柑橘CitPG34的克隆、定位与表达分析
葛廷,黄雪,谢让金
柑橘的克隆、定位与表达分析
葛廷,黄雪,谢让金
(西南大学柑桔研究所/中国农业科学院柑桔研究所,重庆 400712)
【】多聚半乳糖醛酸酶是一类参与细胞壁降解的水解酶,在植物生长发育和器官脱落过程中发挥着重要作用。本研究克隆柑橘及其启动子(CitPG34-P)并进行表达分析,为深入研究柑橘在幼果脱落过程的生物功能奠定基础。以‘塔罗科’血橙(L. Osbeck)为材料,克隆及其启动子,利用ProtParam、Cello、CLUSTALX、MEGA5.2、PlantCARE等软件对其蛋白特性及启动子顺式作用元件进行分析预测;利用实时荧光定量PCR(qRT-PCR)分析在不同组织以及柑橘幼果脱落过程中的表达水平。采用同源重组的方法构建pCAMBIA1302--融合蛋白表达载体和启动子表达载体(CitPG34-P::),分别用于亚细胞定位和启动子活性分析。从‘塔罗科’血橙幼果离层中克隆获得,其ORF为1 194 bp,编码397个氨基酸,预测蛋白分子量为41.47 kD,理论等电点为5.19,其不稳定系数为30.23,表明CitPG34属于稳定蛋白;通过在线软件TMHMM分析发现:CitPG34为跨膜蛋白,具有一个跨膜结构,位于第7—29位氨基酸之间。在CitPG34二级结构中,α-螺旋结构约占15.37%,扩展链约占29.72%,无规则卷曲约占54.91%,与其三级结构预测基本一致。NJ树分析显示CitPG34与西洋梨PcPG3(BAF42034)亲缘关系最近,表明CitPG34可能与果实脱落和软化相关。qRT-PCR分析表明,在花中表达量最高,在根、叶、离层A、离层C中表达量较低,在幼果中几乎不表达。1-氨基环丙烷羧酸(ACC)处理果梗后能显著提高离层A中的表达水平,相反IAA抑制其转录。此外,在柑橘幼果正常脱落过程中,表达明显升高。亚细胞定位发现,CitPG34主要位于细胞壁。克隆获取起始密码子(ATG)前2 075 bp启动子序列(CitPG34-P),PlantCare预测发现,在CitPG34-P序列上存在多种顺式调控元件,如核心启动元件TATA-box、增强子元件CAAT-box以及脱落酸响应元件ABRE等。将CitPG34-P::转入烟草,通过GUS组织化学染色发现,该启动子受乙烯诱导,主要在叶脉和毛状体中表达。的ORF长度为1 194 bp,可编码397个氨基酸,其蛋白主要位于细胞壁;该基因具有明显的组织特异性,在花中表达最高;表达量与柑橘幼果脱落显著相关。上述结果表明,在柑橘幼果脱落和花发育过程中可能发挥着重要的生物功能。
柑橘;多聚半乳糖醛酸酶;基因表达;亚细胞定位;启动子;幼果脱落
0 引言
【研究意义】柑橘(Citrus)是全球重要的经济作物之一,但大量异常落花落果,严重影响了柑橘产量,降低了果农的收入[1-2]。植物多聚半乳糖醛酸酶(polygalacturonases,PGs)作为细胞壁水解酶之一,通过降解离层细胞中间层果胶,从而促进植物器官脱落[3]。本研究基于柑橘全基因组,克隆获得了柑橘及其启动子序列,采用基因表达、亚细胞定位和遗传转化等方法对其进行分析,研究结果为进一步阐明CitPG34生物功能奠定了前期基础,同时也为深入解析柑橘幼果脱落机制提供参考。【前人研究进展】植物器官脱落发生在离层区(AZ),脱落过程中需要大量细胞壁水解酶参与,包括多聚半乳糖醛酸酶、β-1, 4-葡聚糖酶、纤维素酶、半纤维素酶以及扩展蛋白等[4]。其中,关于PGs的研究最为广泛,该酶含有4个典型的保守结构域,即结构域Ⅰ(NTD)、结构域Ⅱ(DD)、结构域Ⅲ(GHG)和结构域Ⅳ(RIK);结构域Ⅰ和结构域Ⅱ中的天冬氨酸(D)以及结构域Ⅲ中的组氨酸(H)参与了蛋白酶的催化功能[5]。PGs通过催化裂解果胶分子中的α-(1→4)-D-半乳糖苷键,导致细胞壁结构改变,从而参与果实成熟软化[6-7]、荚果开裂[8]、花粉发育[9]等植物生长发育过程。目前,关于参与果实脱落的研究主要集中在拟南芥[8]、苹果[10]、棕榈[11]和番茄[12]等植物上。Jiang等[12]利用同源片段,通过VIGS技术,诱导番茄基因沉默,结果导致番茄叶片明显延迟脱落。相反,超表达苹果,造成转基因植株叶片中的果胶降低,使未成熟叶片异常脱落并导致成熟叶片形态及其气孔发育异常[10]。乙烯处理导致棕榈树果实大量脱落,随后通过表达分析从14个中鉴定出一个成员即参与了脱落过程[11]。在荔枝中也有类似报道,从荔枝幼果离层中克隆获得,研究发现该受乙烯诱导,却被2, 4-二氯苯氧乙酸(2, 4-dichlorophenoxyacetic acid,2, 4-D)抑制,环剥摘叶后发现的表达水平与幼果脱落成正相关[13]。RIOV等[3]和RASCIO等[14]研究发现柑橘叶片和桃果实的脱落与PGs的活性变化有关,TAYLOR等[15]在接骨木叶片脱落过程中发现PGs活性的增强与其基因表达水平一致。KALAITZIS等[16]在番茄叶和花中克隆了、、,表达分析发现、、只在叶和花脱落区中表达,表明这些成员可能与脱落相关。上述结果表明,PGs在植物器官脱落过程中发挥着重要作用。【本研究切入点】目前,关于PGs参与器官脱落的研究主要集中在拟南芥、番茄、水稻等模式植物中。在柑橘中,关于参与脱落的研究仅有少量报道[17-19],特别是其在柑橘幼果脱落过程中发挥的生物功能还有待进一步解析。【拟解决的关键问题】克隆获得及其启动子,通过生物信息学、基因表达、亚细胞定位和GUS化学组织染色等手段,了解及其蛋白质的基本特性,初步明确在柑橘幼果脱落过程中的生物功能,及其对脱落诱导因子的响应机制。
1 材料与方法
试验于2018年1月至2019年1月在中国农业科学院柑桔研究所进行。
1.1 试验材料
1.1.1 植物材料 选取10年生‘塔罗科’血橙(L. Osbeck)为试材,其砧木为枳橙((L.) Osb.(L.) Raf.);烟草W38(),均由笔者实验室保存。
1.1.2 试验主要试剂 植物RNA提取试剂盒(RNA prep pure Kit)、植物基因组DNA提取试剂盒(Plant Genomic DNA Kit)、质粒提取试剂盒购自天根生化科技有限公司。高保真酶(PrimeSTAR Max Premix)、反转录试剂盒(Prime ScriptTM RT Reagent Kit)、胶回收试剂盒(MiniBEST Agarose Gel DNA Extraction Kit)、Marker、pMD19-T Vector、I、d III和I购自宝生物工程(大连)有限公司。qRT-PCR染料(iTaqTMUniversal SYBR® Green Supermix)购于伯乐生命医学产品有限公司。植物PBI121表达载体和农杆菌EHA105菌株由笔者实验室保存,大肠杆菌DH5α和农杆菌GV3101购于全式金生物技术有限公司。
1.2 材料处理
花后一周,选取长势一致的有叶单果,从离层A下部4 cm处剪取,随后立即带回实验室。去除叶片和子房,用清水(对照)、2 mmol·L-1IAA,2 mmol·L-1ACC分别处理果梗后,在0、6、12、18和24 h收集离层A(ACC处理的果梗在18 h时已完全脱落,因此在24 h时未能收集离层A)。液氮速冻,-80℃保存。
采集‘塔罗科’血橙的根(砧木)、叶、幼果(花后1周)、花、离层A及离层C用于研究的组织特异性(图1),液氮速冻,-80℃保存。
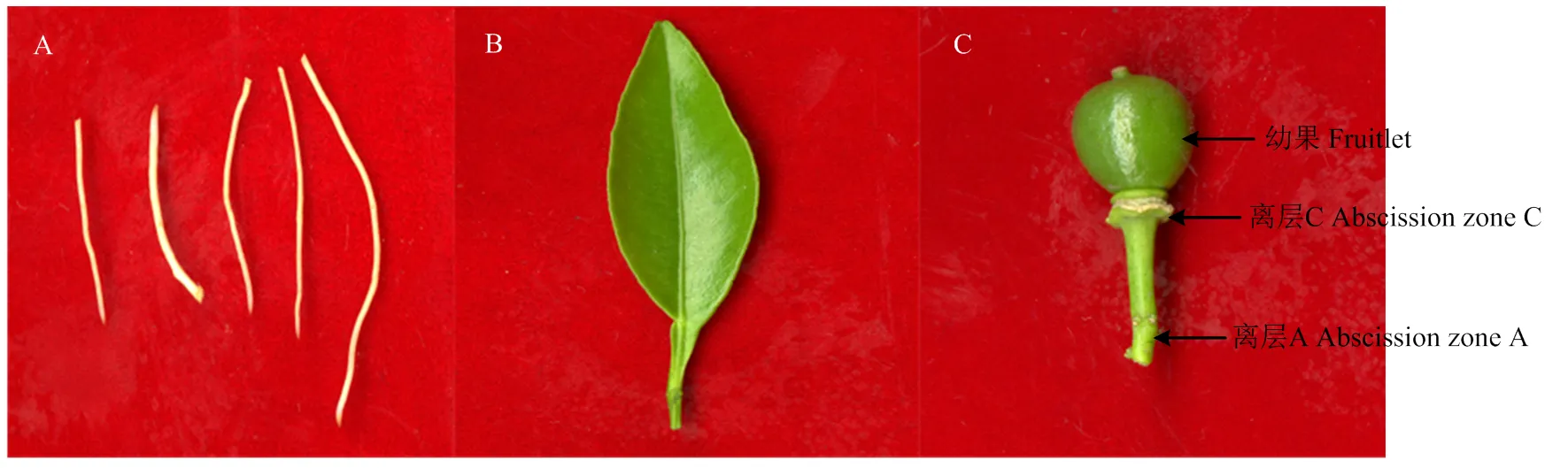
A:根(砧木);B:叶;C:幼果 A: Root (Rootstock); B: Leaves; C: Fruitlet
1.3 总RNA提取与cDNA合成
参照RNA prep pure Kit试剂盒说明书提取样品总RNA,随后,在1.0%琼脂糖凝胶上电泳检测其质量。总RNA浓度由Denovix超微量紫外可见分光光度计DS-11(DS-11 Series Spectrophotometer/ Fluorometer)检测。根据Prime ScriptTM RT Reagent Kit试剂盒说明书,反转录合成第一条cDNA链,并置于-20℃冰箱中保存,用于检测目的基因的相对表达量。
1.4 引物设计与合成
本试验所用引物由Primer5.0设计,英潍捷基生物技术有限公司(上海)合成。引物信息见表1。
1.5 柑橘CitPG34的克隆
利用cDNA为扩增模板获得,反应体系为50 µL:cDNA 2 µL、上下游引物(OE-CitPG34-F/R)各1 µL、高保真酶25 µL、ddH2O 21 µL。反应程序为:94℃ 5 min;94℃ 30 s,56℃ 30 s,72℃ 1 min 30 s,38个循环;72℃ 5 min。产物经1.0%琼脂凝胶电泳检测,回收纯化。将回收产物与pMD19-T Vector连接,转化大肠杆菌感受态DH5α,涂板,挑取单克隆进行菌液PCR检测,将阳性克隆送英潍捷基生物技术有限公司测序验证。采用Editseq软件将测序正确的序列进行拼接,得到基因ORF全长。

表1 本试验所用引物
1.6 生物信息学分析
采用EXPASY(https://web.expasy.org/protparam/)分析CitPG34的理化性质。利用TMHMM(http://www. cbs.dtu.dk/services/TMHMM/)预测CitPG34蛋白跨膜结构。CitPG34蛋白的二级结构和三级结构分别由HNN(https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl? page=/NPSA/npsa_hnn.html)和Phyre2(http://www.sbg. bio.ic.ac.uk/phyre2/html/page.cgi?id=index)预测;利用CELLO(http://cello.life.nctu.edu.tw)预测CitPG34蛋白的亚细胞定位。应用ClustalX软件对CitPG34蛋白进行多重序列比对。基于邻接法(NJ),利用MEGA5.2软件构建PGs的系统进化树(Bootstrap=1 000)。采用PlantCARE(http://bioinforma tics.psb.ugent.be/ webtools/plantcare/html/)对CitPG34启动子元件进行预测分析。
1.7 CitPG34启动子克隆
利用Plant Genomic DNA Kit的试剂盒说明书提取柑橘叶片DNA,其质量和浓度分别用1.0%琼脂糖凝胶电泳和Denovix超微量紫外可见分光光度计DS-11检测。随后以该DNA为模板,克隆启动子序列(CitPG34-P),反应体系为50 µL:cDNA 2 µL、上下游引物(P-CitPG34-F/R)各1 µL、高保真酶25 µL、ddH2O 21 µL。反应程序为:94℃ 5 min;94℃ 30 s,56℃ 30 s,72℃ 2min 10 s,38个循环;72℃ 5 min。经1.0%琼脂凝胶电泳检测,回收纯化,产物置于-20℃保存。
1.8 基因表达分析
以柑橘为内参基因,利用qPCR分析在柑橘不同组织中以及吲哚乙酸(IAA)和1-氨基环丙烷羧酸(ACC)处理下的表达水平。反应体系为10 µL:5 µL iTaqTMUniversal SYBR® Green Supermix、ddH2O 3 µL、引物各0.5 µL、cDNA 1 µL。反应程序为:95℃ 2 min;95℃ 15 s,53℃ 5 s,72℃ 15 s,共39个循环。每个处理3次重复。基因相对表达量采用2-∆∆Ct法计算,用SPSS19.0对数据进行显著性分析,最后用Excel绘制图表。
1.9 亚细胞定位
以cDNA为模板,用Sub-CitPG34-F/R引物(表1)克隆获得去除终止子的全长,通过I单酶切pCAMBIA1302-表达载体,采用同源重组技术将其与去除终止子的连接,转化大肠杆菌感受态DH5α,经测序验证,得到载体命名为pCAMBIA1302--。将质粒pCAMBIA1302-和pCAMBIA1302-(对照)分别转化农杆菌GV3101,涂板,挑取单克隆利用引物Sub-CitPG34-F/R及pCAMBIA1302-F/R进行菌液检测。活化菌液,并用含有1 mol∙L-1MES、2.5 mol∙L-1MgCl2、100 mmol∙L-1乙酰丁香酮的重悬液重悬,调整菌液OD600为0.75,分别将菌液注射到洋葱内表皮,暗培养3 d,用清水和0.3 g∙mL-1蔗糖处理内表皮细胞并制作装片,置于倒置荧光显微镜下观测、拍照。
1.10 CitPG34-P::gus表达载体的构建
用dIII和I酶切去除植物表达载体PBI121的35S启动子序列,随后用infusion重组技术将上述经PCR扩增回收后得到的CitPG34-P片段连接在PBI121表达载体上,连接产物转化大肠杆菌感受态DH5α,涂板,挑取单克隆进行菌液PCR检测,将阳性克隆送英潍捷基生物技术有限公司测序验证。测序正确的表达载体命名为CitPG34-P::。将载体CitPG34-P::提取质粒,利用冻融法转化农杆菌EHA105菌株,涂板,挑取单克隆,经PCR鉴定为阳性后保存菌种。
1.11 烟草遗传转化
首先将含有CitPG34-P::载体的农杆菌活化,直至菌液OD600值在0.8—1.0,离心收集菌液,去掉上清液并加入重悬液MS。将烟草叶片切成大小约为1 cm2的方块,置于重悬液中7—10 min,期间摇晃2—3次,随后取出,用无菌滤纸吸干叶片残余菌液。将侵染后的烟草叶片置于MS共培养基中暗培养2 d,之后转到筛选培养基上继续培养,直到愈伤组织长出幼芽。切取幼芽插入生根培养基,约20 d后,将生根幼苗转移至营养土中继续培养4—5周。利用Plant Genomic DNA Kit的试剂盒说明书提取烟草叶片DNA,并用1.0%琼脂糖凝胶电泳检测转基因植株。
1.12 GUS组织化学染色
利用打孔器,将转基因烟草叶片切成半径为0.25 cm的小圆片,置于滤纸上;用0和5 mmol∙L-1乙烯利溶液处理,然后放入组培瓶中封闭;在25℃条件下处理72 h,取出圆片置入GUS溶液中避光染色;用75%酒精漂洗脱色后,光学显微镜观察。
2 结果
2.1 CitPG34的克隆及生物信息学分析
以OE-CitPG34-F/R为引物进行PCR扩增检测,获得了序列全长,其ORF长度为1 194 bp(图2-A),可编码397个氨基酸。生物信息学分析发现,CitPG34属于稳定蛋白,其相对分子量为41.47 kD,理论等电点为5.19,脂肪系数为88.78,不稳定系数为30.23;此外,在该蛋白7—29氨基酸之间存在跨膜结构。在CitPG34蛋白的二级结构中,α-螺旋结构约占15.37%,扩展链约占29.72%,无规则卷曲约占54.91%,其三级结构中α-螺旋结构占9%,β-链约占55%,TM螺旋占4%,其他结构约占8%。图3显示,CitPG34与其他植物PGs类似,含有典型的4个保守结构域,即SPNTDG(Ⅰ)、GDDC(Ⅱ)、CGPGHGISIGSLG(Ⅲ)、RIK(Ⅳ)。聚类分析显示,所有PG可划分为3个分支(A、B、C),其中CitPG34与西洋梨PcPG3亲缘关系最近(图4)。

M:DL2000分子标记。A:柑橘CitPG34;B:柑橘CitPG34的启动子

AtPG1:拟南芥,NP191310;AtPG2:拟南芥,NP850359;AdPG:猕猴桃,L12019;BrnPG:欧洲油菜,NP001302495;CmPG1:甜瓜,AF062465;CmPG2:甜瓜,AF062466;CpPG4:番木瓜,GQ479796;DkPG3:柿,EU816199;LcPG:荔枝,AFW04075;GmPG6:大豆Glycine max,ABD62085;MaPG3:小果野蕉,AY603339;NtPG1:烟草,AHG12641;MdPG:苹果,L27743;PaPG:杏,ADT82706;PcPG:西洋梨,CAH18935;PcPG1:西洋梨,AB084461;PcPG3:西洋梨,BAF42034;PdPG1:欧洲李,DQ375247;PpPG:碧桃,EF568784;PpPG1:碧桃,BAH56488;PpPG2:碧桃,CAA54448;TAPG1:番茄,NM001246866;TAPG2:番茄,U70480;TAPG4:番茄,U70481;VvPG1:葡萄,AY043233;VvPG2:葡萄,EU078975;ZmPG:玉米,P26216
I:SPNTDG结构域;II:GDDC结构域;III:CGPGHG结构域;IV:RIK结构域
I: SPNTDG domain, II: GDDC domain, III: CGPGHG domain, IV: RIK domain
图3 CitPG34与其他物种PG蛋白的氨基酸系列比对
Fig. 3 Amino acid sequence alignment of CitPG34 with other PG proteins
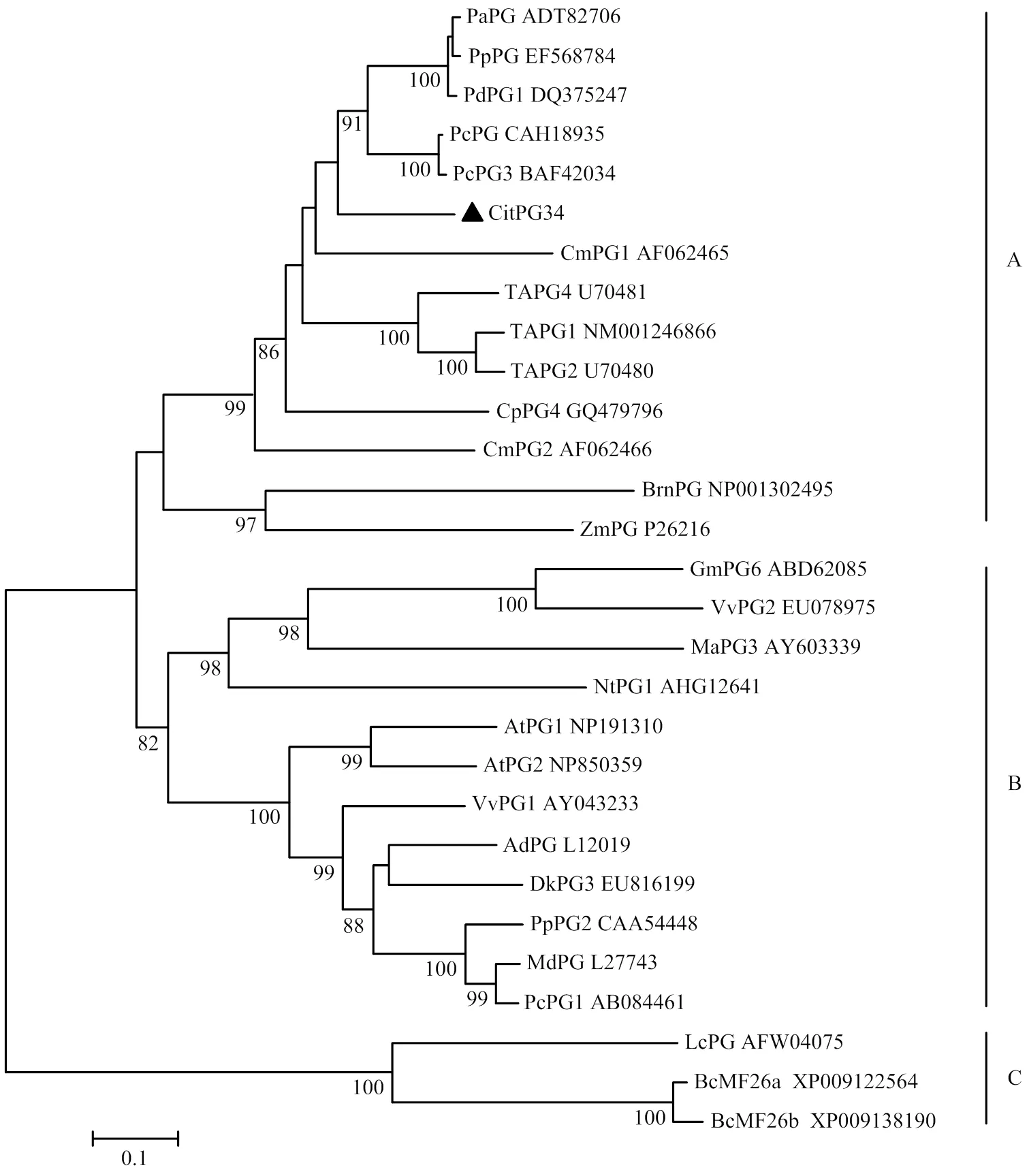
图4 植物PGs蛋白系统进化树
2.2 CitPG34的表达分析
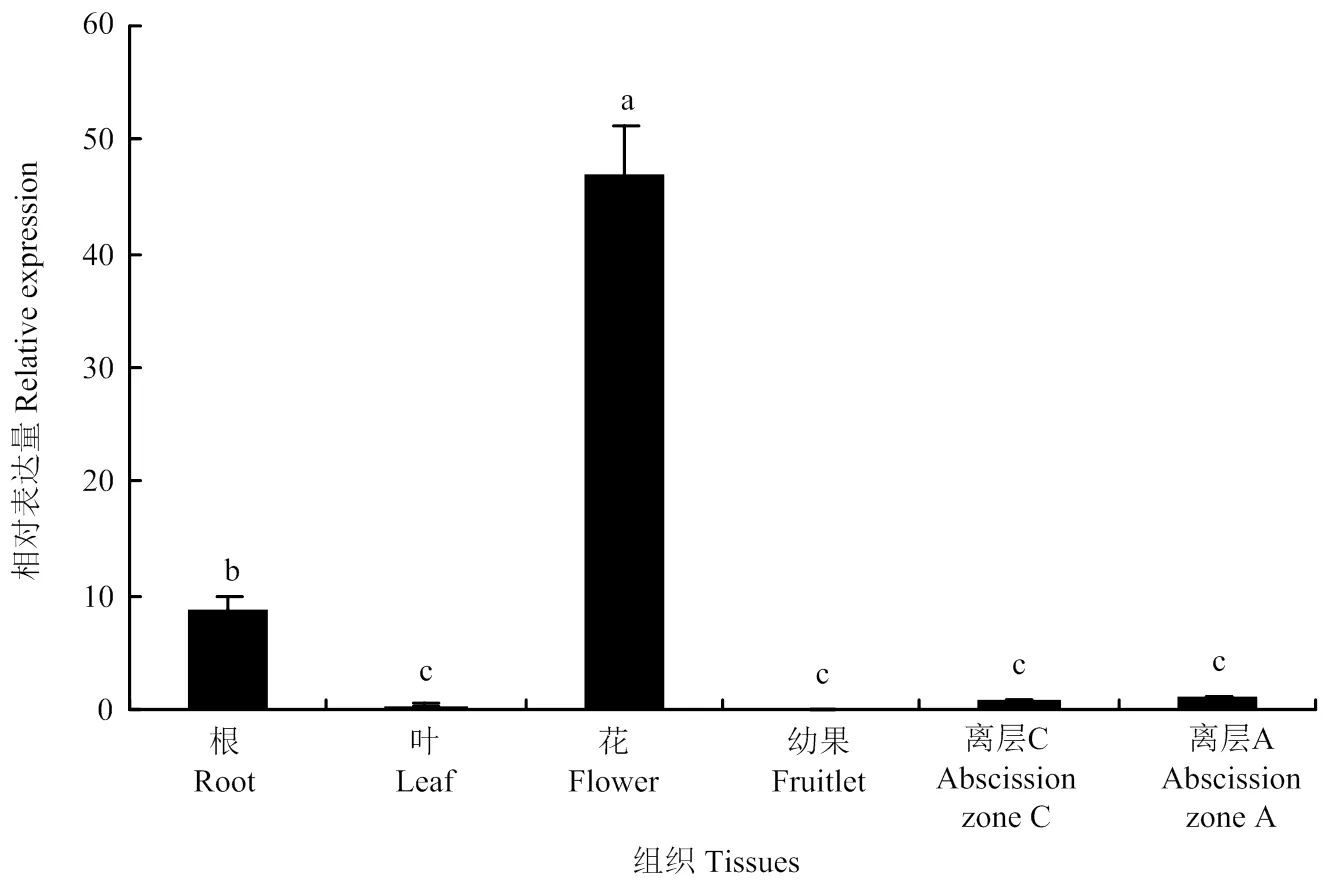
在柑橘不同组织中的表达存在显著差异,即在花中表达量最高,其次为根,在离层A和C中表达量较低,在叶和幼果中几乎不表达(图5)。上述结果表明,在柑橘花发育过程中发挥着重要作用。
在幼果第一次脱落过程中,在离层A中的表达水平显著上升,在24 h达到最高水平;而在ACC处理后,其表达水平在12 h就受到明显诱导,在18 h达到最高水平,表明受到了ACC诱导调控;相反,IAA处理后的离层A中的表达水平始终保持在较低水平,说明该基因的转录受到了IAA的抑制(图6)。上述结果表明,明显与柑橘幼果脱落相关。
2.3 亚细胞定位
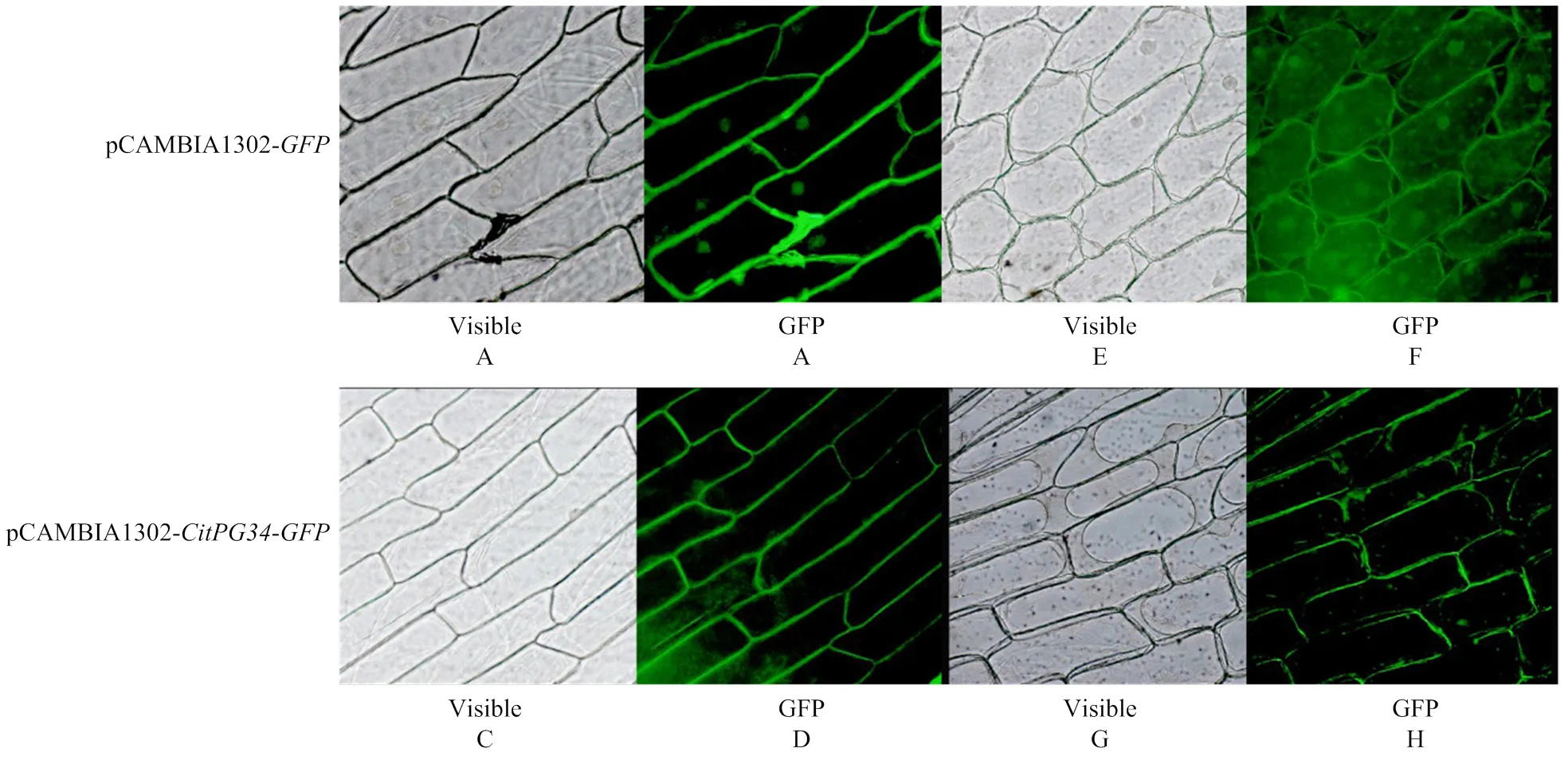
CELLO软件预测表明CitPG34属于胞外蛋白。为了进一步验证该分析结果,本试验利用洋葱表皮细胞对CitPG34进行了亚细胞定位。结果发现,在转入空白载体pCAMBIA1302-的洋葱表皮细胞中,其细胞核、细胞质膜及细胞壁中均出现绿色荧光,而转入pCAMBIA1302--的表皮细胞中,绿色荧光主要在细胞壁上(图7)。该结果与CELLO软件预测一致,表明CitPG34属于胞外蛋白,主要位于细胞壁上。
2.4 CitPG34启动子的克隆与分析
以‘塔罗科’血橙DNA为模板,克隆获得起始密码(ATG)前2 075 bp的启动子片段(CitPG34- P)。Plant CARE分析结果显示,CitPG34-P序列除包含启动子核心元件TATA-box和增强子保守元件CAAT-box外,同时还存在光响应元件、激素响应元件、逆境响应元件等,如Box4、ATCT-motif、ABRE、TGACG-motif、MBS、ARE和Box-W1等(表2)。
不同小写字母表示差异显著(P<0.05)。下同 Different lowercase letters indicate significant difference (P<0.05). The same as below
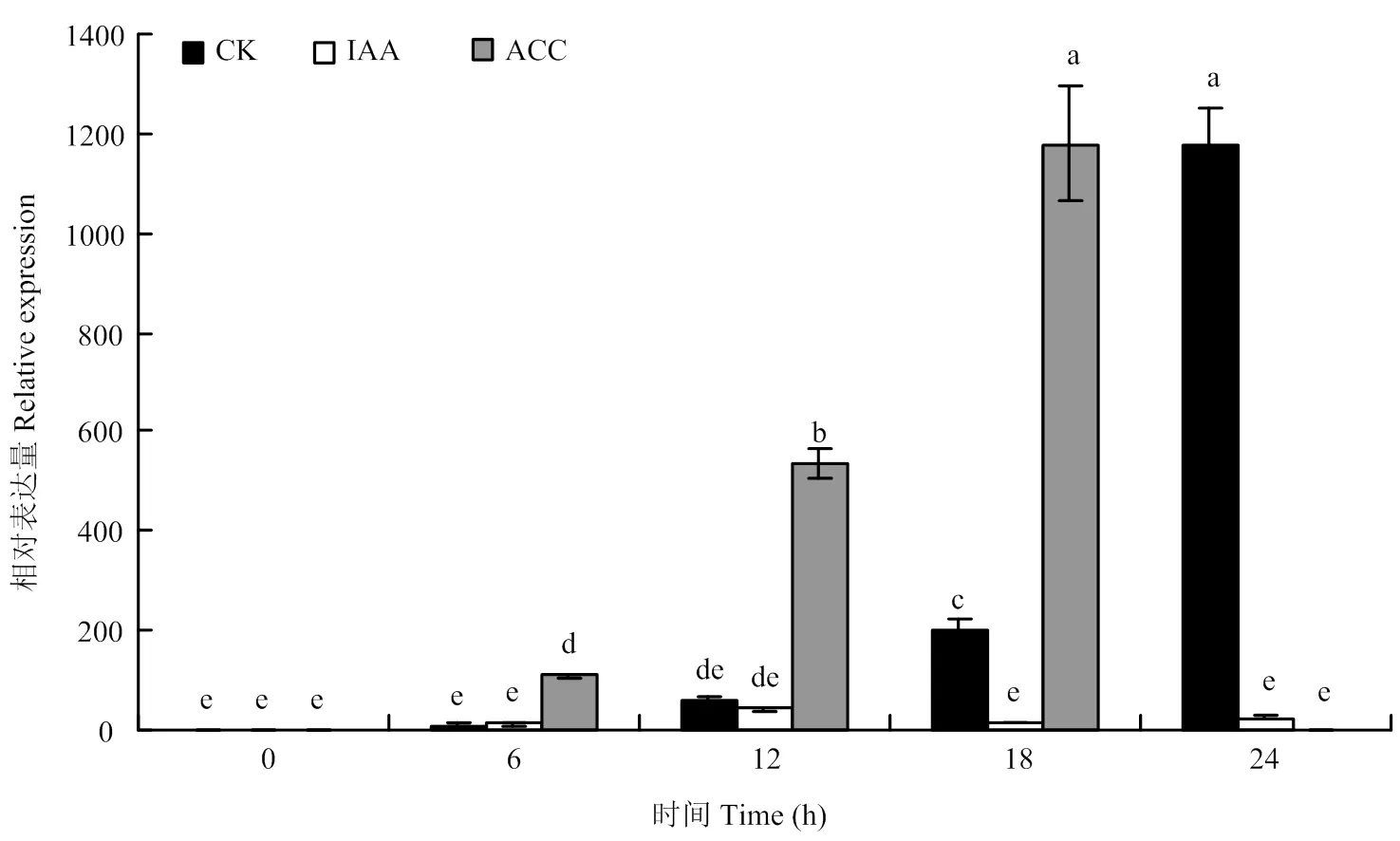
图6 在柑橘幼果脱落过程中CitPG34的表达水平
通过农杆菌介导法,将CitPG34-P::表达载体导入烟草,获得CitPG34-P::转基因植株。GUS组织化学染色表明,在正常条件下,未能检测到转基因植株叶片中的GUS酶活力;然而,在乙烯利处理后,GUS酶活力显著增强,其中叶脉和毛状体上最为明显(图8)。
3 讨论
在植物细胞壁水解酶家族中,PGs由多基因编码,成员之间具有功能特异性,参与植物生长发育的多个过程,如器官脱落、果实成熟软化、种子萌发、花粉粒成熟、木质部细胞发育及花药或荚果开裂等[20-24]。目前,已有大量PG基因在不同物种中被鉴定,如苹果[25]、桃[26]、番茄[27]、拟南芥[28]、水稻[29]、大豆[30]、杨树[31]、番木瓜[32]、葡萄[33]等,但柑橘中相关基因的克隆和功能分析还鲜有报道。本研究成功克隆获得了全长,经多重蛋白序列比对显示,CitPG34含有PGs基因家族典型的保守结构域,即SPNTDG(Ⅰ)、GDDC(Ⅱ)、CGPGHGISIGSLG(Ⅲ)、RIK(Ⅳ)。聚类分析发现,来自不同物种的29个PGs形成3个分支(A、B和C),这与HADFIELD和BENNETT[34]的结果一致,分支A中的PGs成员主要参与器官脱落和果实软化[35-36];分支B中PGs主要涉及果实软化以及荚果开裂[21,26];而分支C主要由参与花粉发育的PGs组成[37]。在本研究中,CitPG34位于分支A中,与PcPG3关系最近,推测该基因可能与柑橘果实软化或脱落相关。亚细胞定位表明CitPG34位于细胞壁,属于胞外蛋白,这与油菜[37]、甘蓝[9]和拟南芥[38]等的报道一致。
A、B、C、D:表皮细胞未发生质壁分离;E、F、G、H:表皮细胞发生质壁分离;比例尺=100 μm

图8 CitPG34-P::gus转基因植株的GUS组织化学染色
在植物体内,在不同组织中的表达水平差异明显,具有一定的组织特异性。例如,在拟南芥中,在花中表达量最高,其次是叶和根,在茎中表达量较低[39];主要在花中表达,在荚果、茎、叶中次之,根最低[38];同样,和在荚果中表达较高,其次是花芽和根,在叶中几乎不表达[28]。LYU等[9]发现,甘蓝在花序表达量最高,根次之,在茎、叶和荚果中表达量几乎不表达。与上述结果类似,在柑橘花、根、叶、果等组织中均有表达,其中花中表达量最高,其次是根、离层A和C,在叶和果中表达量最低,由此推测在柑橘的花发育过程中具有重要作用。
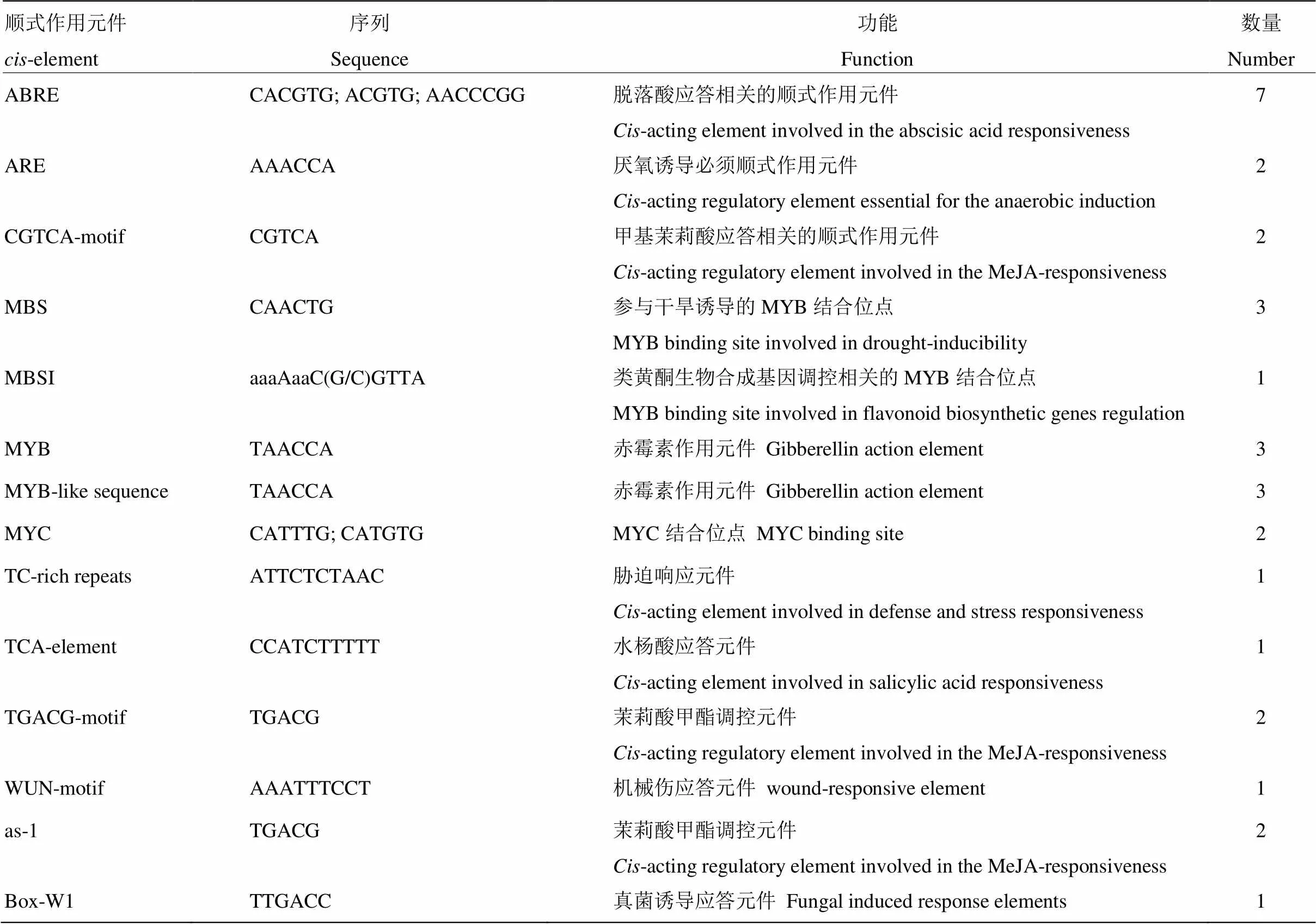
表2 CitPG34启动子序列主要顺式作用元件
研究表明乙烯可以促进柑橘幼果脱落,而吲哚-3-乙酸(Indole-3-acetic acid,IAA)则相反[18,40-41]。VAN DOORN和STEAD[42]认为IAA之所以能抑制器官脱落,是因为IAA降低了离层细胞对乙烯的敏感性。MEIR等[43]利用基因芯片技术研究了番茄花脱落的分子机制,结果显示IAA处理能明显降低、以及在花的离层中的表达。2-萘乙酸(Naphthaleneacetic acid,NAA)通过抑制苹果离层的表达,从而明显降低了成熟果实脱落,表明与果实脱落有关[44]。ZHU等[45]也在苹果上得到类似结果,即通过对苹果幼果喷施NAA后,造成幼果落果率显著增加,转录组分析进一步表明,参与了幼果脱落。XIE等[18]通过转录组分析发现7个PGs成员与柑橘幼果脱落相关,(orange1.1t02532)为其中成员之一。在本研究中,在离层A中的表达水平明显受ACC诱导,却被IAA抑制,在幼果第一次脱落过程中显著上升,与柑橘幼果脱落呈现明显相关。
启动子作为基因结构的重要组成部分,能够调控基因表达的起始时间、空间和表达水平。因此,通过克隆并分析基因启动子序列,可以阐述基因的表达水平及其调控的分子机制。本研究中克隆获得了起始密码(ATG)前2 075 bp的启动子片段(CitPG34-P)。顺式作用元件分析结果表明,CitPG34-P含有对光、干旱胁迫、厌氧胁迫、真菌诱导和激素(脱落酸、茉莉酸、赤霉素)应答元件,说明外界环境的变化可能会诱导基因的转录活性发生相应的变化。HONG等[36]将番茄、以及启动子连接报告基因并转入番茄中,通过GUS染色分析发现,、以及启动子对GUS活力的调控特征基本与相应基因的表达模式相一致。本研究中,启动子的转基因烟草叶片经乙烯利诱导处理后,在叶脉和毛状体中的活性较强,暗示受乙烯诱导,可能与叶脉和毛状体的生长发育有关。
4 结论
从‘塔罗科’血橙中克隆得到及其启动子序列,其蛋白具有SPNTDG(Ⅰ)、GDDC(Ⅱ)、CGPGHGISIGSLG(Ⅲ)、RIK(Ⅳ)4个特征保守结构域。亚细胞定位分析显示CitPG34主要位于细胞壁。在花中表达最高,根次之,在其他组织中表达极低或不表达;另外,其表达量受IAA抑制,被ACC诱导,说明该基因与柑橘幼果脱落相关。GUS组织化学染色结果表明,受乙烯诱导,主要在叶脉和毛状体中表达。
[1] 刘志良. 柑橘过量落花落果的原因及防止对策. 中国农技推广, 2013, 29(9): 28-30.
LIU Z L. Reasons for excessive flowering and fruit dropping of citrus and preventive measures., 2013, 29(9): 28-30. (in Chinese)
[2] 潘小婷, 张静, 葛廷, 马岩岩, 邓烈, 何绍兰, 易时来, 郑永强, 吕强, 谢让金. 柑橘CitCEP基因家族的鉴定及对逆境和激素的响应. 中国农业科学, 2018, 51(16): 3147-3158.
PAN X T, ZHANG J, GE T, MA Y Y, DENG L, HE S L, YI S L, ZHENG Y Q, LÜ Q, XIE R J. Identification of citrus CitCEP genes and their transcriptional response to stress and hormone treatments., 2018, 51(16): 3147-3158. (in Chinese)
[3] RIOV J. A polygalacturonase from citrus leaf explants: role in abscission., 1974, 53(2): 312-316.
[4] NAKANO T, ITO Y. Molecular mechanisms controlling plant organ abscission., 2013, 30(3): 209-216.
[5] CARMEN RODRÍGUEZ-GACIO M D C, NICOLÁS C, MATILLA A J. Cloning and analysis of a cDNA encoding an endo-polygalacturonase expressed during the desiccation period of the silique-valves of turnip-tops (L. cv. Rapa)., 2004, 161(2): 219-227.
[6] QUESADA M, BLANCO-PORTALES R, POSÉ S, GARCÍA-GAGO J A, JIMÉNEZ-BERMÚDEZ S, MUN˜OZ-SERRANO A, CABALLERO J L, PLIEGO-ALFARO F, MERCADO J A, MUÑOZ-BLANCO J. Antisense down-regulation of thegene reveals an unexpected central role for polygalacturonase in strawberry fruit softening., 2009, 150(2): 1022-1032.
[7] POSE S, PANIAGUA C, CIFUENTES M, BLANCO-PORTALES R, QUWSADA M A, MERCADO J A. Insights into the effects of polygalacturonasegene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits., 2013, 64(12): 3803-3815.
[8] GONZÁLEZ-CARRANZA Z H, ELLIOTT K A, ROBERTS J A. Expression of polygalacturonases and evidence to support their role during cell separation processes in., 2007, 58(13): 3719-3730.
[9] LYU M L, LIANG Y, YU Y J, MA Z M, SONG L M, YUE X Y, CAO J S. Identification and expression analysis of, a novel polygalacturonase gene involved in pollen development of., 2015, 28(2): 121-132.
[10] ATKINSON R G, SCHRODER R, HALLETT I C, COHEN D, MACRAE E A. Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion., 2002, 129(1): 122-133.
[11] ROONGSATTHAM P, MORCILLO F, JANTASURIYARAT C, PIZOT M, MOUSSU S, JAYAWEERA D, COLLIN M, GONZÁLEZ- CARRANZA Z H, AMBLARD P, TREGEAR J W, TRAGOONRUNG S, VERDEIL J, TRANBARGER T J. Temporal and spatial expression of polygalacturonase gene family members reveals divergent regulation during fleshy fruit ripening and abscission in the monocot species oil palm., 2012, 12(1): 150.
[12] JIANG C Z, LU F, IMSABAI W, MEIR S, REID M S. Silencing polygalacturonase expression inhibits tomato petiole abscission., 2008, 59(4): 973.
[13] PENG G, WU J Y, LU W J, LI J G. A polygalacturonase gene clustered into clade E involved in lychee fruitlet abscission., 2013, 150: 244-250.
[14] RASCIO N, CASADORO G, RAMINA A, MASIA A. Structural and biochemical aspects of peach fruit abscission (L. Batsch)., 1985, 164(1): 1-11.
[15] TAYLOR J E, WEBB S T J, COUPE S A, TUCKER G A, ROBERTS J A. Changes in polygalacturonase activity and solubility of polyuronides during ethylene-stimulated leaf abscission in., 1993, 44(1): 93-98.
[16] KALAITZIS P, TUCKER S M L. Three different polygalacturonases are expressed in tomato leaf and flower abscission, each with a different temporal expression pattern., 1997, 113(4): 1303-1308.
[17] CHENG C Z, ZHANG L Y, YANG X L, ZHONG G Y. Profiling gene expression in citrus fruit calyx abscission zone (AZ-C) treated with ethylene., 2015, 290(5): 1991-2006.
[18] MERELO P, AGUSTÍJ, ARBONA V, COSTA M L, ESTORNELL L H, GÓMEZ-CADENAS A, COIMBRA S, GÓMEZ M D, PÉREZ-AMADOR M A, DOMINGO C, TALÓN M, TADEO F R. Cell wall remodeling in abscission zone cells during ethylene- promoted fruit abscission in citrus., 2017, 8: 126.
[19] XIE R J, GE T, ZHANG J, PAN X T, MA Y Y, YI S L, ZHENG Y Q. The molecular events of IAA inhibiting citrus fruitlet abscission revealed by digital gene expression profiling., 2018,130: 192-204.
[20] GONZÁLEZ-CARRANZA Z H, WHITELAW C A, SWARUP R, ROBERTS J A. Temporal and spatial expression of a polygalacturonase during leaf and flower abscission in oilseed rape and., 2002, 128(2): 534-543.
[21] MARKOVIC O, JANECEK S. Pectin degrading glycoside hydrolases of family 28: sequence structural features, specificities and evolution., 2001, 14(9): 615-631.
[22] SITRIT Y, HADFIELD K A, BENNETT A B, BRADFORD K J, DOWNIE A B. Expression of a polygalacturonase associated with tomato seed germination., 1999, 121(2): 419-428.
[23] SANDER L, CHILD R, ULVSKOV P, ALBRECHTSEN M, BORKHARDT B. Analysis of a dehiscence zone endo-polygalacturonase in oilseed rape () and: evidence for roles in cell separation in dehiscence and abscission zones, and in stylar tissues during pollen tube growth., 2001, 46(4): 469-479.
[24] ROBERTS J A, ELLIOTT K A, GONZÁLEZ-CARRANZA Z H. Abscission, dehiscence, and other cell separation processes., 2001, 53(1): 131-158.
[25] CHEN H F, SHAO H X, FAN S, MA J J, ZHANG D, HAN M Y. Identification and phylogenetic analysis of thegene family in apple., 2016, 2(5): 241-252.
[26] MING Q, YIKE Z, YAN X Y, HAN M Y. identification and expression analysis of polygalacturonase family members during peach fruit softening., 2016, 17(11): 1933.
[27] KE X B, WANG H S, LI Y, ZHU B, ZANG Y X, HE Y, CAO J S, ZHU Z J, YU Y J. Genome-wide identification and analysis of polygalacturonase genes in., 2018, 19(8): 2290.
[28] OGAWA M, KAY P, SWAIN W S M.,, andare polygalacturonases required for cell separation during reproductive development in., 2009, 21(1): 216-233.
[29] KIM J, SHIU S H, THOMA S, LI W H, PATTERSON S E. Patterns of expansion and expression divergence in the plant polygalacturonase gene family., 2006, 7(9): R87.
[30] WANG F F, SUN X, SHI X Y, ZHAI H, TIAN C G, KONG F J, LIU B H, YUAN X H. A global analysis of the polygalacturonase gene family in soybean ()., 2016, 11(9): e0163012.
[31] YANG Z L, LIU H J, WANG X R, ZENG Q Y. Molecular evolution and expression divergence of the populus polygalacturonase supergene family shed light on the evolution of increasingly complex organs in plants., 2013, 197(4): 1353-1365.
[32] FABI J P, BROETTO S G, DA SILVA S L G L, ZHONG S L, LAJOLO F M, DO NASCIMENTO J R O. Analysis of papaya cell wall-related genes during fruit ripening indicates a central role of polygalacturonases during pulp softening., 2014, 9(8): e105685.
[33] DEYTIEUX-BELLEAU C, AMÉLIE V, BERNARD D, GENY L. Pectin methylesterase and polygalacturonase in the developing grape skin., 2008, 46(7): 638-646.
[34] HADFIELD K A, BENNETT A B. Polygalacturonases: many genes in search of a function., 1998, 117(2): 337-343.
[35] BONGHI C, RASCIO N, RAMINA A, CASADORO G. Cellulase and polygalacturonase involvement in the abscission of leaf and fruit explants of peach., 1992, 20: 839-848.
[36] HONG S B, SEXTON R, TUCKER M L. Analysis of gene promoters for two tomato polygalacturonases expressed in abscission zones and the stigma., 2000, 123(3): 869-881.
[37] LYU M L, YU Y J, JIANG J J, SONG L M, LIANG Y, MA Z M, XIONG X P, CAO J S.andare duplicated polygalacturonase genes with divergent expression patterns and functions in pollen development and pollen tube formation in., 2015, 10(7): e0131173.
[38] RUI Y, XIAO C W, YI J, KANDEMIR B, WANG J Z, PURI V M, ANDERSON C T.functions in seedling development, rosette growth, and stomatal dynamics in., 2017, 29(10): 2413.
[39] XIAO C, SOMERVILLE C, ANDERSON C T.functions in cell elongation and flower development in., 2014, 26(3): 1018-1035.
[40] KÜHN N, SERRANO A, ABELLO C, ARCE A,ESPINOZA C, GOUTHU S,DELUC L,JOHNSON P A. Regulation of polar auxin transport in grapevine fruitlets (L.) and the proposed role of auxin homeostasis during fruit abscission., 2016, 16(1): 234.
[41] YUAN R C, WU Z C, KOSTENYUK I A, BURNS J K. G- protein-coupled alpha2A-adrenoreceptor agonists differentially alter citrus leaf and fruit abscission by affecting expression of ACC synthase and ACC oxidase., 2005, 56(417): 1867.
[42] VAN DOORN W G, STEAD A D. Abscission of flowers and floral parts., 1997, 48(4): 821-837.
[43] MEIR S, PHILOSOPH-HADAS S, SUNDARESAN S, SELVARAJ K S, BURD S, OPHIR R, KOCHANEK B, REID M S, JIANG C Z, LERS A. Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion., 2011, 154(4): 1929-1956.
[44] LI J G, YUAN R C. NAA and ethylene regulate expression of genes related to ethylene biosynthesis, perception, and cell wall degradation during fruit abscission and ripening in ‘Delicious’ apples., 2008, 27(3): 283-295.
[45] ZHU H, DARDICK C D, BEERS E P, CALLANHAN A M, XIA R, YUAN R C. Transcriptomics of shading-induced and NAA-induced abscission in apple () reveals a shared pathway involving reduced photosynthesis, alterations in carbohydrate transport and signaling and hormone crosstalk., 2011, 11(1): 138-138.
Cloning, Subcellular Localization and Expression Analysis ofCitrus
GE Ting, HUANG Xue, XIE RangJin
(Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712)
【】Polygalacturonases (PGs) play important roles in plant growth and development as well as organ abscission by degrading pectin in cell wall.In this study, a citrus PG gene (i.e.,) and its promoter (CitPG34-P) were cloned and expression analyzed based on our previous data, which would provide a basis for further elucidating the function of【】The full length ofgene and its promoter was cloned from ‘Tarcocco’ blood orange (L. Osbeck). The protein characteristics and-acting elements on promoter were analyzed by ProtParam, Cello, CLUSTALX, MEGA5.2, and PlantCARE, etc. The gene expression level was detected by real-time Quantitative PCR (qRT-PCR). The PCAMBIA1302--fusion protein expression vector for subcellular localization and CitPG34-P expression vector (CitPG34-P::) for promoter activity analysis were constructed by homologous recombination, respectively.【】The ORF ofwas 1 194 bp in length, encoding 397 amino acids. The predicted molecular weight of CitPG34 was 41.47 kD, the theoretical pI was 5.19, and the instability coefficient was 30.23, indicating that CitPG34 belonged to stable protein.TMHMM analysis showed that CitPG34 was a transmembrane protein, the transmembrane domain locating between the amino acid residue 7 and 29. In the secondary structure of CitPG34, the alpha-helix structure, extended chain and random coil account for 15.37%, 29.72% and 54.91%, respectively, which were nearly consistent with its tertiary structure. NJ tree analysis showed that CitPG34 was close to PcPG3 (BAF42034), a pear PG, indicating that it might be related to fruit abscission and softening. qPCR analysis showed thatdominantly expressed in flowers, followed by roots, leaves, abscission zone A (AZ A) and C (AZ C), and almost undetected in fruits. In AZ A, the expression level of CitPG34 was significantly up-regulated by ACC, whereas inhibited by IAA, showing the role in citrus fruitlet abscission. Subcellular localization revealed that CitPG34 was mainly located in cell wall. A 2 075 bp promoter sequence ofwas cloned, which contained several cis-regulatory elements, including TATA-box, enhancer CAAT-box and ABRE, etc. GUS histochemical staining revealed that the GUS activity in vein and trichomes was remarkably up-regulated by ethylene. 【】The ORF length ofgene was 1 194 bp, encoding 397 amino acids. CitPG34 was mainly located in cell wall. qPCR analysis showed thatdominantly expressed in flowers and was significantly associated with citrus fruitlet abscission. Taken together, these results indicated thatplayed important roles in citrus fruitlet abscission and flower development.
citrus;Polygalacturonases; gene expression; subcellular localization; promoter; fruitlet abscission
10.3864/j.issn.0578-1752.2019.19.011

2019-05-05;
2019-07-12
重庆市基础研究与前沿探索项目(cstc2018jcyjAX0564)
葛廷,E-mail:m13364018674@163.com。
谢让金,E-mail:xierangjin@cric.cn
(责任编辑 赵伶俐)

