A New Method to Improve the Product Stability and Performance of Soap-based Cleanser at Two Different Temperatures
Han Qiao, Feng Chunbo
Shanghai Jahwa United Co., Ltd., China
Abstract
Key words
soap-based; facial cleanser; stability; non-ionic emulsifier; thermotropic liquid crystal
Background
Facial cleanser has been widely accepted by consumers as a frequently-used cleaning product.The purpose of skin cleansing is to remove the dirt staying on the skin to keep skin clean and healthy.The dirt generated from the body should be cleaned away in time, such as sebum secreted by the skin, sweat, and dead stratum corneum cells.And also the dirt from outside, like dust, various kinds of microorganisms, and cosmetic residues, etc.[1]
The sebum on skin surface consists of ceramide and cholesterol that derived from intercellular lipids of the stratum corneum cells, and the lipids derived from sebaceous glands including squalene, triglyceride, wax, and free fatty acid.These lipids form a sebum film on skin surface, protecting skin from external stimuli and keeping the skin hydrated and moisturized.However, the secreted sebum will attach to dust from outside and be decomposed by Staphylococcus epidermidis over time.After been oxidized and deteriorated, the sebum turns into the dirt that should be washed away.Otherwise, it could be turned into lipid peroxides that irritating to the skin if further gets exposed to UV light.[1,2]
Sweat secreted from the sweat glands, with the evaporation of water, remains on the surface of skin as salt or urea, which causes irritating to the skin.The stratum corneum cells that protect human body from external stimuli are eventually peeled off with metabolism as dirt.[1,2]If the stratum corneum cells stay on skin with sebum and dust for long time, it will promote bacterial growth and even cause skin diseases.Thus, it is necessary to clean properly.
The lipids from sebaceous glands could be recovered in short time with temporarily removal by cleansing products.In contrast, the lipids from stratum corneum take long time to recover.Moreover, as the lipids derived from the stratum corneum are closely related to skin barrier function including water retention and adhesion of keratinocytes, it is desirable that skin cleanser does not permeate into the stratum corneum and dissolve the lipids.Skin moisture is maintained by natural moisturizing factors (NMF) such as free amino acids, pyrrolidone carboxylic acid (PCA), and lactate.In short, an ideal skin cleaner is designed to avoid the excessive removal of sebum membranes and NMF, while cleansing dirt and dust selectively[2].Cellon et al.have discussed dermatological problems caused by skin cleansing products, for instance, skin pH change, changing of skin microbial flora, stratum corneum dehydration, increase of transepidermal water loss (TEWL) and skin irritation.[3-5]It is important to consider the adverse effects of skin cleanser when designing formulas.
There are many kinds of facial cleaners on the market, which could be divided into emulsifying base, surfactant base and soap-based facial cleanser according to formula structure.The emulsified facial cleanser doesn't generate rich foam, which provides inadequate cleansing capability to consumer.Surfactant-based facial cleanser has creamy and rich foam, but the residue feel is poor after rinsing. Soapbased cleanser is one of the most popular types of facial cleanser on market.Compared with surfactant or emulsified based cleansers, soap-based cleanser is preferred for its pearlescent appearance, rich foam and excellent cleansing effect.In recent years, amino acid surfactant-based cleanser has attracted more and more attention for safety and mildness.Nevertheless, the growing of amino acid surfactant cleanser is limited by the high ingredient cost and difficulty to be thickened.[6]So far, it is mainly used in the high-end cleansing market, while soap-based facial cleanser remains the leading position in facial cleanser category in domestic market.
Therefore, it is meaningful for both cosmetic company and consumer to better improve product stability and performance of soap-based facial cleanser.Its poor product stability including water separation or cheesy texture at room temperature, viscosity dropping at high temperature and viscosity increasing at low temperature heavily impact consumer's usage.Many formulators made efforts to improve soap-based cleanser stability and consumer usage, mainly focused on 3 approaches to adjust the formula 1) the ratio of fatty acids; 2) saponification ratio; 3) adding anionic surfactants and suspension thickener.Until now, it is still difficult to balance formula stability and consumer's usage.
In this work, we developed a new method to improve high and low temperature stability by regulating the microstructure of soap-based cleanser.Meanwhile, product performance including mildness and tube extrusion performance at low temperature were demonstrated to be improved at the same time.
Methods and procedure
Formulation design and preparation
Viscosity, gelling point and pearlescent appearance of the soap are determined mainly by the mixture of fatty acid and potassium fatty acid.It forms a semi-solid appearance because the limited solubility of the mixture in water solution.A basic soap formula is included as benchmark formula with all attributes and parameters measurement for comparison.Detail formulation is given in Table 1.In the purpose of improving the stability at high and low temperatures and the usage of soap-based cleanser, while maintaining its excellent cleansing capability, this work explored following approaches to adjust the formula, including the optimization of the combination of fatty acids with different carbon chain lengths, introduction of non-ionic emulsifier to the system, and the reduction of alkaline (potassium hydroxide) in formula.Both optimized formula and benchmark formula are prepared following typical saponification reaction process.
The benchmark formula is formulated with higher ratio of short-chain fatty acids as the main soap-based formula system, including C12 acid and C14 acid.It was suspected that the secondary recrystallization of free short-chain fatty acids (C12 acid and C14 acid) caused the significant viscosity increase at low temperature.Thus, we increase the ratio of long-chain fatty acids, mainly C18 acids, in the optimized formula, which encourages the formation of crystal structure of long-chain fatty acids at low temperatures.Free short-chain fatty acids could found it difficult to insert into long-chain fatty acids crystal structure to form secondary recrystallization.With this change, the formula viscosity is supposed to be more stable at low temperature.Meanwhile, the adjustment might raise the gelling point of the system, which is conducive to the improvement of high temperature stability.
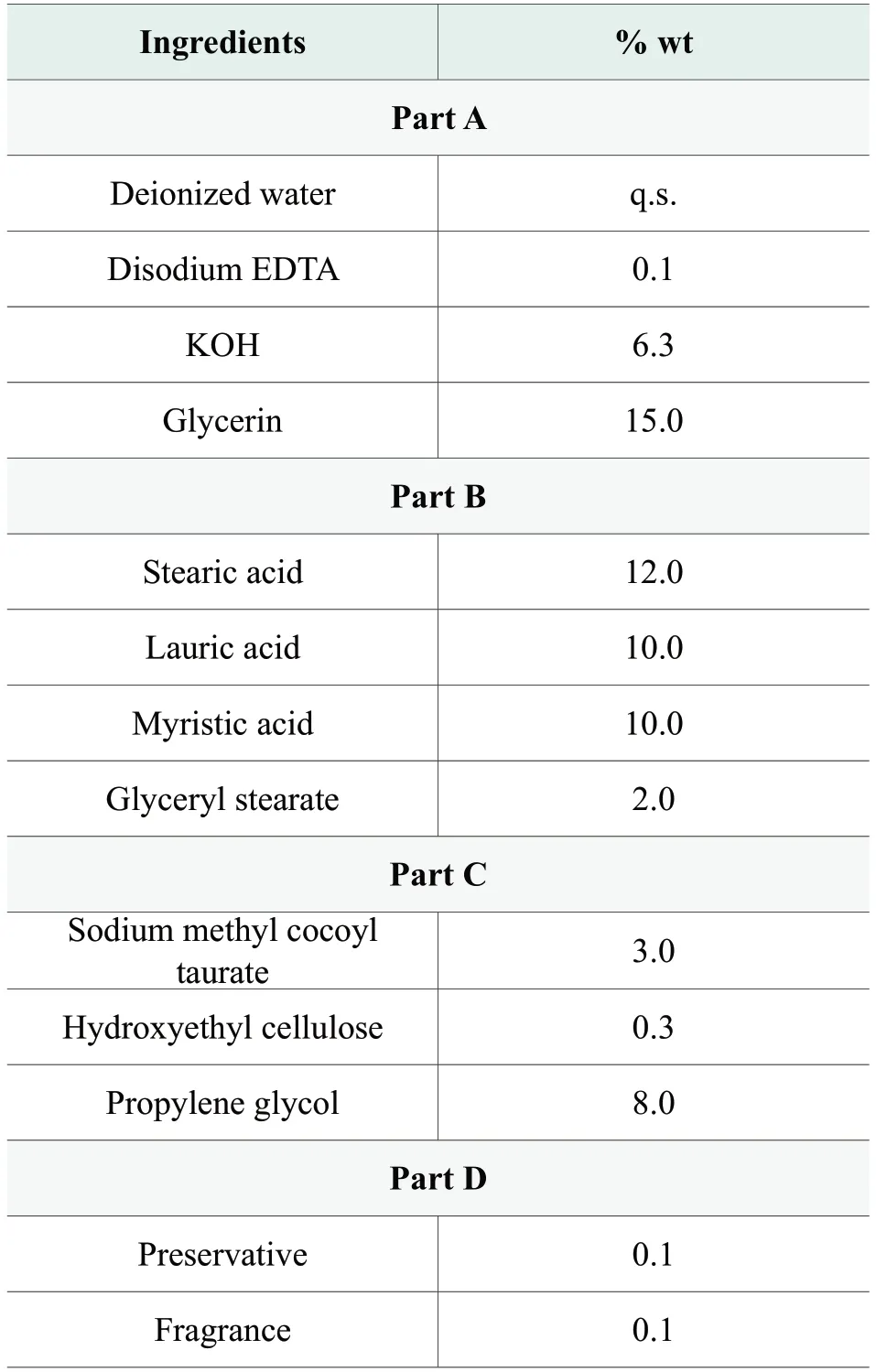
Table 1.Formulation of benchmark soap-based cleanser
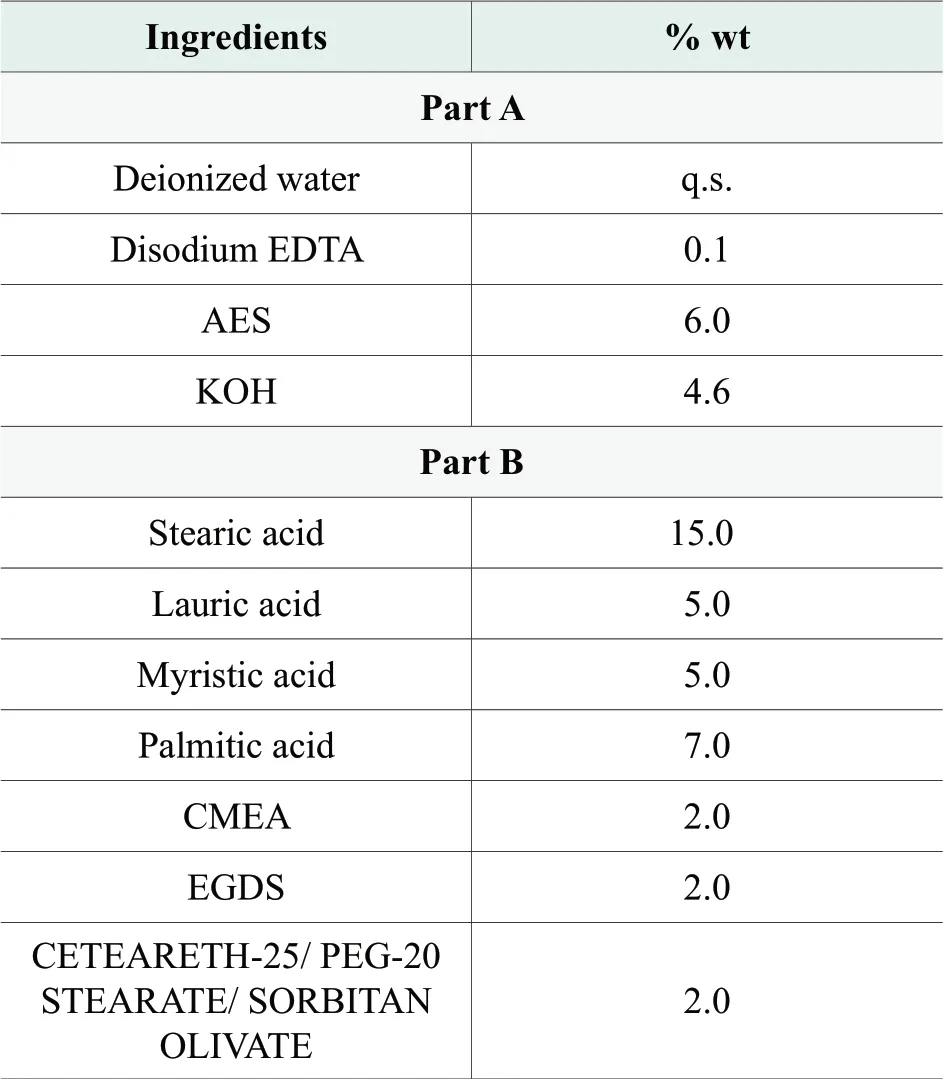
Table 2.Formulation of optimized soap-based cleanser
Ingredients% wt Part C Hydroxyethyl cellulose 0.3 Glycerin 15.0 Part D Preservative 0.1 Fragrance 0.1
With the purpose of relieving the skin dryness after washing, we reduced the saponification rate of soap system by decreasing the amount of potassium hydroxide added.It was believed that high PH of soap-based cleanser led to skin dehydration, and even caused skin disfunction and irritation.With incomplete saponification of the soap system, formula pH could be lowered down and the free fatty acids could act as skin moisturizing agent to improve the skin feel after rinsing.Furthermore, in order to better stabilize the free fatty acid left in the system, a nonionic emulsifier (CETEARETH-25/ PEG-20 STEARATE/ SORBITAN OLIVATE) was utilized to enhance the suspension of the free fatty acids.Final formulation is shown in Table 2.
Viscosity measurement
The thermal stability of two formulations was studied by placing in glass tubes and they were placed in 4 ℃, 25 ℃, 40 ℃ and 48 ℃ chamber.Their physical stability including viscosity was inspected for a period of 3 months at interval of one month.The viscosity of the soap cleanser samples were determined by using Brookfield viscometer (BROOKFIELD PV-S) set at different spindles and speeds.[7]
Microscopic observation
Microstructure of both benchmark formula and optimized formula were monitored along with t emperature change.A drop of each sample was placed on a microscope slide and then covered with a cover slip.The microstructure was then observed using polarized light microscope (OLYMPUS BX53) and heating platform (CAIKON CK-300).The images were obtained using a CCD camera with Digital Image Processing Software installed on a computer.[8]
Rheology evaluation
Rheological measurements for the viscoelastic properties by oscillatory measurements from 0.01 to 10 Hz were performed, for which the deformation was controlled to be in the linear region.Temperature in the measuring system was controlled to ± 0.1 ℃.
Tube extrusion test
Product extrusion performance was measured by a texture analyzer (TA.XT Plus).Both formulas were filled in soft tube (3 mm-calibe/30 g) to stimulate consumer daily use.Soft tubes were kept in 4 ℃ refrigerator for 48 hours before measuring.Product weight (formula and tube) was recorded before and after the test.As shown in Figure 1, a card slot was used to fix the tube during the squeezing of texture analyzer probe.The P1S probe was used.The probe pressed against the tube and traveled 1.5 cm.The recognition force is 1 g; probe travelling speed is 0.5 mm/s and 1mm/s after contact.The maximum force was used to characterize the easiness of formula extrusion in the tube.The reduction of product weight after the test is calculated, which also explained the extrusion performance of benchmark formula and optimized formula.

Figure 1.Texture analyzer (TA.XT Plus and P1S probe) to detect the softness of formula in tube at low temperature
Panel test
Texture and sensory properties are some of the key aspects influencing consumers' choice of personal care products.To evaluate the texture and sensory of two formulations, expert panel test was run with 30 trained volunteers aged from 20 to 45 through an arm test.Each formula was used on one arm with tap water.Volunteers had to rate on a scale of 1 to 10, 10 corresponding to the highest performance.Foaming speed, foam richness, rinsing feel etc.were evaluated after single use.To measure the product extrusion performance at low temperature, test samples were kept in 4 ℃ refrigerator for 48 hours before test.
Results and discussion
Viscosity-temperature correlation and microstructure of benchmark formula
The results of viscosity measurement show that viscosity of benchmark formula change significantly with temperature.The viscosity of 48 ℃ sample was measured by using spindle#3, setting at 20rpm, 60 s.The viscosity of 25 ℃ sample was measured by using spindle T95, setting at 12 rpm, 60 s.The viscosity of 4 ℃ sample was measured by using spindle T96, setting at 12 rpm, 60 s.Measurements were performed three times for each sample.Mean results with standard deviation are presented in Table 3.25 ℃ sample with mean viscosity values 275400mPa·s is easily manageable, while 48 ℃ sample is to fluid.We consider that viscosity values under 3000 mPa·s are not appropriate for a facial cleanser, because it flows very easily and can get into the eyes.4 ℃ sample with mean viscosity values 552500 mPa·s makes it very difficult to take the desired amount from the package tube.
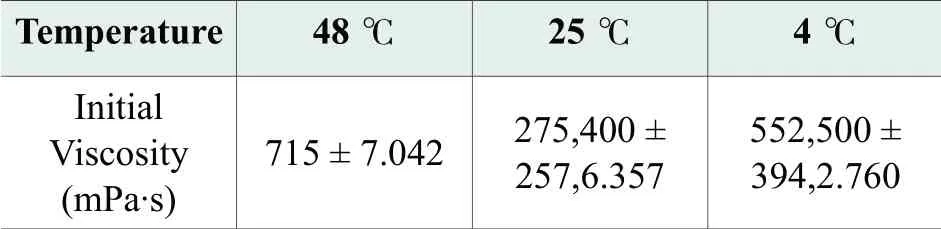
Table 3.Viscosities of benchmark formula at different temperatures
Polarized light microscope with fixed heating apparatus is used to monitor the change of formula microstructure from 4 ℃ to 80 ℃.Figure 2.shows the microstructure of the benchmark formula at 25 ℃ and 4 ℃.As indicated by the arrows in the picture, the crystallization structures become thicker and denser at 4 ℃, which suggests that free fatty acids in formula recrystallize at 4 ℃.The secondary recrystallization leads to viscosity increasing of the final product.As soap-based cleansers are generally packed in tubes, significant viscosity increase may affect product extrusion and performance.
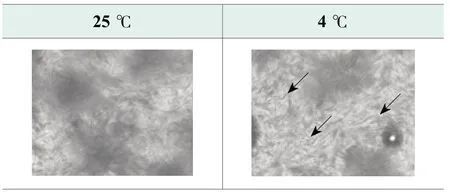
Figure 2.Photos of benchmark formula at 25 ℃ and 4 ℃ by polarized microscope
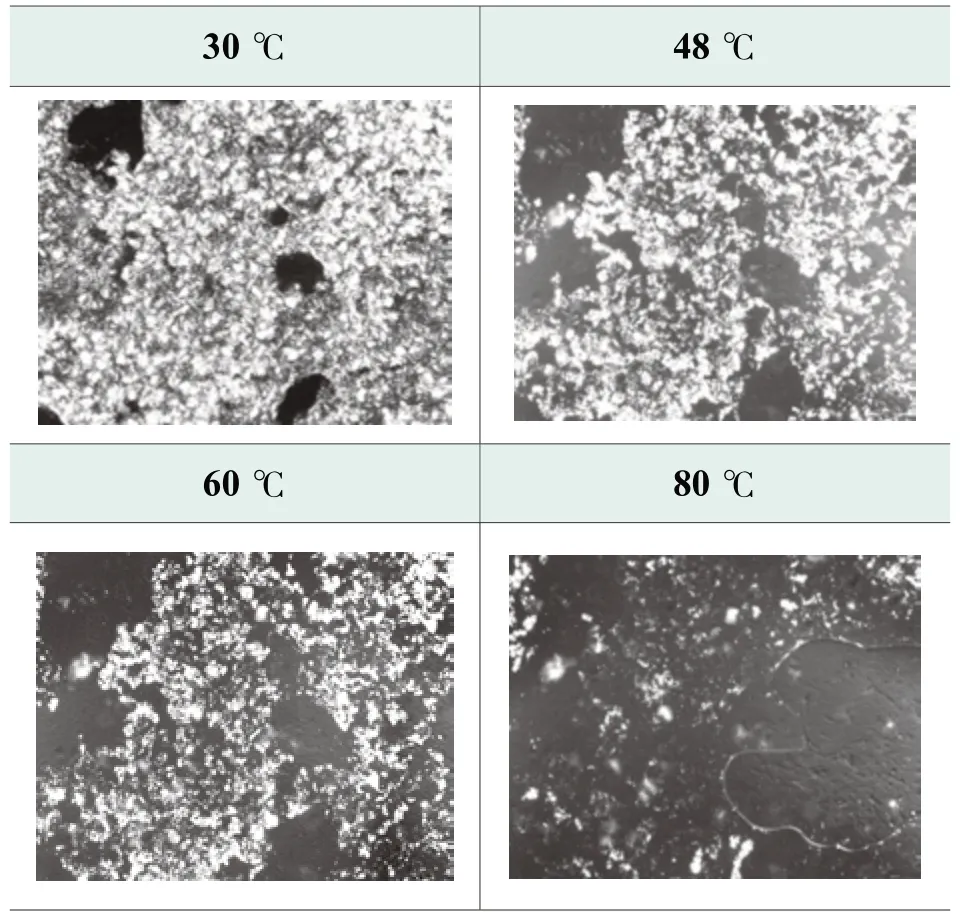
Figure 3.Photos of benchmark formula at high temperature by polarized microscope
The change of microstructure on the formula at high temperature range is monitored using polarized light microscope with heating platform.Figure 3.presents the microstructure change on benchmark formula from 30 ℃ to 80 ℃.Layered crystal is observed in microstructure which is mostly composed of potassium salt of fatty acids[8].The solubility of potassium salt of fatty acids increases along with the temperature.With temperature rising up to 60 ℃, layered crystals begin to disappear that corresponds to product viscosity dropping at high temperature.The insoluble fatty acids could even rise up to the surface and cause phase separation.Although the separation at high temperature could be solved by adding thickening agents as well, the product viscosity still drops significantly.Low viscosity may cause many product issues such as package leaking and eye irritation.
Viscosity-temperature correlation and microstructure of optimized formula
The ratio of long-chain fatty acids is increased in optimized formula by adding C16 acid and C18 acid, which helped to generate long-chain fatty acid crystal structure at higher temperature.This change inhibits the secondary recrystallization at low temperature which causes undesirable viscosity increasing of a proper soap cleanser.The addition of KOH is reduced to lower saponification value, the product pH is lowered down from 10.5 to 9.0 and stability might be improved.It is reported that lower pH improves the mildness of soap-based cleanser and reduces the product-induced skin barrier disfunction even skin irritation.[9]A non-ionic emulsifier (CETEARETH-25/ PEG-20 STEARATE/ SORBITAN OLIVATE) is introduced into the formula to stabilize unneutralized free fatty acids in system.
The results of viscosity measurement show that viscosity of optimized formula having a reduced variation of values over the temperature change.Measurements were performed three times for each sample.Mean results with standard deviation is given in Table 4.Soap cleanser must have a good consistency in order to facilitate consumer use, low enough to ensure a facile removal from the package and facile foaming on palm, but high enough to prevent from reaching the eyes.As we can see from Table 4, the optimized formula has greater viscosity values at both low and high temperatures.
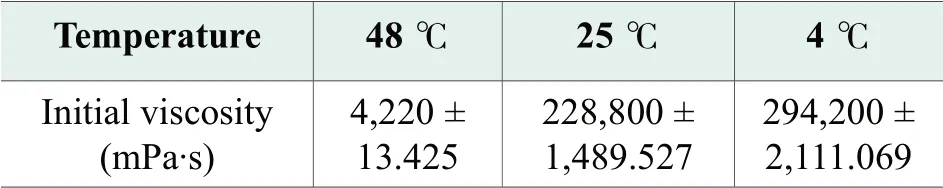
Table 4.Viscosities of optimized formula at different temperatures
The microstructure change at 4℃ confirms the improvement of viscosity-temperature correlation of optimized formula.There is no obvious secondary recrystallization occur at 4℃ as shown in Figure 4.The crystal structure shows better integrity compared to benchmark formula.
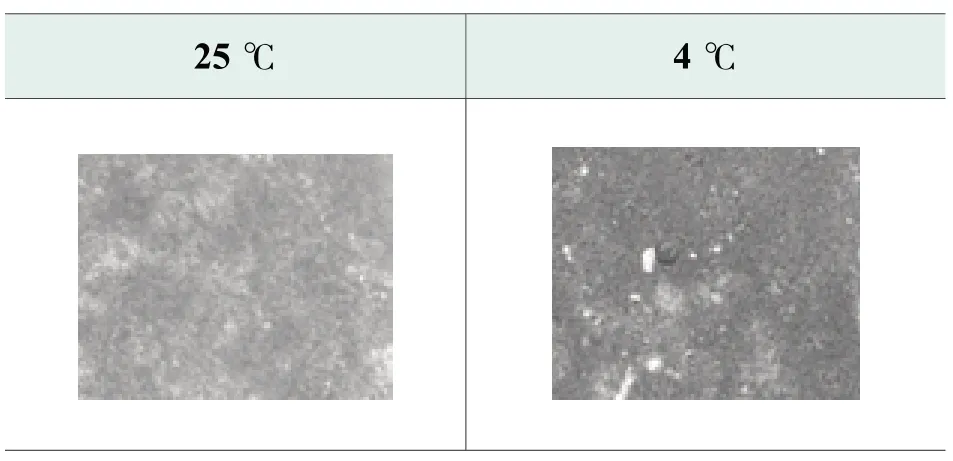
Figure 4. Photos of optimized formula at 25 ℃ and 4 ℃ by polarized microscope
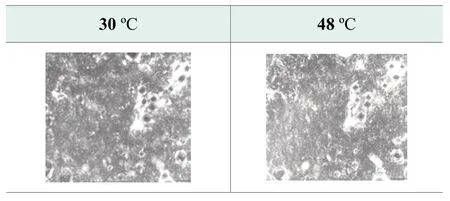
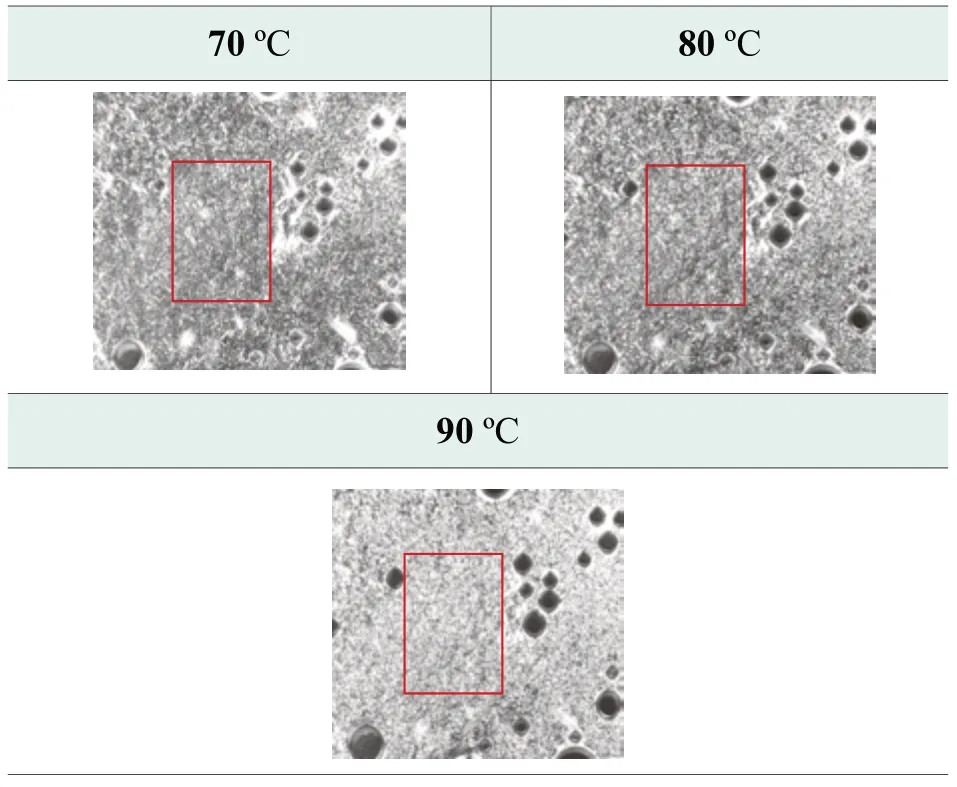
Figure 5.Photos of optimized formula at high temperature by polarized microscope
An unexpected finding is that the unique thermotropic liquid crystal structure formed in optimized formula at high temperatures.The density of liquid crystal structure is increasing with the rise of temperate from 70 ℃ to 90 ℃, which is indicated in red rectangle in Figure 5.This well explains the excellent high temperature stability.With temperature decreasing, thermotropic liquid crystals are replaced by layered crystals, and the system texture remains uniform and stable.
Rheological behavior of benchmark formula and optimized formula
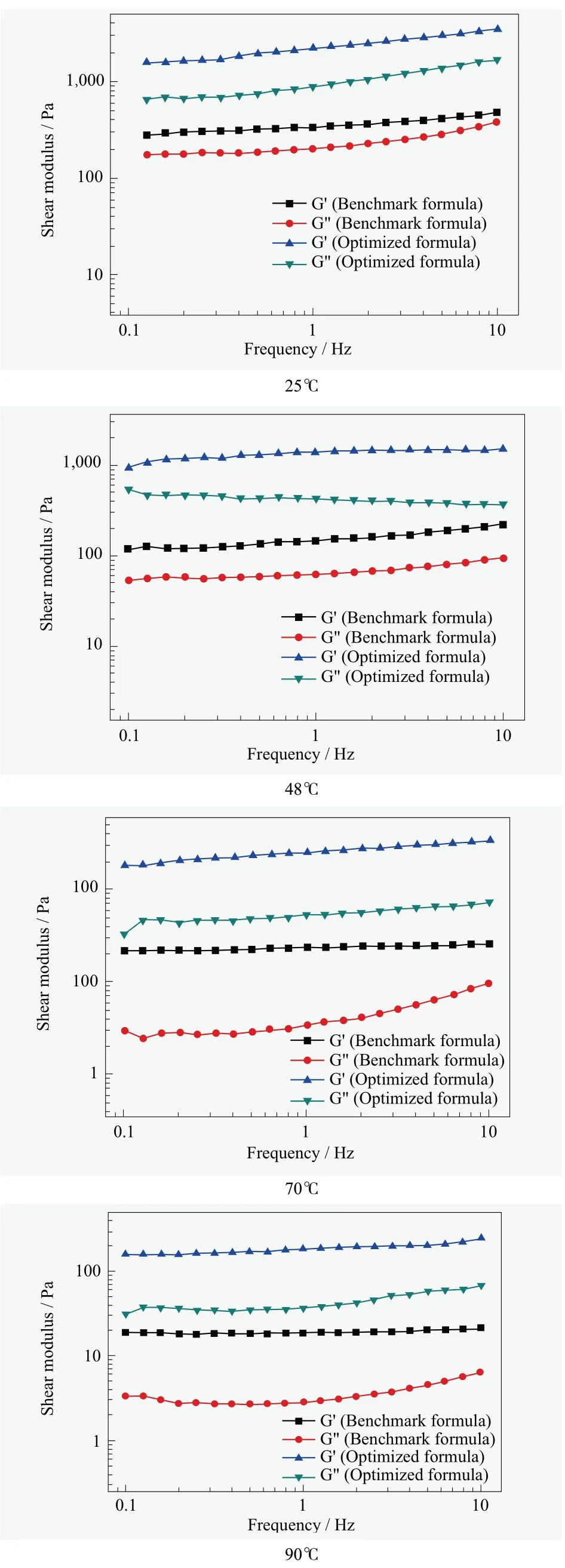
Figure 6. Dynamic rheological measurements for both benchmark formula and optimized formula at different temperature
Rheological behavior can reflect the interaction in complex fluids.As for both benchmark formula and optimized formula, the storage modulusG'dominates in the 0.01~10 Hz range for all temperature, which indicates the formula exhibit strong interactions with each other and they are mostly elastic.[10]As shown in Figure 6, at 4 ℃ and 25 ℃, both the storage modulusG'and the loss modulusG”vary with shear frequency, which are typical Lɑ-phase that has frequency independent moduli with the storage modulus higher than the loss modulus.The microstructure of optimized formula has a more regular arrangement than benchmark formula that also support the hypothesis of free fatty acids in formula recrystallization at 4℃, this deformation process impacts the structural regularity and leads to the viscous modulusG”increasing close toG', so that the benchmark formula behaves more like the pure compound itself.[11]In contrast, Optimized formula contains some nonionic surfactants to stabilize the system and stop the possible deformation at low temperature.
With the temperature increasing, the storage modulus decreases, which is an indication of swelling of the Lɑ-phase.Since the storage modulusG'is 10 times higher than the loss modulusG”for optimized formula, which indicate the formula still remains the liquid crystal structure and there is no big change forG'andG”from 70 ℃ to 90 ℃, that the occurrence of liquid crystal configuration changes.[10,11]
Product extrusion performance at low temperature
Figure 7 represents the force change during the tube extrusion test recorded by texture analyzer.The benchmark formula generates a much higher maximum force (4,278) than the optimized formula (3,201).Reduction in product weight is calculated and listed in Table 5.With same pressuring force, the tube filled with optimized formula gives proper amount of cleanser while the benchmark formula does not.We could affirm that the optimized formula outperforms the benchmark formula on extrusion performance in package tube at low temperature.The room temperature usually approaches freezing point in some part of China in winter, which challenges the extrusion performance of soap cleanser in package.Therefore, low-temperature extrusion performance becomes an important criterion in evaluating soapbased cleanser.
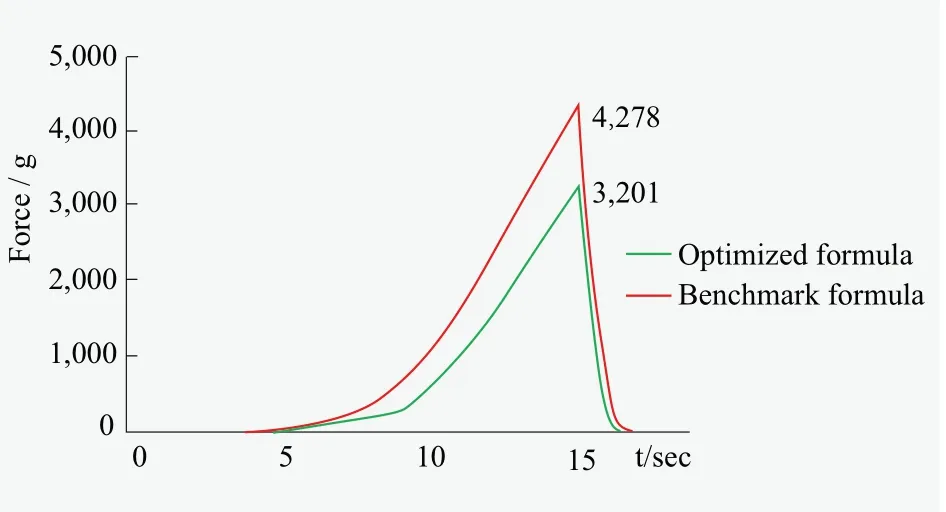
Figure 7.Force measurement of texture analyzer for both benchmark formula and optimized formula at low temperature

Table 5.Calculated extrusion output and recorded maximum force of benchmark formula and optimized formula
Consumer evaluation
Sensory evaluation was carried out in the sensory laboratory of Shanghai Jahwa R&D Center.30 volunteers graded on 9 sensory attributes after single use for 2 formulas using both room temperature and low temperature samples.
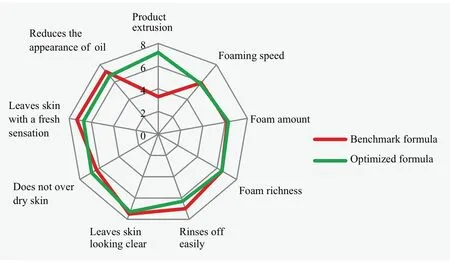
Figure 8.Graphical evaluation of the sensory attributes for both formulas using low temperature sample
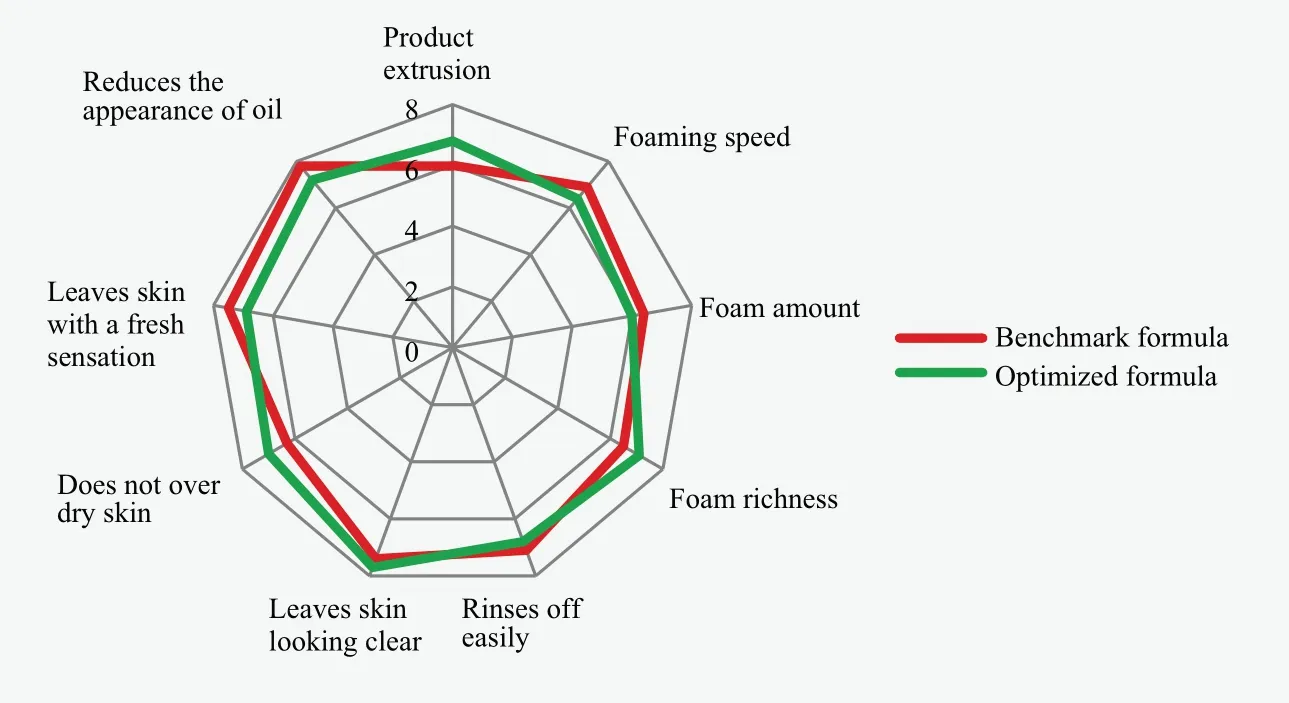
Figure 9. Graphical evaluation of the sensory attributes for both formulas using room temperature sample
The results are graphically depicted in Figure 8 and Figure 9.As shown in Figure 8, although the two formulations shows similar foaming characteristics, product extrusion of optimized formula is superior to benchmark formula (significant difference under the 95% confidence limit).It has been mentioned before that poor package extrusion is considered poor quality.According to the results in Figure 9, the optimized formula is demonstrated to provide enhanced skin moisturization (does not over dry skin) and desirable rich foam using room temperature sample.Thus, it could be concluded that the optimized formula offers an alternative solution to solve soap-based cleanser stability, addressing a major manufacturer concern, while exhibiting excellent foam properties and better conditioning benefits after wash.introduction of non-ionic emulsifier and ratio of fatty acids contributes the most to the formation of thermotropic liquid crystal.
To evaluate product performance, both equipment and panel test were conducted for both formulations.Although two soap formulations show comparable foaming properties in panel test, the optimized formula presented significant difference in package extrusion.Meanwhile, the optimized formula improved foam richness and enhanced skin moisturization after rinsing.In summary, this work successfully developed an approach to improve formulation stability and sensorial properties for soap-based facial cleanser at both low and high temperatures.
Conclusion
The optimized soap cleanser formulation is not only more stable than the benchmark soap cleanser formulation at different temperatures, but also reduces potential skin irritation by lower pH with incomplete saponification.The microscopic pictures of the benchmark formula indicate that the secondary recrystallization of free short-chain fatty acids has been the cause of viscosity increase at low temperature.Therefore, we increased the ratio of long-chain fatty acids in the optimized formula, which successfully stabilize the viscosity at low temperature and improve package extrusion.The microscopic pictures of optimized formula indicate the formation of thermotropic liquid crystal from 70 ℃ to 90 ℃.With the thermotropic liquid crystal, the optimized formula demonstrates greater stabilizing power than the benchmark formula at high temperatures.This study suggests that
 China Detergent & Cosmetics2019年3期
China Detergent & Cosmetics2019年3期
- China Detergent & Cosmetics的其它文章
- Experimental Design of Cosmetics Human Efficacy Evaluation
- Application Research of Bamboo Vinegar in Daily Chemicals
- Determination of Paeonol in Detergent by High Performance Liquid Chromatography
- Fluorinated Surfactants and Fluorinated Polymer Materials (III): Personal Opinion on the Problems of PFOS
- Safety and Risk Management of Cosmetic Products
- Innovation Reshapes the Future of Detergent Industry in China
