships with their enthalpies of formation.Regarding to all the mentioned cases,increasing of energy content of the studied explosives leads to increase of the relative explosive strength or initiation reactivity,respectively.Exception with the opposite trend,the outputs of VST are for BCHMX,where in PBXs are matrices with the esteric plasticizers or the energetic poly-glycidyl azide.Admixture of RDX or HMX,respectively,into the BCHX PBXs gives ternary PBXs whose thermal stability,in the sense of applied VST,is higher comparing to the original binary explosives.
1. Introduction
The highly filled polymers by nitramines make a large and significant group of energetic materials,commonly known as the plastic bonded explosives(PBX).From the point of view of the reliability and safety of their application,their initiatory reactivity is a topic of research interest.Relationship between this reactivity and performance of energetic materials in general was widely discussed in literature(see papers[1—9]and references therein).In 2000,analysis of Licht‘s research[1],has shown that a high level of performance is usually accompanied by higher sensitive properties and that an insensitive explosive will not exhibit top performance.It has subsequently been shown that this can be considered as a general rule[2,3,5—9]but the author has stated that this result was not proved by any theory[1].With using detonation characteristics as a measure of performance,our team have verified this relation in many of our papers(see for example papers[2,3,5—9],and references therein)and a few exception were found for several pure nitramines[5],in addition to some misgivings,which appeared in literature[4].However,in our recent paper[8]we have tried preliminarily to explain the mentioned rule for cis-1,3,4,6-tetranitrooctahydroimidazo-[4,5-d]imidazole (BCHMX) and its PBXs.However,all the above mentioned studies and corresponding conclusions were made on the base of performance characteristics,resulted from detonation of the studied explosives.
Performance of energetic materials should be related to their energy content[9].Relationship between heats of combustion and explosion was already specified[9,10]but direct relationships between other characteristics of the energy content in explosives and their initiatory reactivity was not described,yet.Therefore,in this paper we would like to describe relationships between impact and thermal reactivities(sensitivities),on one side,and enthalpies of formation of the nitraminic PBXs,on the other side.Emphatic attention is paid also to the dependence of the relative explosive strength on heat of combustion of the mentioned PBXs which was not studied yet.
2. Experimental
2.1. Net energetic materials used
The nitramines used were 1,3,5-trinitro-1,3,5-triazinane(RDX)and β-1,3,5,7-tetranitro-1,3,5,7-tetrazocane (β-HMX) obtained partially from Eurenco,Paris,France with an average particle sizes of about 64 and 42 μm respectively and RDX partially from the Slovak company Chemko Str′aˇzske(in the case of our older papers cited here). In addition, ε-2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane(ε-HNIW or ε-CL-20)of technical(common)quality with impact sensitivity of 4.2 J and also with reduced sensitivity(RS-ε-HNIW,impact sensitivities of 9.0,10.8 and 11.2 J,respectively)as reported in Ref.[11].Also cis-1,3,4,6-tetranitrooctahydroimidazo-[4,5-d]imidazole(BCHMX)have been prepared according to patents[12,13].3-Nitro-1,2,4-triazol-5-one(NTO)was a product of Eurenco company and was recrystallized from water before incorporation into PBX. 1,1-Diamino-2,2-dinitroethene(DADNE or FOX-7)was prepared at our laboratories by the published procedure[14].These individual energetic materials were used for the plastic bonded explosives preparation in Framework of papers,cited in Table 1.
2.2. Preparation of plastic bonded explosives(PBXs)
The studied PBXs and their required characteristics are summarized in Table 1 with references to the sources of their preparation and the methods used to determine the presented characteristics.Therefore,hereinafter only briefly the coding of samples in Table 1 and also of their composition is explained.Coding of mixtures in Table 1 by the suffix—C4 means PBXs,bonded by 9%wt.of softened polyisobutylene(PIB)-the prepared mixtures were then marked as RDX-C4,HMX-C4,BCHMX-C4,ε-HNIW—C4 and RS-ε-HNIW—C4.
Silicone-based matrix,marked by suffix—Si,is formed by mixing two kinds of the oily polydimethylsiloxane(PDMS)polymer,Wacker®AK 10 000 and Wacker®AK 60 000(both terminated by trimethylsilyl groups)in a ratio of 1:1.The silicone matrix is incorporated in mass of 12%by wt.in the corresponding puttyplastic PBXs which are designated as RDX-Si,HMX-Si,BCHMX-Si and HNIW—Si.In another case a mixtures with 44%of BCHMX,44%of NTO and 12%of PDMS and similarly 44%of BCHMX,44%of FOX-7 and 12%of PDMS were prepared under code designation BCHMX/NTO-Si and BCHMX/FOX7-Si, respectively. Samples bonded by the PMDS(Sylgard binder),i.e.BCHMX-Sylgard with 15%wt.of this binder,was prepared according to paper[10].
The same procedure,as in the case of matrices—C4 and—Si was used for preparation of putty-plastic PBX bonded with softened acrylonitrile-butadiene rubber binder(NBR)which forms of 15%wt.of the corresponding PBXs with the code designations RDXsem,HMX-sem,BCHMX-sem and HNIW-sem.Also PBXs with 14%wt.of softened styrene-butadiene rubber(SBR)were obtained by this method and resulted mixtures are marked as RDX-form,HMXform,BCHMX-form and HNIW-form.
Samples bonded by polyfluorinated binders,Viton A 200 and fluoroelastomer Dynenon FT 2481(Fluorel),and the softened polymethyl methacrylate(PMMA)were prepared by a modified watersolvent slurry method(see in Refs.9 and 15).PBXs bonded by Viton A 200 were marked by suffix-5 V for content of 5%wt.and suffix-9 V for content of 9%wt.of binder in final PBX.Mixtures bonded by 9%wt.of Fluorel are marked by suffix—F while PBXs bonded by 9%wt.of the softened PMMA are marked by suffix—PA.
The linear energetic glycidyl azide polymer was prepared in our laboratory in the sense of paper[17]and then was used for preparation of the corresponding PBXs which contained of 13%wt.of cured binder.Samples thus obtained are designated RDX-GAP,HMX-GAP,BCHMX-GAP and HNIW-GAP.
Coding of mixtures in Table 1 by the suffix—HTPB means the cast cured PBXs,bonded by 18%wt.of polyurethane matrix on the basis of a hydroxyl-terminated polybutadiene(HTPB),prepared according to paper[18].
The melt cast explosives with the 2,4,6-trinitrotoluene(TNT)were based on the mixture of 40%wt.of TNT and of 60%wt.of nitramine,corresponding PBXs are marked as RDX-TNT(Composition B),HMX-TNT,BCHMX-TNT and HNIW-TNT.
2.3. Heat of combustion and enthalpy of formation
An automatic high pressure Bomb calorimeter,model BCA 500,OZM Company,Czech Republic,was used to measure the heat of combustion of the BCHMX/GAP as well as the other PBXs.The sample was placed in a closed bomb filled with an excess of oxygen and ignited(the heats of combustion were determined for each prepared PBX because of these data were one from the needed treasures for the computational assessment of these explosives).The output data was used for calculation the enthalpy of formation of the PBXs which was used for determining the detonation characteristics(all procedures see Ref.15 and references therein).The results of the elemental analysis,needed for this calculation,were recalculated to match the N content to the individual explosive as a hypothetical formula.This formula calculated in this way was used as if it was individual explosive and it was used[7,15].
2.4. Sensitivity to impact
The standard impact tester with exchangeable drop weight of BAM impact sensitivity instrument was used[7,15]the amount of substance tested was 50 mm3,and drop hammers of 2 and 5 kg weight were used.The probit analysis was used to determine the probability levels of the initiation.Only the 50%probability of initiation is used and is expressed as drop energy in Joule units as Table 1 shows.
2.5. Vacuum stability test(VST)STABIL
A modernized STABIL 16-Ex apparatus(manufactured by OZM Research)was used with the procedure of measurement presented in paper[19]:the amount of the samples used for measurement was 2 g.Tests were performed over 360 min.The temperature for the isothermal measurements was chosen to be 120°C.The samples in evacuated glass test tubes were placed into the heating block and heated to the desired temperature.Straight lines were obtained by linearization of each curve for isothermal exposure over 60—360 min the data(details see in Ref.19)for which are presented in Table 1;the slopes of these lines,k,correspond to the reaction velocity of evolution of gaseous products in a zero-order reaction[19]and,therefore,k represents the specific rate constant(here the k values are in kPa⋅g-1min-1).
2.6. Relative explosive strength measurement
A ballistic mortar test was used for the determination of the relative explosive strength of the samples studied,using TNT asreferences[7,9]A fixed amount of a tested explosive(10 g)was wrapped in polypropylene foil and inserted into the mortar enclosed by a steel projectile and then fired using a non-electric detonator(No.8).The measurements are based on obtaining a calibration curve for the standard explosive(TNT)at different masses,then the explosive strength of the tested explosive is expressed relative to the calibration curve of TNT(%TNT)[7,9].For each measurement,a part of the non-electric detonator is inserted in the plastic sample and fired by match.Three measurements were made for each sample and the mean values are reported in Table 1.
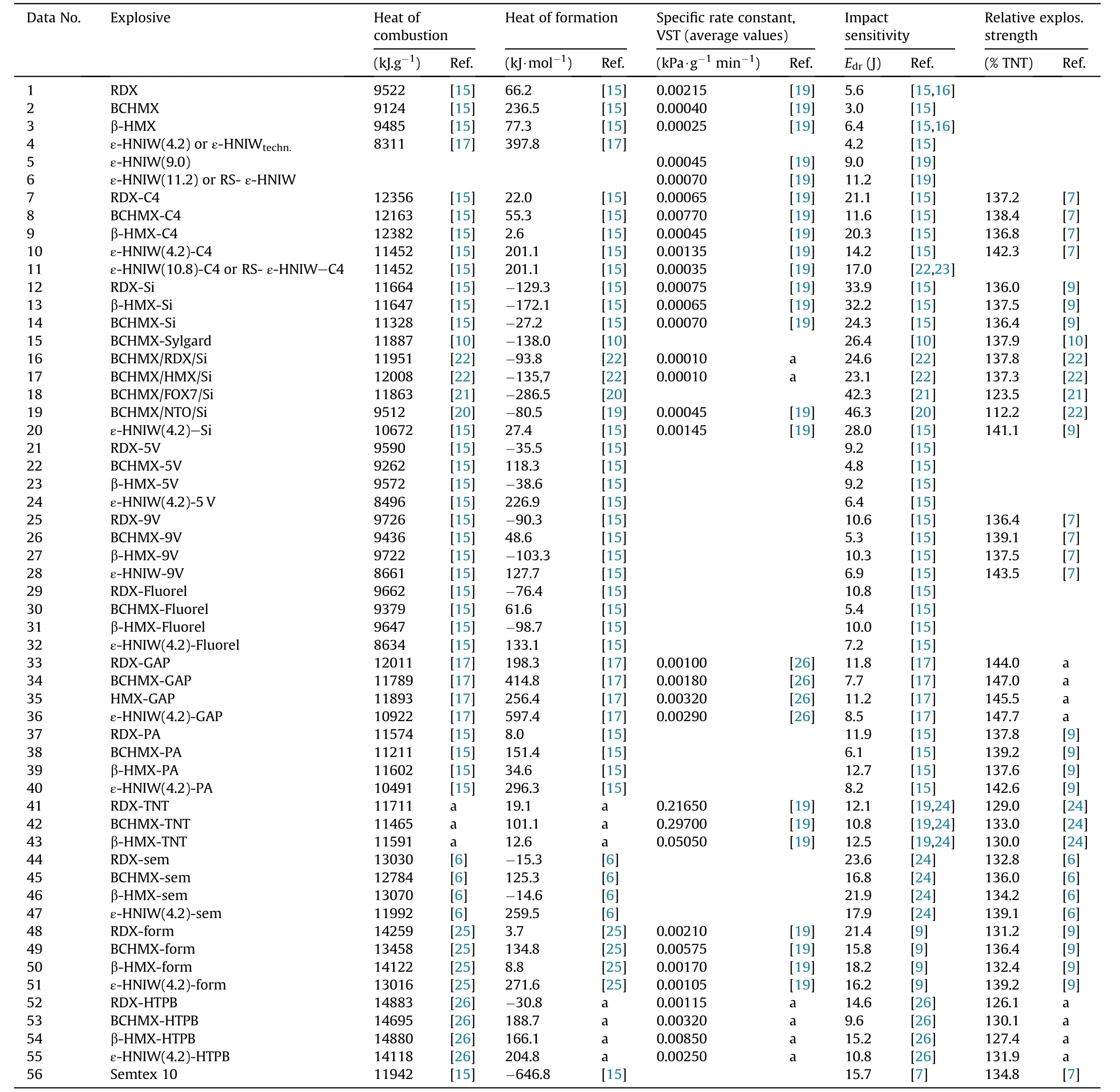
Table 1 A survey of the heats of combustion and formation,specific rate constants from Vacuum stability test,impact sensitivity and relative explosive strengths from ballistics mortar,all for the studied explosives(numbers in parentheses at HNIW signify impact sensitivity in J of the used nitramine).
3. Results and discussion
3.1. Correlations on the base of heat of combustion
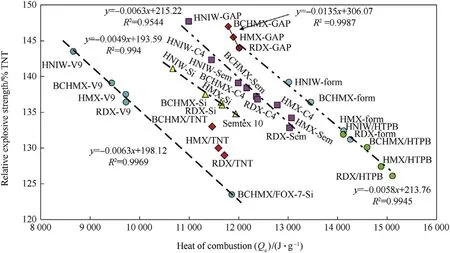
Fig.1.Relationship between relative explosive strength and heat of combustion of the studied PBXs.
The relationships between heats of combustion and explosion have already been mentioned[9,10].In continuity to them,Fig.1 shows a new relationship between the heat of combustion,Qc,and the relative explosive strength(RES).Since that the values of Qcdescend with increasing of the enthalpies of formation so Fig.1 documents the increase of RES with the growth of the energy content of the explosive molecule.It is very interesting that correlation in this Fig.are relatively very tight.
Distribution of the studied explosives into groups according to Fig.1 is primary given by the thermochemical aspects of their fission and burning in the detonation wave.Additional influence is possible to find out in PBXs with the Qcvalues lower then of 12000 J g-1in which their binders'macromolecules contain functional groups with oxidizing character(—F,—NO2,—O-bridges-the first and third mentioned see Figs.2 and 3).With the data of the polydimethyl siloxane PBXs thus the Semtex 10 data perfectly correlate.This putty extruded plastic explosive on the base of pentaerythritol tetranitrate(PETN)has the—O—bridge in the PETN molecule(as a part of the nitroxyl groups)and here is this Czech explosive presented as a comparative standard.
A correlation of the BCHMX/FOX-7/Si data with those for PBX-V9 is interesting;it was observed that a combination of the BCHMX/FOX-7 mechanical mixture with PDMS strongly increases thermal reactivity of the resulting mixture[27]even if the siloaxane binder seems to be one from the best binders for PBX stability.The increased thermal reactivity might be a reason of the previously mentioned correlation(here is not secreted creation of HO-radicals as intermediates of this mixture decomposition).
As well,a similarity in the binders’macromolecules structure can be the second reason of aggregation of different PBXs into groups in sense of Fig.1.Thus the PIB and NBR polymers(see Figs.4 and 5)contain vinylidene or methyl-vinylidene grouping in their basic molecular chain and,therefore,their behaviour in chemistry of detonation might be very similar;this might be a reason for association of the corresponding extruded PBX-C4 and PBX-sem into one group as of Fig.1 shows.
Beside vinylidene groupings,also vinyl groups are bonded at the basic chain of the NBR and HTPB macromolecules(see Figs.6 and 7)which means that their reactivity in detonation might be mutually comparable and should differentiate from both the PIB and SBR analogues.Then it is logical that extruded PBX-form and casted PBX-HTPB generate one group in Fig.1.
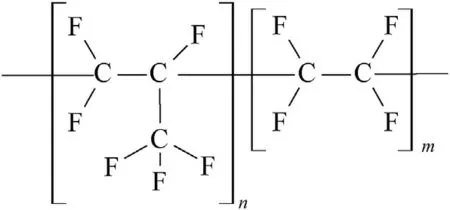
Fig.2.A fragment of the Viton A macromolecule.
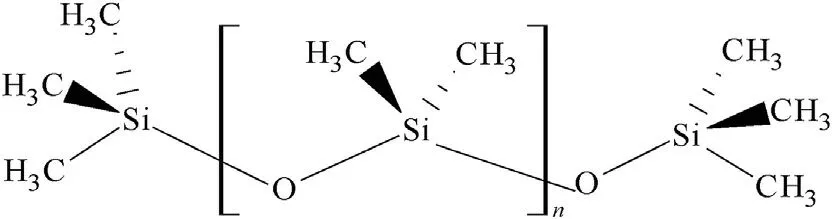
Fig.3.Polydimethyl siloxane(PDMS)terminated by trimethylsilyl groups(Wacker®AK).
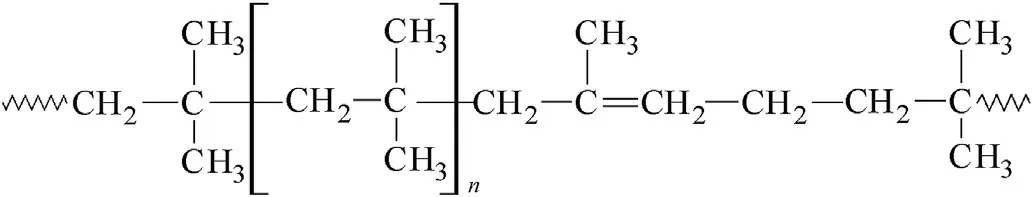
Fig.4.A fragment of the polyisobutylene(PIB)macromolecule.
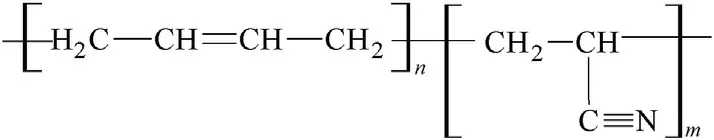
Fig.5.A fragment of the poly-acrylonitrile-butadiene(NBR)macromolecule.
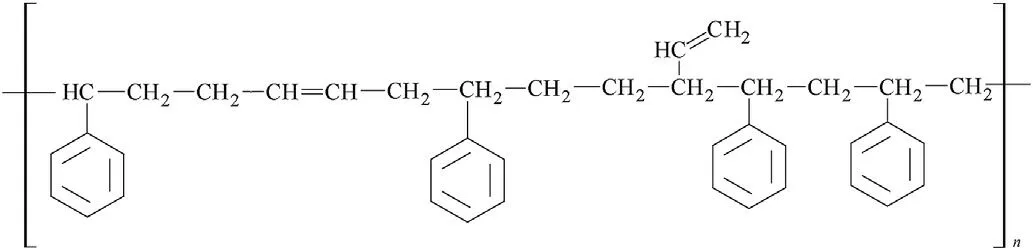
Fig.6.A fragment of the polystyrene-butadiene(SBR)macromolecule.
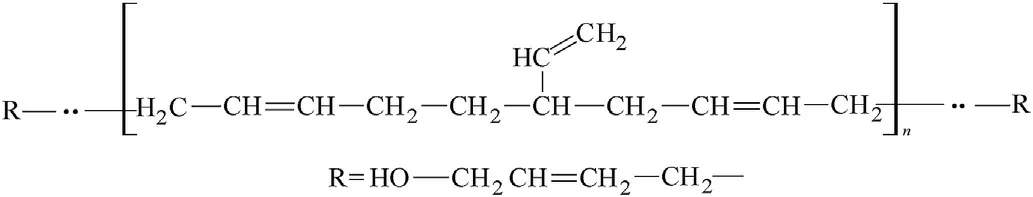
Fig.7.Hydroxyl-terminated polybutadiene(HTPB)macromolecule.
Position in Fig.1 of the casted GAP-bonded explosives are interesting(binder see in Fig.8).This group mostly lies almost at the straight line for the PBX-form and PBX-HTPB mixtures where this binder acts as if decreasing the difference in performance of the particular nitramines used.However,mixture of HNIW-GAP practically correlates with extruded explosives PBX-sem and PBX-C4;this result is due to its heat of combustion which might be influenced by creation of some complex between HNIW and polyglycidyl azide(it is in research).The above-mentioned proximity of PBX-HTPB and PBX-GAP may also be related to the same method of crosslinking of both these binders,i.e.with the polyurethane's linking.
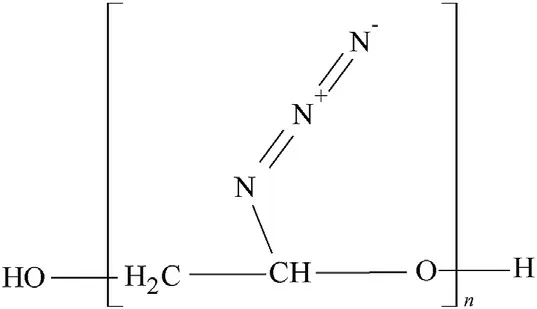
Fig.8.Poly-glycidyl azide(GAP)macromolecule.
The relationships within the meaning of Fig.1 are thus likewise limited by the molecular structure not only of the nitramine components but also the binders of the studied PBXs.In the other words:beside the known conception of the primary fission of nitramines(see Ref.2 and references herein)thus a new notion appears about the possible reaction centers of the binders’macromolecules in initiation of the corresponding PBXs.
3.2. Correlations on the base of enthalpy of formation
Heats of combustion are the data source for the enthalpies of formation which give information about energy content of compounds in general.How it is possible to use these enthalpies for the study of the initiation reactivity as shown in Figs.9—11.
Fig.9 expressly documents the increasing of impact sensitivity with raising of the enthalpy of formation values.Unlike the relationships in Fig.1,the influence of the binder molecular structure is not clearly evident here,perhaps with the exception of the data presented on the straight line I.This line concentrates data for polyfluorinated binders.It is also possible to draw attention to the straight line IV,which is predominantly linked to data of HNIW.The rest of the relationships in Fig.9 are a mix of different molecular structures as so nitramines thus bindand PBXs.This Fig.is rather complicated and therefore the data for BCHMX and PBXs on its base were extracted and inserted into Fig.10.
In Fig.10,the PBXs mixtures with polyfluorinated and PDMS binders make a separate groups(straight lines A and C,respectively).The Sylgard data correlate here with straight line B shows that there is a need to consider the thermochemical influence-this special kind of the PDMS binder is in the BCHMXSylgard mixture presented in 18%wt.in comparison with 12%wt.of PDMS in the other PBX-Si explosives,whereby corresponding difference reduces enthalpy of formation in this case.A smaller reduction in this enthalpy causes the replacement of the 50%BCHMX in a mixture of BCHMX-Si by NTO which,together with expressive decreasing the impact sensitivity of the resulting BCHMX/NTO-Si assigns this explosive into data of the straight line D.For comparison the PBX-GAP explosives were inserted in this Fig.The BCHMX-GAP does not correlated with the other mixtures of this group(straight line E),probably due to insufficient desensitization of BCHMX by the used amount of GAP.However,data of this cured explosives perfectly correlate with those for cured mixtures BCHMX-HTPB, BCHMX-TNT and extruded explosive BCHMX-C4(straight line F). Similarly to Fig. 1 also in Fig. 10 the corresponding correlations are relatively tight.
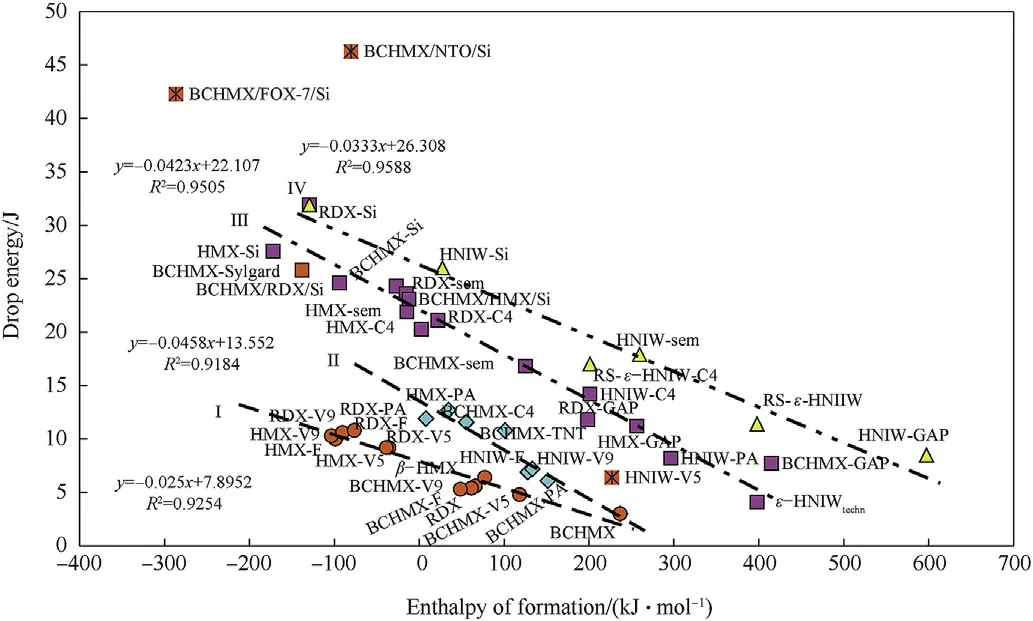
Fig.9.Approximate relationship between impact sensitivity,expressed as drop energies,and enthalpy of formation of the most studied nitramines and PBXs.
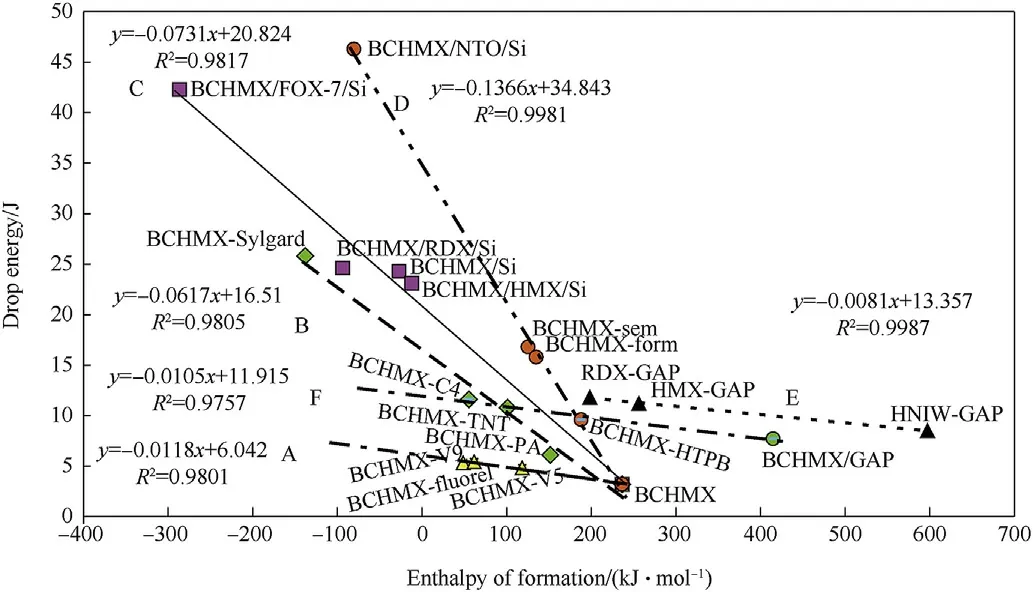
Fig.10.Relationship between impact sensitivity,expressed as drop energies,and enthalpy of formation of the BCHMX and PBXs on its base with inserted PBXs-GAP also of RDX,HMX and HNIW.
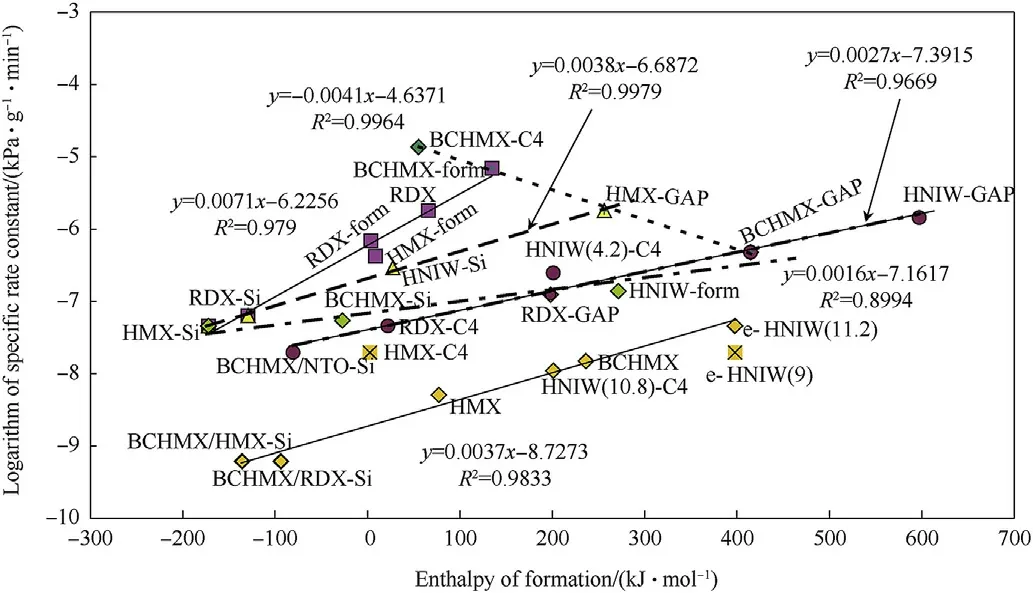
Fig.11.Semilogarithmic relationship between specific rate constants from Vacuum stability test and enthalpy of formation of the studied nitramines and most PBXs;numbers in parentheses near HNIW codes mean impact sensitivity in J;code designations BCHMX/RDX-Si and BCHMX/HMX-Si labels ternary PBXs in which wt.ratio of BCHMX to RDX or to HMX is 1:1.
3.3. Outputs of the vacuum stability test
Another case of this kind of relationships can be illustrated here by means of the outputs of the Czech vacuum stability test(VST)STABIL,which is more particularly described in papers[19,28](and references therein).Under conditions of this measurement,i.e.by the sample exposure at 120°C for 6 h in vacuum[19,28]even the most stable nitramines undergo to the very weak but evident decomposition[19],characterized by the corresponding very low values of specific rate constants(see in Table 1).Logarithmic relationships were described between detonation velocities and impact sensitivities,on the one side,and the mentioned rate constants,on the other side[19,28].In this sense with taking the enthalpies of formation of the studied explosives,we have found their semilogarithmic relationship with these constants as shown in Fig.11.Here a presented relative tight relationships show a raising of the thermal reactivity with the increasing of the energy content for majority of the studied explosives.Exception from this trend are the data of BCHMX-GAP,HMX-GAP,BCHMX-form and BCHMX-C4.However,using of the calculated reaction constants for unimolecular decomposition at 200°C,k200,from outputs of TG/DTG[29—31]the order of these“exceptional PBXs”are almost opposite,concretely(the value k200in s-1):BCHMX/GAP(14.8),HMX-GAP(8.2),BCHMX-form(1.3×10-6)and BCHMX-C4(2.2×10-4).In the VTS a problem is a relatively long time of the sample decomposition at low temperature(in comparison with non-isothermal TG,combustion or detonation)such that the kinetic of decomposition is more influenced by contact of nitramine namely with the esteric plasticizers(including with the crosslinked GAP)which perform a function of solvent(removing of the stabilizing effect of the crystal lattice)unlike of the PDMS oily binder.This fact is unfavorable mainly for sterically crowded BCHMX molecule(its crystallography and initiation reactivity see paper[32]).
In Fig.11,it is also appropriate to draw attention to the differences in the reactivity of the different kinds of the HNIW and PBXs on their bases:a small difference is between RS-ε-HNIW with impact sensitivity of 9 and of 11.2 J,but a large difference is demonstrated on PBXs-C4 filled by ε-HNIW with this sensitivity of 10.2 J,on the one hand,and with“normal”sensitivity of 4.2 J,on the other.From the point of view of thermal stability and impact sensitivity the PDMS binder seems to be the best from the used matrices while GAP appears to have an opposite character.The GAP logically increases relative explosive strength (RES) of corresponding PBXs and markedly decreases mutual differences between these RESs of the individual nitramines incorporated into the mentioned PBXs.
3.3.1. Stabilizing influence of the RDX or HMX admixtures to the binary BCHMX PBXs
Fig.11 also documents,that binary mixtures RDX-Si,HMX-Si and BCHMX-Si have roughly the same thermal stability.However,if 50%wt.of BCHMX in the last mentioned one was substituted by RDX(BCHMX/RDX-Si)or by HMX(BCHMX/HMX-Si),resulting ternary PBXs had higher thermal stability comparing to the original binary ones.This stabilizing effect of the RDX or HMX admixture to the BCHMX PBXs exists also for other ratios these nitramines to BCHMX and for other kinds of binders[33]and needs further study.
4. Conclusion
Heat of combustion and/or enthalpy of formation were taken as representatives of the energy content of the nitraminic plastic bonded explosives(PBXs).A linear and relative tight relationships were found between the Relative Explosive Strengths(RES)of PBXs and their heats of combustion, Qc. These relations divide the studied PBXs into subgroups not only according to molecular structure of the nitraminic fillers and thermochemical factors of the mixture burning,but,mainly according to the mutual structural similarities of the binder's macromolecules.The found relationships in this case thus perhaps indicate the potential reaction centers in the binders'macromolecules of the studied PBXs,which might primarily participate in their initiation.
Unlike the case of heat of combustion,specified linear relationships between enthalpy of formation and impact sensitivity are without a clear influence of the binder molecular structure,perhaps with exception of polyfluorinated elastomers whose ability to protect the explosives against impact is the lowest from the studied binders.By dividing of the studied PBXs in the sense of this relationships into subgroups the thermochemical factors are here dominating.
Thermochemical aspects seem to be a limiting factor in combining of the studied PBXs into subgroups on the basis of the relationships between the specific rate constants,derived from Vacuum Stability Test (VST), and the enthalpies of formation.Growing energy content in the studied PBXs here evokes the increasing of the reaction rate of their decomposition.An opposite trend was found in PBXs, which are filled by cis-1,3,4,6-tetranitrooctahydroimidazo-[4,5-d]imidazole (BCHMX), bonded by azidoglycidyl polymer or matrices with the esteric plasticizers because of these admixtures damage the stabilizing effect of crystal lattice of its crowded molecule.
Adding RDX or HMX to a binary BCHMX PBX will increase the resilience of the resulting ternary PBX against thermal decomposition in conditions of the used VST.This effect remains inexplicable and needs further attention.
Acknowledgement
This paper was supported by means of the financial resources of Students Grant Projects No. SGS_2018_002 of the Faculty of Chemical Technology at the University of Pardubice.
- Defence Technology的其它文章
- Modeling on the shock wave in spheres hypervelocity impact on flat plates
- Insensitive high explosives:IV.Nitroguanidine—Initiation&detonation
- Effect of wave shaper on reactive materials jet formation and its penetration performance
- A comparative study for the impact performance of shaped charge JET on UHPC targets
- The role of crystal lattice free volume in nitramine detonation
- Optimizing the defensive characteristics of mild steel via the electrodeposition of Zn—Si3N4 reinforcing particles

