不同氧化硅前驱体熔盐反应制备莫来石晶须
马雪冬 韩霁昌 杜 炜 王 伟*,,3
(1长安大学环境科学与工程学院,旱区地下水文与生态效应教育部重点实验室,西安 710054)
(2国土资源部退化及未利用土地整治工程重点实验室,西安 710075)
(3长安大学,陕西省土地整治重点实验室,西安 710054)
0 Introduction
Mullite is a strong candidate material for advanced structural applications at high temperature,because of its low thermal expansion,low thermal conductivity,high temperature creep resistance,good chemical and thermal stability[1].Mullite is the only stable compound in the Al2O3-SiO2system at atmosphere pressure,and it is a peritectic phase,but under certain conditions it can solidify metastable without prior alumina nucleation.The crystal structure of mullite is orthorhombic and is generally viewed as a defect from sillimanite,Al2O3.SiO2.The mullite structure consists of chains of edge-sharing AlO6octahedra running parallel to the caxis.These chains are cross-linked by alternating(Si,Al)O4tetrahedra forming chains,which also run parallel to the c-axis[2].Each octahedron shares two oxygen atoms,along an edge,with the octahedron just above it.And the tetrahedra share corner atoms in both the ab-plane (forming the double-chain)and the c-axis. Because the tetrahedrally coordinated aluminium and silicon are no longer present in the ratio of 1∶1,these sites necessarily become chemically disordered.Furthermore,crystal structure analysis have identified the presence of a second tetrahedral site-displaced from the original by approximately 0.13 nm,whch is presumably occupied by cations that have lost bridging O atoms[3].Ghate et al.firstly reported kinetics of mullite about densification and grain growth[4].They assumed that diffusion of Si4+controlled densification and grain growth process.Some research literatures revealthatthe needle-like shape is common to mullite formed in the presence of a liquid,whereas sintering of Al2O3-SiO2compounds in the absence of liquid cerated aggregates or agglomerations of mullite.In the case of acicular grain growth,mullite whiskers grow in the c-axis direction and are bounded by (110)surfaces.It is believed that the prismatic (110)planes have a lower surface energy than c-axis growth,and thus,mullite grains grow in the[001]direction for thermodynamic reasons[5].
Ceramic whiskers are commonly used as reinforcements in metal matrix composites(MMCS)and ceramic matrix composites (CMCS). For toughening of MMCS,it is required that high stresses are needed to fracture fibers at the tip of composite cracks and that the stress concentrations at the crack tips are as low as possible.In the whisker reinforced composites,high whisker stiffness implies high modulus at low temperature,which means both high modulusand high creep resistance undertimedependent deformation conditions at high temperature[6].Mullite whiskers have unique properties which result from their near-perfect structure,and it can be used to improve the mechanicalstrength,the creep resistance,chemical stability and thermal properties[7-8].Numerous methods have been developed to produce mullite whiskers[9-12],and most of them are expensive.Wang et al.synthesized CeO2-doped mullite whiskers using sol-gel process[13],and the mullitization activation energies calculated based on non-isothermal differential scanning calorimetry (DSC)are 473 and 722 kJ.mol-1for the 2%(n/n)CeO2-doped and undoped samples,respectively.Kong et al.[14-15]obtained the anisotropic microstructure of mullite ceramics by high-energy ball milling method.Wang et al.[16-17]prepared mulllite whiskers with diameters ranging from 30 to 150 nm and lengths of over several microns through molten salts reactions.Mullite whiskers were also obtained by means of the thermal decomposition of natural colorless topaz doped with rare earth oxides[18].Molten salt reaction has been employed to synthesis ceramic powders because itdecreases reaction temperature and gives powders of homogeneous morphology[19-20]. Molten salts provide liquid environment in which the nucleation and growth of grains are dependent on the dissolution of chemical reagents in the molten flux.Yang et al.prepared highly ordered mullite nanowhiskers using B2O3-doped molten salt synthesis method,and the reaction mechanism is attributed to local concentration gradient[21].Zhu et al.studied the mullite growth mechanism using aluminum sulfate and silica as raw materials in molten sodium sulfate by the differential scanning calorimetry[22].Zhang et al.synthesized mullite whiskers with Al2(SO4)3and Al(OH)3as alumina precursors by molten salt synthesis,and experimental result indicate that amorphous Al2O3is beneficial for the formation of mullite[23].When amorphous Al2O3is produced by the decomposition reaction of Al2(SO4)3,the total reaction processes are comprehensive and complicate paths included solid-liquid-gas phase transformation,the thermal pyrolysis mechanism of aluminum sulfate have been researched somewhat,but the decomposition reaction hasn′t been extensively investigated from the thermodynamic and kinetic view.
Herein,mullite whiskers were prepared using kieselguhr or silica fume as silica precursors in molten salt system,and α-Al2O3was also obtained in Na2SO4flux through decomposition reaction of aluminum sulfate without silica involved in comprehensive reactions.Silica fume emerges as a byproduct from the melting ferrosilicon alloy,and this solid waste presents serious problems of storing and environmental pollution.Kieselguhr is a kind of siliceous sedimentary rock originated from ancient biologicalcells,which can be used asthermal insulation materials,filler sand catalyst carriers in industry field. Although chemical composition,morphology and structure have great differences,SiO2is the mainly ingredients forsilica fume and kieselguhr.Influence on the resulting product can be shown through differentsilica sources selecting,interesting for this contrast,the same product(mullite whiskers)was fabricated in our experiments.Decomposition kinetics of aluminum sulfate was explored using dynamic thermal analysis at different heating rates(β)of 5,10 and 15 K.min-1,respectively.Moreover,amorphous Al2O3can be transformed into mullite phase because mullite is more stable state for Al2O3-SiO2eutectic phase while silica precursors are used as raw materials.
1 Experimental
1.1 Preparation
Selected silica fume (Xi′an Linyuan silica fume Ltd.)and kieselguhr(Tianjin Bodi Chemical Co.,Ltd.)were used as the silica precursors to fabricate mullite whiskers,and physical parameters of kieselguhr and silica fume are listed in Table 1.Al2(SO4)3and Na2SO4were weighed accurately according to the molar ratio of SiO2,Al2(SO4)3and Na2SO4(nSiO2∶nAl2O3∶nNa2SO4=6∶1∶10).The mixture was grinded in a ceramic mortar for 20 minutes,and then heated to final temperature of 800℃ (or 900℃)for 2 h.The samples were washed with hot water to remove sodium sulfate (non reacting solvent).Then,white mullite powders were obtained after filtration,washed and dried.
Amorphous alumina,composition ofmullite(3Al2O3.2SiO2),came from the pyrolysis of Al2(SO4)3in this experiment.In order to investigate the influence of aluminum sulfate,the product of Al2(SO4)3decomposition was obtained in molten salt system,and the preparation process is described as follows:Firstly,Al2(SO4)3and Na2SO4were weighed accurately according to molar ratio of Al2(SO4)3to Na2SO4being 1∶5,andthe mixed powder(Al2(SO4)3+Na2SO4)was grinded in a ceramic mortar for 20 min.Subsequently,the mixed powders was placed in the bottom of alumina crucible and slowly heated to 900℃for 2 h and then cooled down to room temperature in a furnace.Finally,the product was obtained after boiling in distilled water,filtration and drying.

Table 1 Physical parameters of kieselguhr and silica fume
1.2 Characterization
Crystalline phase of the sample was examined by using X-ray diffractometer(XRD,D/MAX-RA),with monochromated Cu Kα radiation (λ=0.154 18 nm)operating at 40 kV and 30 mA,scanning range(2θ)from 10°to 80°.Scanning electronic microscope(SEM,S-4800)coupled with energy dispersive spectrometer(EDS,INCA-350)was used to characterize and analyze the microstructure,which was operated at 20 kV and 20 mA.High-resolution TEM (HRTEM)and selected area electron diffraction (SAED)were conducted on the JEOL-2100F to characterize the microstructures of the whiskers.The sizes and distributions of particles were analyzed usingMalvern laserparticle size analyzer (Mastersizer 2000).The decomposition of Al2(SO4)3was performed in a simultaneous thermogravimetry and differential scanning calorimetry(TGDSC)(STA 449F5,Netzsch,Germany).About 15 mg of sample was taken in a platinum crucible and heated from 300 to 1 273 K in nitrogen environment with a constant flow rate of 40 mL.min-1(with 99.99%purity)at different heating rates(β)being 5,10 and 15 K.min-1.
2 Results and discussion
2.1 XRD analysis
Fig.1 shows XRD patterns of final products using silica fume(or kieselguhr)as raw materials for molten salt synthesis.Mullite phase has been formed in the samples heated at 800 and 900℃,since there exist several strong peaks at 2θ=16.52°,26.32°,31.08°,33.36°,35.36°,40.92°and 49.52°,which are attributed to the(111),(210),(001),(220),(111),(121)and(311)planes of the orthorhombic type(mullite)phase,respectively[24].A series of peaks are showed in Fig.1a at 28.03°,38.08°,39.63°,40.72°and 46.91°,which can be ascribed to the(211),(202),(022),(220)and(131)planes of Al2SiO5,respectively.In addition,some weak peaks in Fig.1(b)were also checked out,which result from the impurities of kieselguhr.

Fig.1 XRD patterns of mullite powders synthesized by silica fume(a)and kieselguhr(b)
2.2 SEM analysis
TheSEM photographsandEDSspectraof prepared mullite whiskers are shown in Fig.2.It can be seen that the morphologies of samples (Fig.2a~b)synthesized at 800℃are not perfect in comparison with the samples (Fig.2c,2e)synthesized at 900℃.No matter which SiO2-containing material is used,mullite whiskers will grow better at higher temperatures.Although the chemical composition of silica fume and kieselguhr is different,the final product is mullite(3Al2O3.2SiO2)whiskers.It is well known that edge-shared AlO6octahedral chains align in the cdirection and are crosslinked by corner-shared(Si,Al)O4tetrahedra in unit cell of mullite crystal[25].And the mullite crystal growth may be faster in crystallographic direction parallelto the c-axis than in other directions,resulting in a high degree of orientation.The whiskers that prepared at 800 and 900℃both possess nanometer-sized diameters and micrometersized lengths.The corresponding EDS spectra of the whiskers fabricated from silica fume in Fig.2(d)and kieselguhr in Fig.2(f)indicate that the sample consist of Al,Si,O,Na,S and C.Moreover,the quantitative analysis shows that their average atomic ratio(nAl∶nSi∶nO=6 ∶2 ∶13)approximates the stoichiometric ratio of mullite.The signals of elemental Na,S and C came from molten saltsmedia (Na2SO4)and carbonconductive tape for sample uploading.
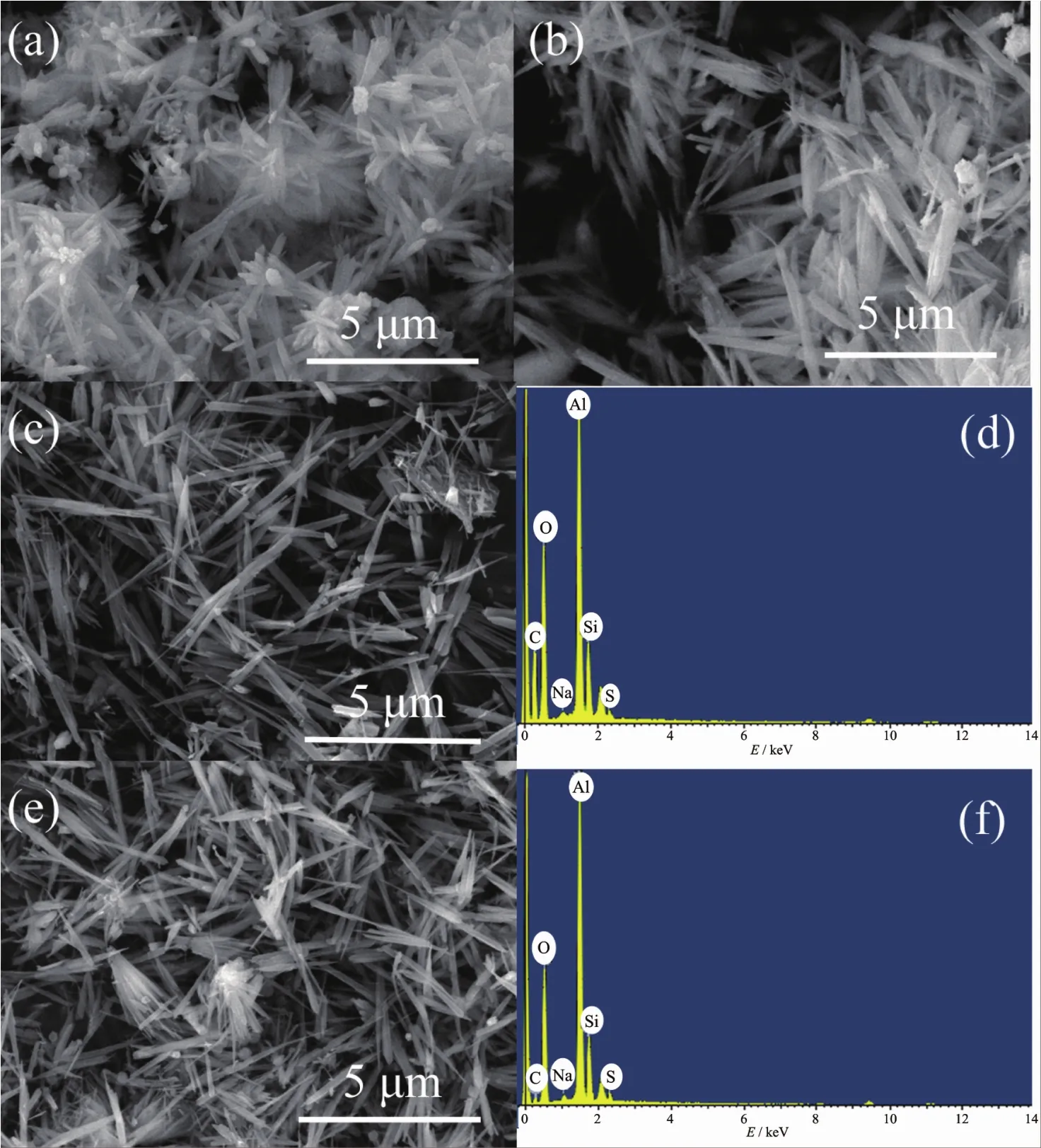
Fig.2 SEM micrographs and EDS spectra of the mullite whiskers prepared from silica fume at 800℃(a),kieselguhr at 800℃(b),silica fume at 900℃(c~d)and kieselguhr at 900℃(e~f)
2.3 TEM analysis
Fig.3 shows TEM image,HRTEM image and SAED pattern of the samples prepared from silica fume and kieselguhr.It can be seen that the average diameter of mullite whiskers was in a range of 50~150 nm and the length was over several microns.The selected-area electron diffraction patterns from the mullite whiskers in Fig.3(a,c)can be indexed to the orthorhombic structure.HRTEM image showed that the spacing was 0.539 nm,in accordance with(110)crystal plane spacing of mullite,which indicates that the mullite crystal grows along c-axis firstly and develops into fibrous microstructure[26].

Fig.3 TEM images(left)and HRTEM images(right)of mullite whiskers prepared from silica fume(a~b)and kieselguhr(c~d)at 900℃
2.4 TG-DSC,XRD,SEM and particle size distributions analysis for aluminum sulfate decomposition
It can be seen that mullite whiskers formed in Al2(SO4)3+Na2SO4molten salts system using Kieselguhr or silica fume as silica precursors.If silica is not existed in molten sulfate flux,what will be happened in the system?According to some literatures[23],the decomposition reaction of Al2(SO4)3take place firstly and amorphous γ-Al2O3forms above 700 ℃ .In the second step,the γ-Al2O3subsequently reacted with silica and transformed into other product.Therefore,two interesting questions are how the Al2(SO4)3is decomposed in sodium sulfate (non-reactive solvent)and how to analyze the resulting product to confirm this decomposition reaction mechanism.
Fig.4(a)shows the TG-DSC curve of aluminum sulfate octadecahydrate pyrolysis.It can be seen that the weight loss mainly takes place in the following three main ranges.The first weight loss (23.59%)is attributed to the dissociation of absorbed water from 75 to 150℃,and one endothermic peak appeared in theDSC curve simultaneously.Thesecond one occured between 150 and 400℃and the relevant weight loss ratio was 16.56%,which is ascribed to the removing of intramolecular crystal water.The third one(Weight loss:37.60%)happened in a temperature range from 700 to 900℃,corresponding to an endothermic peak on the DSC curve,indicating that the decomposition of aluminum sulfate[23].
The synthesized product is characterized by X-ray diffraction (XRD)to determine phase.As can be seen from Fig.4(b),all of the diffraction peaks can be assigned to rhombohedral α-Al2O3(PDF No.46-1212).No residue or contaminant has been detected,indicating the high purity of the sample[27].Fig.4(c)presents the SEM image of α-Al2O3particles,from which the α-Al2O3is seen to be several hundred nanometers in diameter.Fig.4(d)shows the size distribution of assynthesized α-Al2O3particles.The average diameter was found to be 400~500 nm.Sodium sulfate is a kind of high temperature solvent medium,which is beneficial for ion diffusion and stable phase growth.Amorphous alumina from Al2(SO4)3decomposition transformed into α-Al2O3due to the lack of silica precursor.

Fig.4 (a)TG-DSC curves of aluminum sulfate octadecahydrate decomposition;(b)XRD pattern of as-synthesized α-Al2O3powders;(c)SEM morphology of as-synthesized α-Al2O3powders;(d)Particle size distributions for α-Al2O3powders
2.5 TEM analysis of as-synthesized α-Al2O3powder
Fig.5 shows TEM image,HRTEM image and SAED pattern of the as-synthesized α-Al2O3powder.It can be seen that the width of this powder was in a range of 400~600 nm and the length was over one micron.The selected-area electron diffraction patterns in Fig.5(a)can be indexed to the rhombohedral crystal system.HRTEM image shows that the crystal plane spacing is 0.237 9 nm,in accordance with(110)crystal plane spacing of α-Al2O3,which confirms that α-Al2O3powder is formed in Al2(SO4)3-Na2SO4molten salt system.

Fig.5 (a)TEM image of individual α-Al2O3powder after ultrasonic treatment;(b)HRTEM image of the powder
2.6 Thermodynamic analysis
The utilization ofaluminum sulfate thermal pyrolysis to prepare mullite whiskers has been researched somewhat[28],but the decomposition process hasn′t intensively been investigated from thermodynamic and kinetics point of view.Almost all the literatures think that amorphous γ-Al2O3firstly forms in the liquid after decomposition of Al2(SO4)3according to reaction(2)[29-30].But α-Al2O3also forms as confirmed by the above mentioned characterization,indicating that α-Al2O3phase derives from the decomposition reaction (1).According to traditional knowledge,the higher temperature is necessary for α-Al2O3phase formation[31].Thus,we try to explain α-Al2O3formation through the thermal pyrolysis of Al2(SO4)3in high temperature solventmedium (Na2SO4)from the thermodynamic view.

The decomposition of Al2(SO4)3is controlled by pressure and temperature.For the reaction path(1)and (2),their free energy changes with temperature are shown in Fig.6,which is derived from ΔG=ΔG⊖+ΔnRTln(p/p⊖)with ΔG⊖=ΔH⊖-TΔS⊖.As pressure and temperature are two interrelated variables about the reaction free energy change,the effect of pressure on the reaction free energy change is weak and the reaction free energy change(ΔG)can be calculated in p=p⊖condition.Therefore,when p=p⊖(constant pressure),ΔG=ΔG⊖=ΔH⊖-TΔS⊖with ΔH⊖=ΔH⊖f(α-Al2O3or γ-Al2O3)+3ΔH⊖f(SO3)-ΔH⊖
f(Al2(SO4)3)and ΔS⊖=S⊖
(α-Al2O3or γ-Al2O3)+3S⊖(SO3)-S⊖(Al2(SO4)3).The data of ΔH⊖and S⊖are obtained from the reference[32].The
ffree energy changes(ΔG)of reaction(1)and reaction(2)with temperature are shown in Fig.6.The reaction path (1)could not happen in the lower temperature range (G>0),and the reaction would process vigorously when the temperature is up to 993 K(ΔG<0). With the similar changing tendency, the equilibrium point of reaction path(2)is 1 023 K,and this chemical equilibrium could exist according to positive direction at higher temperature range(>1 023 K).It is well known that alumina exists in various stable and metastable phases[33],such as alumina hydrate(Al2O3.nH2O),transition state alumina(β-Al2O3, θ-Al2O3,η-Al2O3,γ-Al2O3,etc.)and stable phase alumina(α-Al2O3).Among them,the firsttwo typesare metastable phases.The metastable phases(β-Al2O3,θ-Al2O3,η-Al2O3,γ-Al2O3,etc.)can be converted into α-Al2O3by high temperature sintering irreversibly.

Fig.6 Changes of free energy ΔG for reactions(1)and(2)depending on temperature
It can been seen that γ-Al2O3is a kind of metastable phase for alumina,in addition,γ-Al2O3could be transformed into more stable mullite phase when some silica sources coexist in molten salt system.The reaction of mullite formation can be described as reaction(3).

Where γ-Al2O3comes from the decomposition of Al2(SO4)3,and γ-Al2O3subsequently transforms into mullite through Eq.(3),which is a commonly accepted mechanism of mullite formation.The mixture of γ-Al2O3and SiO2is converted in a liquid molten salts environment,especially when they are intrinsic mixed in a molecular level.The reaction kinetic need not be considered because the diffusion paths of ingredient is so fast through the L-S growth mechanism[34].Free energy change of reaction (3)reveal that mullite formation is a spontaneous process(Fig.7).Therefore,reaction(1),(2)and(3)should be considered together,and the decomposition of aluminum sulfate is the most important controlling step.

Fig.7 Change of free energy ΔG for reactions(3)depending on temperature
In the Al2(SO4)3-Na2SO4molten salts,aluminum sulfate decomposition and transformation into γ-Al2O3determine the growth rate of mullite whiskers.The γ-Al2O3is more active in the molten salts system because the combination reaction (3)involves mullite formation.Atthe same time,mullite whiskers formation reaction can consume γ-Al2O3and SiO2,which accelerate the progress of reaction (2).The mullite whiskers growth mechanism can be described asfollows:aluminum cationsfirstly exsitwhen temperature increase to the eutectic point(Al2(SO4)3-Na2SO4),and then amorphous γ-Al2O3is produced by the decomposition reaction of Al2(SO4)3.Subsequently,SiO2coming from silica fume or kieselguhr can be dissolved in sulfate flux and the mullite nuclei are formed in this process.Then,mullite crystal grow quickly along the specific prismatic planes because it is the lowest surface energy barrier.The silica is consumed continuously in accordance with the abovementioned combination reaction(3).
It is well known that the diffusion of Si4+controlls densification and grain growth process of mullite whiskers[4].Some literatures reveal that the needle-like shape is common to mullite formed in the presence of a liquid environment,and molten salts system provide a high ionic conductivity environment for crystal growth.Mullite whiskers growth requires the supply of silica species.In the absence of silica taking part in reactions,α-Al2O3formsasthe productofthe decomposition of Al2(SO4)3,while silica fume(or kieselguhr)is used as the starting reactant,then reaction(3)takes place in molten salts system,which eventually affects the decomposition of aluminum sulfate and the growth of mullite whiskers.
2.7 Kinetic analysis


Fig.8 Plot of DTG vs T(a),ln(β/Tp2)vs 1/Tp(b)for Al2(SO4)3decomposition reaction
In non-isothermal decomposition study,kinetic parameters are easily calculated from thermo-kinetic analysis.Among the integral methods,Kissinger-Akahira-Suno method gives more accurate apparent activation energy(Ea)as compared to other methods[35-36].Therefore,different heating rates(β)of 5,10 and 15 K.min-1were selected to measure in this experiment and the apparent activation energy of Al2(SO4)3decomposition reaction wascalculated usingKissinger-Akahira-Suno method.The equation is expressed as follows:Where Eais the apparent activation energy and R is the gas constant,T denotes temperature,Tpis the peak temperature of DTG curves(Fig.8a)and β is the heating rate(β=dT/dt).Peak temperatures(Tp)appeared at 1 116,1 141 and 1 159 K,which were ascribed to β=5,10 and 15 K.min-1,respectively.With the different heating rates,the plots(Fig.8b)of ln(β/Tp2)against 1/Tpgave a straight line,and the slope of the corresponding line gave the value of apparent activation energy(Table 2).The apparent activation energy of Al2(SO4)3decomposition reaction is 257.2 kJ.mol-1.

Table 2 Decomposition peak temperature and apparent activation energy for main decomposition of Al2(SO4)3
3 Conclusions
Mullite whiskers had been prepared by molten salt synthesis,and α-Al2O3was formed in sulfate flux without silica species taking part in molten salt reactions.Aluminum sulfate decomposition and mullite formation reaction pathshad been studied with thermodynamic and kinetic view.The main conclusions are summarized as follows:
(1)Mullite whiskers are single crystalline and possess uniform morphology with 200~400 nm in diameter and several microns in length.HRTEM image reveals that the interplanar spacing of 0.539 nm is in accordance with the spacing of the(110)crystal plane of mullite.
(2)According to the thermodynamic calculation,aluminum sulfate decomposition reaction is the most important controlling step,and α-Al2O3form in sulfate flux at 720℃without silica species introducing in raw materials.
(3)The apparent activation energy(Ea)of Al2(SO4)3decomposition is 257.2 kJ.mol-1which is calculated through Kissinger-Akahira-Suno method with various heating rates(β=5,10 and 15 K.min-1).

