Smallanthus sonchifolius roots ameliorate non-alcoholic fatty liver disease by reducing redox imbalance and hepatocyte damage in rats fed with a high fructose diet
Mariano Nicolás Alemán, Sara Serafina Sánchez, Stella Maris Honoré
Instituto Superior de Investigaciones Biológicas (INSIBIO), Consejo Nacional de Investigaciones, Científicas y Técnicas - Universidad Nacional de Tucumán (CONICET-UNT), Chacabuco 461, T4000ILI San Miguel de Tucumán, Argentina
Keywords:Smallanthus sonchifolius roots Non-alcoholic fatty liver disease Oxidative stress Apoptosis Liver
ABSTRACT Objective: To evaluate the potential of Smallanthus sonchifolius (S. sonchifolius) roots in ameliorating hepatic damage of rats fed with a high fructose diet.Methods: The effect of S. sonchifolius roots on energy intake, body weight, fat and liver mass was determined in male rats fed with a high-fructose diet. Plasma glucose, triglycerides, total cholesterol, lipoproteins and aspartate aminotransferase and alanine aminotransferase were analyzed. Histological changes of the livers were evaluated by electronic microscopy and apoptosis was examined using the TUNEL method. The levels of malondialdehyde, reducedglutathione and antioxidant enzymes (catalase, superoxide dismutase, glutathione peroxidase,glutathione S-transferase) activities were also determined.Results: S. sonchifolius roots significantly decreased energy intake, body weight, fat and liver mass (P < 0.05). S. sonchifolius roots ameliorated liver steatosis and mitochondrial morphology,avoiding cellular apoptosis and normalizing transaminase activity in the liver of rats fed with high fructose. Enzymatic assays revealed that S. sonchifolius roots had a modulatory effect on the oxidative stress induced by fructose-feeding by reducing lipid peroxidation (P < 0.05) and antioxidant enzyme activities (P < 0.05) in liver.Conclusions: S. sonchifolius roots can ameliorate non-alcoholic fatty liver disease by improving oxidative stress and liver injury.
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of advanced liver disease in coming decades. The prevalence of NAFLD is raising as a result of increasingly sedentary lifestyle and high carbohydrate diets, particularly by excessive fructose consumption[1]. It is strongly associated with many metabolic disorders such as obesity, dyslipidaemia, insulin resistance, and hypertension and is now considered as the hepatic manifestation of the metabolic syndrome[2]. This disease includes a wide spectrum of histologic findings ranging from isolated steatosis or non-alcoholic fatty liver to non-alcoholic steatohepatitis, which may progress in some patients to fibrosis and more severe liver complications such as cirrhosis, hepatocellular carcinoma[3]. Accumulating data suggest that redox imbalance is one of the most important mechanisms leading to hepatic injury in many metabolic diseases, thus playing a fundamental role in NAFLD progression[3,4]. The overproduction of reactive oxygen species (ROS) and the decrease of antioxidant factors can damage proteins, lipids, and even alter the DNA,compromising their biological functions[5].
Many studies strongly support a role for polyphenols in the prevention of metabolic and chronic degenerative diseases by the modulation of oxidative stress[6-8]. These bioactive compounds present in fruits and vegetables which can act as natural dietary antioxidants protect cell membranes against damage mediated by free radicals[5]. Thus, there is growing interest directed toward the identification of plant foods with favorable nutritional characteristics,antioxidant activity, high fiber content, and prospective potential for the development of safe functional food products for human consumption.
Smallanthus sonchifolius (S. sonchifolius) (Poepp. and Endl.) H.Robinson is a traditional crop from South America since ancient times[9]. S. sonchifolius (yacon) tubers contain significant amounts of phenolic compounds, fructooligosaccharides (FOS) and low sugar content[10]. Five caffeic acid derivatives were detected in S. sonchifolius roots: chlorogenic (3-caffeoylquinic), 3,5-dicaffeoylquinic acids and three caffeic and altraric acids esters identified as 2,5-dicaffeoylaltraric, 2,4 or 3,5-dicaffeoylaltraric, and 2,3,5 or 2,4,5-tricaffeoylaltraric acids[11,12]. In addition, derivatives of octulosonic acid, ferulic acids, quercetin and two other flavonoids have also been isolated from S. sonchifolius tubers[12-15].
S. sonchifolius roots have low caloric value and sweet flavor, and are usually consumed fresh and dehydrated or dried as flour to maintain the adequate properties for a longer period of time[16]. We have previously reported that S. sonchifolius roots have hypolipidemic properties without long-term side effects in diabetic and obese animals[16-18]. The present study was designed to determine whether S. sonchifolius roots may be effective in ameliorating hepatic damage,reducing redox imbalance and oxidative stress in the liver of high fructose-fed rats.
2. Materials and methods
2.1. Plant material and root flour preparation
The S. sonchifolius (Poepp. and Endl.) H. Robinson (yacon) roots were obtained from the 2018 harvest of a field at Barcena, Jujuy,Argentina. Voucher specimens were deposited in the herbarium of“Instituto Miguel Lillo”, Tucuman, Argentina (No. 600982LIL).Roots were washed, dried, peeled, sliced and desiccated at 50 ℃in a forced air circulation oven. Partially dried slices were milled according to Genta et al[16]. S. sonchifolius roots flour contained 45.20% FOS and 20.12% free simple sugar determined by Fructan Assay Procedure AOAC Method 999.03, AACC Method 32-32.01(Megazyme International Ireland Ltd). Total polyphenol content of the flour was (19.21 ± 2.24) (mg gallic acid equivalent/g flour)estimated by the Folin-Ciocalteu method[19].
2.2. Animals
Adult male Wistar rats between 180-220 g were purchased from the colony bread at INSIBIO (CONICET-UNT), Tucumán, Argentina.Rats were kept in plastic cages in a room under controlled conditions at (23 ± 1) ℃ room temperature and (approximately 60%) humidity,with a 12 h dark/light cycle. The rats had unlimited access to chow(Standard Food-Asociación Cooperativas Argentinas-S.E.N.A.S.A.)and tap water. The protocol for animal experiments was approved(No. 0004-17) by the Animal Care and Use Committee from the Universidad Nacional de Tucuman and was according to the Guide for the care and use of laboratory animals (USA, National Institute of Health).
2.3. Experimental design
The experimental animals were randomly divided into two groups:the control group (n=6) and the high-fructose group (n=18). Both groups were fed with a standard diet ad libitum, and while the animals from control group had free access to tap water, and those of highfructose group were allowed free access to freshly prepared 10% (w/v) fructose solution in tap water for twelve weeks to induce metabolic syndrome[20]. Then the animals from high-fructose group were randomly divided into 3 subgroups and further supplied for eight weeks with the fructose solution in drinking water. One group of rats(high fructose+S. sonchifolius) received a tablet of S. sonchifolius flour containing 340 mg FOS/kg body weight[16], the other group (high fructose+fenofibrate) received fenofibrate (30 mg/kg bw; Craveri Lab, Argentina) as a positive control, once a day for eight weeks. The remaining rats served as high fructose control group. Body weight was determined once a week along the study. At the end of the experiment,rats were anaesthetized (1:1 xylazine-ketamine) and blood samples were collected from the heart. Liver and white adipose tissues were dissected and weighed.
2.4. Oral glucose tolerance test
The oral glucose tolerance test was developed in the experimental groups at week 12 (data not shown) and at the end of the experiment(week 20). After an overnight fast, baseline blood glucose (time 0) was determined. Then a glucose solution (2 g/kg glucose, 50%glucose solution) was administered via gavage, followed by the determination of blood levels at 15, 30, 60, and 120 min[18]. The area under the curve of glucose was calculated.
2.5. Biochemical assays
Fast glucose concentrations were determined from tail vein blood using an Accu-Check®glucometer (Roche Diagnostics, GmbH,Mannheim, Germany). Triglycerides, total cholesterol, high-density lipoproteins (HDL-C), low-density lipoproteins (LDL-C), very low-density lipoproteins (VLDL-C) and hepatic enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assessed using enzymatic methods (Wiener lab Group, Argentina)according to the manufacturer’s instructions.
2.6. Electron microscopic study
A small piece of the right lobe of liver of each animal was fixed in 2.5% phosphate-buffered glutaraldehyde and post fixed in 1%phosphate buffer osmium tetroxide[21]. Ultrathin sections were cut and stained with uranyl acetate and lead citrate and examined using a Zeiss EM109 transmission electron microscope.
2.7. Processing of tissue and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)assay
Liver tissue samples were processed for histology, fixed in 4%phosphate-buffered formalin and embedded in paraffin. Fourmicrometer tissue sections were subjected to TUNEL assay using an in situ cell death detection kit (DeadEnd™Fluorometric TUNEL System, Promega) according to the manufacturer’s instructions.Apoptosis was quantified by counting the number of TUNELpositive cells in twenty-five representative 400× microscopic fields throughout the section. The total number of cells per field was determined by counting the number of nuclei stained with DAPI.The slides were taken with a fluorescent microscope (Olympus,Tokyo, Japan).
2.8. Assessment of hepatic lipid peroxides
Liver samples from each rat were homogenized in freshly prepared cold sodium phosphate buffer saline. Protein concentration in liver homogenates was estimated by Bradford method[22].Malondialdehyde (MDA) content was investigated as a biomarker of lipid peroxidation, using the spectrophotometric method of Beuge and Aust[23].
2.9. Antioxidant capacity estimation
The antioxidant capacity was determined by the estimation of superoxide dismutase (SOD)[24], catalase (CAT)[25], glutathione peroxidase (GPx)[26]and glutathione-S-transferase (GST)[27]activities. Reduced glutathione (GSH) was measured as levels of endogenous antioxidants according to the method described by Ellman[28]. All of the compounds used for the assay of different biochemical parameters were obtained from Sigma Inc., USA.
2.10. Statistical analysis
All data were expressed as mean ± standard deviation (mean ± SD).Statistical differences between groups were assessed using unpaired Student’s t test two-tailed and one-way ANOVA followed by a Tukey-Kramer post-hoc test to compare all treatments vs. high fructose group with GraphPad Prism software (version 6.0), Inc.,USA. The level of significance was set at P < 0.05.

Table 1. Effect of S. sonchifolius flour on clinical and biochemical parameters.
3. Results
3.1. Effect of S. sonchifolius roots on clinical parameters
As shown in Table 1, the energy intake, body weight and body weight gain in the high-fructose group were higher than those in the control group (P < 0.05). The treatment with S. sonchifolius roots significantly reduced the energy intake and body weight (P < 0.05)exhibiting a weight difference of 6% compared with untreated high fructose group. Consistent with these findings, the abdominal fat and liver weight in the high fructose group was significantly higher than in the control group (P < 0.05), however, the S. sonchifolius treatment decreased the weights of these organs in treated group (P< 0.05). There were no significant differences in the energy intake,body weight, body weight gain, abdominal fat and liver weight between fenofibrate-treated group and high fructose control group (P> 0.05).
3.2. Effects of S. sonchifolius roots on biochemical parameters
In our study, fructose substantially increased fasting plasma glucose(136.00±18.00) mg/dL and led to an impaired glucose tolerance(14 066.36±182.00) mg/dL/min (Table 1). In contrast, S. sonchifolius significantly reduced fasting glucose levels (105.00±10.00) mg/dL and improved glucose tolerance (13 732.31±88.00) mg/dL/min in treated animals (P < 0.05). The administration of fenofibrate did not affect the parameters analyzed (P > 0.05).
Fructose feeding in rats also led to hypertriglyceridemia and significantly increased plasma VLDL-C concentrations (P < 0.05).The addition of S. sonchifolius roots to the diet lowered plasma triglycerides and VLDL-C levels (P < 0.05). Similar effects were observed in high fructose-fed rats treated with fenofibrate (P < 0.05).Total cholesterol, HDL-C and LDL-C concentrations did not differ significantly among the treated group and the high fructose control group (Table 1).
S. sonchifolius showed an improvement in lipid profile compared with high fructose-fed animals. S. sonchifolius significantly decreased AST and ALT activity (P < 0.05), while fenofibrate increased hepatic transaminase activities (P < 0.05) exacerbating hepatocyte injury caused by fructose feeding (Table 1).
3.3. Effects of S. sonchifolius roots on hepatic structure
Microvesicular steatosis was observed in the liver of high fructosefed animals (Figure 1B). S. sonchifolius treatment reduced fat droplets accumulation (Figure 1C), showing a similar phenotype to the control rats (Figure 1A). Fenofibrate also ameliorated lipiddeposition in the liver of high fructose-fed animals (Figure 1D).The development of hepatic steatosis was coincident with changes in gross mitochondrial morphology (Figure 1E-H). Electron micrographs indicated that mitochondria from livers of rats fed with high fructose appeared to be shorter and more round than those in liver of the control group (Figure 1E and F). Moreover, some swollen mitochondria showed a hypodense matrix and abnormal cristae morphology (Figure 1F). In contrast, the mitochondria of the S. sonchifolius treated group showed cristae morphology and matrix density similar to those of control animals (Figure 1E and G). Fenofibrate treated-rats showed an increased number of mitochondria, many of them smaller and with dilated cristae (Figure 1H).
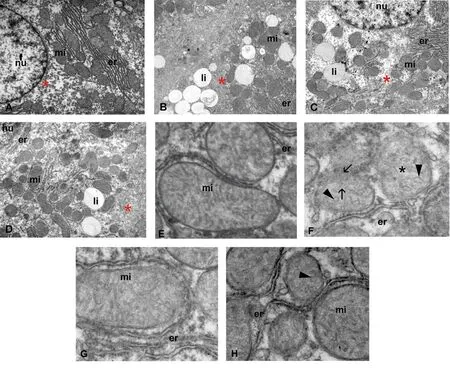
Figure 1. Effects of S. sonchifolius roots on hepatocyte and mitochondrial morphology. Electron micrographs of hepatic tissue from rats of (A, E) control, (B, F)high fructose diet, (C, G) high fructose diet + S. sonchifolius (340 mg FOS/kg) and (D, H) high fructose diet + fenofibrate (30 mg/kg bw) groups are presented.Round-shape mitochondria showing membrane disruption (arrow), a hypodense matrix (black*) and abnormal cristae (arrow head). mi: mitochondria; nu:nucleus; er: endoplasmic reticulum; li: lipid droplet; glycogen (red*). A-D: ×7 500; E-H: ×12 800.

Figure 2. Effects of S. sonchifolius roots on oxidative stress and antioxidant status. A: reduced glutathione (GSH) levels and B: malondialdehyde (MDA) levels in the liver of rat fed with control diet (CD), high fructose diet (FD); high fructose diet + S. sonchifolius (340 mg FOS/kg) (FD+Ss), and high fructose diet +fenofibrate (30 mg/kg bw) (FD+F). Values are expressed as mean ± SD (n=6 animals). P < 0.05, as compared to CD (a) and FD (b) groups using one-way ANOVA followed by Tukey-Kramer test.

Figure 3. Effects of S. sonchifolius roots on liver apoptosis. A-D: Representative images of liver sections from rats of (A) control, (B) high fructose diet, (C)high fructose diet + S. sonchifolius (340 mg FOS/kg) and (D) high fructose diet + fenofibrate (30 mg/kg bw) groups analyzed with TUNEL assay. TUNEL positive cells (arrow). A-D: ×400, E: Apoptotic index (number of TUNEL positive cells per total number of cells per field ×400).Values are expressed as mean± SD (n=6 animals). P < 0.05, as compared to CD (a) and FD (b) groups using one-way ANOVA followed by Tukey-Kramer test. CD: control diet; FD: high fructose diet; Ss: S. sonchifolius; F: fenofibrate.
3.4. Effects of S. sonchifolius roots on oxidative status and apoptosis
In our experimental model, fructose caused a significant increase in hepatic MDA level (P < 0.05) and a reduction in GSH, testifying the intensified lipid peroxidation in the high fructose group (Figure 2A and B). The treatment of S. sonchifolius roots significantly reduced MDA levels (P < 0.05) and increased GSH concentrations similar to those in the control group. These findings proved that S. sonchifolius could also protect mitochondrial morphology by decreasing ROS damage.
In addition, TUNEL-positive hepatocytes were readily observed in livers from the high fructose-fed rats (Figure 3B). In contrast,only a few TUNEL-positive cells were identified in the livers of the S. sonchifolius treated rats similar to those in the control group (Figure 3A and C). Apoptotic index revealed a threefold reduction of apoptosis in S. sonchifolius fed rats (Figure 3E)(P < 0.05), highlighting the beneficial effects of S. sonchifolius roots in ameliorating cellular damage in the liver. However, the treatment with fenofibrate showed significant apoptosis (P < 0.05)in concordance with the high level of AST observed in this group(Figure 3D).
3.5. Effects of S. sonchifolius roots in liver antioxidant capacity
The effects of fructose and S. sonchifolius roots administration on the CAT, SOD, GPx and GST activity in the liver were summarized in Table 2. Fructose caused a significant increase in the activities of all analyzed enzymes in high fructose-fed rats, when compared to the control group (P < 0.05). S. sonchifolius treatment caused a significant decrease in the activities of CAT, SOD, GST and GPx in comparison to the high fructose group.
4. Discussion
In the present study, we provide information regarding the beneficial action of S. sonchifolius roots on hepatic damage and the redox imbalance in the fatty liver of rats administered with high fructose.
Several studies have shown that the consumption of enriched fructose, sucrose or fat diets leads to profound metabolic alterations in the liver, with structural and functional consequences[1,2]. The oxidative stress plays an important role in the establishment of these alterations, determining NAFLD progression. Different studies have also shown that mitochondrial dysfunction-related lipid changes affect the respiratory chain activity and the oxidation process generating a pro-oxidant environment[4,5]. Moreover, enhanced mitochondrial ROS production in metabolic excess can also alter both the structure and function of proteins, modifying mitochondrial permeability and promoting the release of pro-apoptotic factors which in turn increase tissue damage[3]. These data suggest that restoring redox state could be a possible therapeutic target to improve liver alterations[6]. Our results showed that administration of S. sonchifolius roots, as dietary supplement, significantly reduced increased plasma and hepatic lipid accumulation in rats fed with high fructose diet. Moreover, the MDA in liver, a secondary product, during liver lipid peroxidation[23]was also decreased in these animals. Similar effects on metabolic and oxidative status were observed when high fructose-fed rats were supplemented with quinoa seeds[7]or oligofructose[29]. Interestingly, S. sonchifolius treatment was also efficient to recover the GSH levels and the antioxidant enzymes activities even when the animals continued feeding high-fructose diet. Moreover, the decreased liver antioxidant activity observed in our study after S. sonchifolius treatment could be explained by the alleviation of oxidative stress and consequently-less stimulation of antioxidant defenses in this organ as was suggested for quinoa seed ingestion[7]. Previous studies in our laboratory indicate that the administration of S. sonchifolius roots to diabetic rats produced a significant decrease in MDA levels and SOD and CAT activities in the liver and kidney of diabetic rats, however,GPx and GSH levels increased[30]. These results are consistent with the findings of Jarukamjorn et al[31], which showed an increase in mRNA expression and enzyme activities of SOD, CAT and GPx in rats fed a diet high in fat and fructose, suggesting an attempt by the antioxidant system to attenuate the oxidative stress generated by fructose in the liver. A similar protective effect in the liver was described for metformin and resveratrol[32]. However, in our study,we observed that treatment with fenofibrate not only does not improve the oxidative status of animals supplemented with fructose,but it also intensifies the degree of oxidative stress by increasing the levels of MDA and antioxidant enzymes. In agreement with our results, some studies have shown a detrimental effect of the use of fenofibrate[33,34].

Table 2. Antioxidant enzyme activities.
On the other hand, it has been shown that exposure to toxic nutrient levels may induce mitochondrial fragmentation and even hepatocyte apoptosis[35]. In our study, the mitochondrial structure of high fructose-fed rats was improved significantly after S. sonchifolius treatment. In this sense, S. sonchifolius roots could prevent the progression of NAFLD ameliorating altered mitochondrial structure and dynamics, and thus avoiding tissue damage.
The antioxidant activity of S. sonchifolius roots has been associated with the presence of phenolic compounds: caffeic acid, chlorogenic acid (3-caffeoylquinic acid and 3,5-dicaffeoylquinic acid),protocatechuic acid and coumaric acid as well as the amino acid tryptophan[11,12]. These compounds could act as radical scavengers and terminate peroxidation chain reactions, restoring the redox balance and preventing liver damage in treated high fructose-fed rats[6]. This fact could also explain the decrease in the SOD, CAT and GPx activities in the liver of animals treated with S. sonchifolius flour. A recent study has shown the protective influence of caffeic and ferulic acids on the metabolic syndrome induced by the high fructose diet[36].
On the other hand, although the participation of FOS as antioxidant is uncertain, it has been suggested that changes in the gut microbiota,stimulating the growth of probiotic bacteria, exert actions that improve the oxidative damage through communication gut-brain axis[37]. Probably, peptide hormones released from intestinal L cells following FOS consumption, could also be involved in the antioxidant effect of S. sonchifolius roots by suppressing hepatic gluconeogenesis, hypolipidemic effect and antioxidant actions in tissues, as was suggested previously[17,30]. In view of the above,the main phytochemical components of the root FOS and phenolic compounds could act together to improve fatty liver induced by high fructose feeding.
In conclusion, this study has demonstrated that S. sonchifolius roots ameliorate metabolic alterations induced by high fructose feeding in an experimental model of metabolic syndrome in rats. S. sonchifolius treatment improved body weight gain and reduced plasma triglycerides as well as AST and ALT enzyme activities. In addition,S. sonchifolius roots significantly decreased both hepatic steatosis and ROS generation, avoiding mitochondrial damage and reducing cell death by apoptosis in high fructose fed animals. In conclusion, S.sonchifolius has a beneficial effect on oxidative stress and liver injury and therefore can prevent NAFLD progression.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
This research was supported by PIP 2015 (no. 183) (CONICET Argentina), PICT 2013 (no. 1949), PICT-2017 (no. 3941) (ANPCyT,Argentina) and PIUNT2018 D619 (SCAIT-UNT, Argentina), grants to SSS and SMH. MNA received CONICET fellowship.
 Asian Pacific Journal of Tropical Biomedicine2019年9期
Asian Pacific Journal of Tropical Biomedicine2019年9期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Determining endemic values of cutaneous leishmaniasis in Iranian Fars province by retrospectively detected clusters and receiver operating characteristic curve analysis
- Pyrrolidine dithiocarbamate and saxagliptin ameliorate ulcerative colitis in rats
- Antidiabetic effect of Opuntia dillenii seed oil on streptozotocin-induced diabetic rats
- Nrf2-inducing and HMG-CoA reductase inhibitory activities of a polyphenol-rich fraction of Guazuma ulmifolia leaves
- Phytochemical analysis and antibacterial activities of Eleutherine bulbosa (Mill.) Urb.extract against Vibrio parahaemolyticus
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
