Immunomodulatory effect of schisandrae oil in mouse model of autoimmune hepatitis induced by concanavalin A
Wen-Qian Dong, Peng Luo, Da-Peng Lu, Hao Wang, Bao-Long Wang
Immunomodulatory effect of schisandrae oil in mouse model of autoimmune hepatitis induced by concanavalin A
Wen-Qian Dong1, 2, Peng Luo1, 2, Da-Peng Lu1, 2, Hao Wang1, 2, Bao-Long Wang1, 2*
1Department of Clinical Laboratory, The First Affiliated Hospital of USTC, Hefei 230001, China;2Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, China.
: To study the immunomodulatory effect of schisandra oil (SCO) in mouse model of autoimmune hepatitis induced by concanavalin A (ConA).C57BL/6 mice were divided into control group, model group and SCO group. Mice in SCO group were given SCO at 5 mg/kg by intragastric administration every day for 7 days, followed by intravenous injection of ConA at 10 mg/kg. 10 hours after ConA injection, the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were measured by the kits, the expression of inflammatory cytokines like interferon-γ (IFN-γ), interleukin-4 (IL-4), interleukin-17 (IL-17), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in liver was detected by real-time quantitative PCR, and the T cell activation and IFN-γ expression in spleen and MLN were examined by flow cytometry.Compared with control group, each indicator in model group were significantly higher. In SCO preventive treatment group, the levels of serum ALT, AST and LDH were significantly reduced (all< 0.001), the expression levels of inflammatory cytokines in liver were downregulated, the T cell activation in spleen and MLN was inhibited (= 0.006 and= 0.008), the percentages of IFN-γ+CD8+and IFN-γ+CD4+T cells were decreased, and the frequencies of Th2 and Th17 cells in spleen and MLN were also decreased at the same time.SCO has a protective effect on immune liver injury by inhibiting the activation of T cells and reducing the expression of inflammatory cytokines, which reflects that SCO plays a role in the immunomodulation of autoimmune hepatitis, indicating that SCO is of great significance for the maintenance of autoimmune homeostasis.
Schisandra oil, Autoimmune hepatitis, Immunomodulation, Concanavalin A, Inflammatory cytokines, T cells.
Schisandra oil has a protective effect on liver injury in model of autoimmune hepatitis by inhibiting the activation of T cells and reducing the expression of proinflammatory cytokines.
Wuweizi () is the fruit of Chinese magnolia vine, a well-known plant species in traditional Chinese medicine. The first description of Wuweizi () was found in, a famous ancient book of Chinese book written by Li Shizhen in the Ming Dynasty (1552 C.E.-1578 C.E.). The main components of schisandra oil are lignans, which are shown to have hepatoprotective activities.
Background
Wuweizi () is the fruit of a well-known plant species named Chinese magnolia vine, which is widely used in traditional Chinese medicine [1]. The first description of Wuweizi() can be found in the ancient book of Chinese medicine namedwritten by Li Shizhen, a famous medical scientist in the Ming Dynasty (1552 C.E.-1578 C.E.). It is demonstrated that Wuweizi() has been applied in the treatment of body fatigue, insomnia, weakness and excessive sweating, as well as in the treatment of various diseases like respiratory failure, intestinal diseases and cardiovascular diseases [2]. Wuweizi () contains lignans, polysaccharides, essential oils, fatty acids, vitamins, amino acids and so on. The main components of schisandra oil (SCO) are lignans, which are the most important active components of Wuweizi () [3-6]. Hepatoprotective activity is the best-known action of six kinds of schisandra lignans: gomisin A, schisantherin A, schisandrin B, schisandrin C, schisandrin and deoxyschisandrin. The available literature contains many reports on the mechanism of effect of these extracts [7-14]. For example, it has been reported that gomisin A could increase the activity of NADPH cytochrome C reductase, cytochrome B5 and P450 in microsomal, and reduce the activity of 3, 4-dibenzopyrene hydroxylase. Besides, it could accelerate the hepatic flow, the hepatocyte proliferation and the renewal of endoplasmic reticulum [15]. In addition, schisandrin B has been reported to protect liver tissues from oxidative stress injury [10]. Therefore, the role of SCO plays in hepatoprotective activity deserves attention.
Concanavalin A (ConA)-mediated liver injury in mice is a commonly used model of autoimmune hepatitis (AIH), which is a common clinical autoimmune liver disease [16-17]. AIH occurs in children and adults of all ages, which presents as a chronic progressive disease course of unknown cause. The variant forms of AIH have the same characteristics as other hypothetical autoimmune liver diseases, primary sclerosing cholangitis and primary biliary cirrhosis, but abnormal autoresponsiveness is considered to play the most important role in the pathogenesis [18]. Kinds of autoimmune antibodies result in tissue damage and systematic dysfunction, which makes anti-inflammatory or immunosuppressive treatment become lifetime maintenance therapy [19-20]. Since there is no cure, the focus is on prevention. However, there is no good prevention method for AIH so far. According to the record, Wuweizi() extracts have hepatoprotective activity, but much less attention was payed to the protective effect of SCO on ConA-mediated immune liver injury.
In the present study, further evidence is provided that SCO exerts immunomodulatory effect on the liver in ConA-induced model of AIH.
Materials and methods
Animals and reagents
Female C57BL/6 mice (6-8 weeks old) were purchased from the experimental animal center of Anhui medical university and kept in SPF laboratory. Schisandra oil was purchased from Hairui Natural Plant Co., Ltd (Jiangxi, China). ConA was purchased from Sigma Aldrich (USA).
Animal models
C57BL/6 mice were divided into control group, model group and SCO group (n = 16 for each group). Mice in SCO group were given SCO at 5 mg/kg by intragastric administration (i.g.) every day for 7 consecutive days, followed by intravenous (i.v.) injection of ConA at 10 mg/kg. Mice in model group were given i.g. phosphate buffered saline (PBS) as a control for 7 days, followed by an i.v. injection of ConA at 10 mg/kg. Mice in control group were given i.g. PBS for 7 days followed by i.v. injection of PBS. All animal experiments were approved by the Laboratory Animal Control Committee of University of Science and Technology of China, No. 2019-N(A)-078.
Real-time quantitative PCR analysis
Total RNA was extracted from liver tissues with TRIzol reagent (Invitrogen, USA) and was transcribed to cDNA by using a high capacity cDNA reverse transcription kit (Applied Biosystems, USA), both according to the manufacturer's protocols. Real-time quantitative PCR (qPCR) was performed with THUNDERBIRD SYBR qPCR mix (TOYOBO, Japan) on ABI 7500 applied biosystems under the following conditions: 95℃ for 5 min with 1 cycle, 95℃ for 30 s, 58℃ for 30 s and 72℃ for 30 s with 35 cycles, and 72℃ for 2 min with 1 cycle. mRNA levels were normalized to β-actin. The primers were synthesized by Sangon Biotech (China) and the primer sequences were as follows: mouse β-actin, sense 5’-CCTTCTTGGGTATGGAATCCTG-3’, antisense 5’-CAATGCCTGGGTACATGGTG-3’; mouse interferon-γ (IFN-γ), sense 5’-CTCATGGCTGTTTCTGGCTG-3’, antisense 5’-GACCTGTGGGTTGTTGACCT-3’; mouse interleukin-4 (IL-4), sense 5’-TTGTCATCCTGCTCTTCTTTCTC-3’, antisense 5’-TGGACTTGGACTCATTCATGG-3’; mouse interleukin-17 (IL-17), sense 5’-CGATCATCCCTCAAAGCTCAG-3’, antisense 5’-ACACCCACCAGCATCTTCT C-3’; mouse tumor necrosis factor-α (TNF-α), sense 5’-CCTATGTCTCAGCCTCTTCTC-3’, antisense 5’-CAATGACTCCAAAGTAGACCT-3’; mouse interleukin-1β (IL-1β), sense 5’-CCTGTTCTTTGAAGTTGACGG-3’, antisense 5’-AATGAGTGATACTGCCTGCC-3’; mouse interleukin-6 (IL-6), sense 5’-AACAAGAAAGACAAAGCCAGAG-3’, antisense 5’-GTTAGGAGAGCATTGGAAATTGG-3’.
Preparation of single cell suspension of mouse spleen and mesenteric lymph nodes
Mice were sacrificed and the spleen and mesenteric lymph nodes (MLN) were removed and put into PBS with 1% fetal bovine serum. The spleen and MLN were ground into cell suspension with frosted slides and the cell suspension was filtered through a 48 μm cell strainer to a centrifuge tube, followed by centrifugation at 400 g for 5 min. The supernatant was removed and the cell precipitation was resuspended with 3 mL red blood cell lysis buffer (Solarbio, Beijing, China) at room temperature for 5 min. Then 3 mL PBS was added to neutralize the effect of red blood cell lysis buffer followed by centrifugation at 400 g for 5 min. The supernatant was removed and the major components of cell precipitation are lymphocytes, which were resuspended by 3 mL PBS and washed for 3 times. The single cell suspension from spleen and MLN was used for subsequent flow cytometric analysis (BD FACS Calibur, USA).
Flow cytometric analysis
For the surface staining, antibodies were added into 100 μL lymphocyte suspension and incubated at 4 ℃ for 30 min. The surface staining antibodies were as follows: anti-CD3 (17A2, 5 μg/mL), anti-CD4 (GK1.5, 5 μg/mL), anti-CD8a (53-6.7, 5 μg/mL), anti-CD69 (H1.2F3, 10 μg/mL). For intracellular cytokine staining, lymphocytes prepared from spleen and MLN were cultured for 5 hours in the presence of Phorbol-12-myristate-13-acetate (80 nM), ionomycin (1.3 μM), and Brefeldin A (5 mg/mL). Cells were treated with anti-IFN-γ (XMG1.2, 10 μg/mL) on the basis of the cytoplasmic proteins staining protocols (Biolegend, USA). All antibodies were purchased from Biolegend (USA) and flow cytometric analysis was performed with BD FACS Calibur (USA).
Serum transaminase and lactate dehydrogenase measurement
The blood extracted from mouse eyeballs was collected and stood at room temperature for 3 hours, followed by centrifugation at 3000 rpm for 10 min. The serum was obtained and measured for alanine transaminase (ALT), aspartate transaminase (AST) and lactate dehydrogenase (LDH) according to the instructions in the kits. The ALT, AST and LDH assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (China).
Statistical analysis
Results were presented as mean ± SEM and statistical significance was examined by the analysis of variance. All the statistical analysis was performed by the GraphPad Prism 7.0 software. Differences were considered to be statistically significant when< 0.05.
Results
SCO prevents liver injury from ConA-induced AIH
To explore whether preventive treatment of SCO could protect the liver from immune injury, the serum markers of liver injury were measured. As shown in Figure 1A to C, the serum levels of ALT, AST and LDH in the model group are significantly higher than those in the control group (all< 0.001), indicating that ConA injection can absolutely result in liver injury. Interestingly, the levels of serum ALT, AST and LDH are significantly reduced with SCO pretreatment (all< 0.001), suggesting that SCO seems to be capable of preventing immune liver injury induced by ConA.
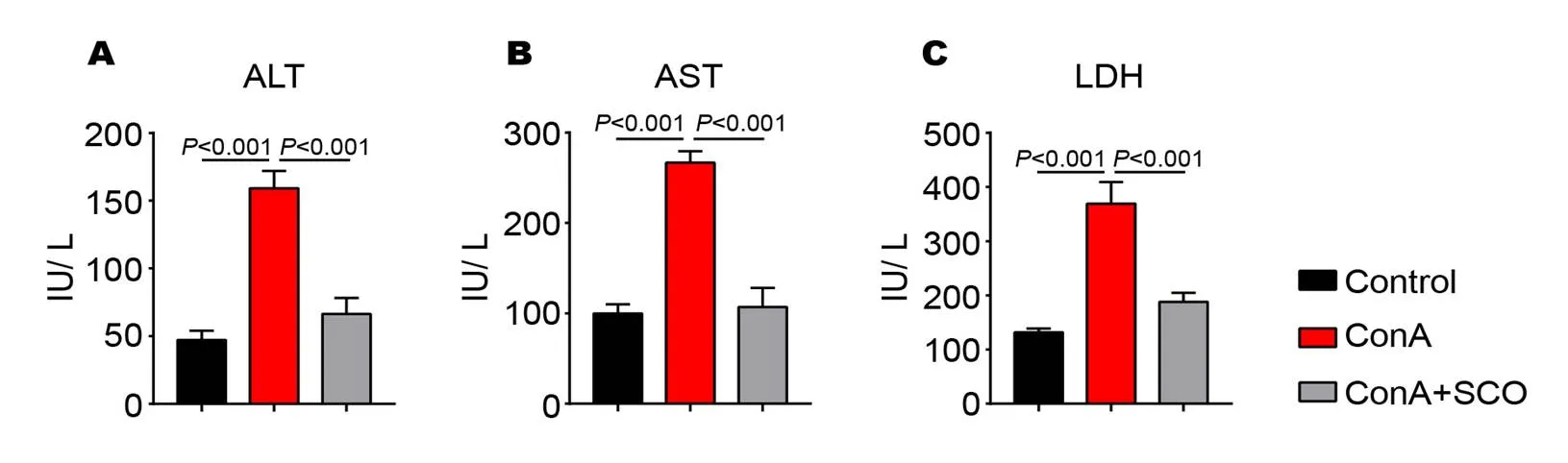
Figure 1 SCO prevents liver injury from ConA-induced AIH
(A to C) C57BL/6 mice were divided into control group, model group and SCO group (n = 10 per group). Mice in SCO group were treated i.g. with SCO for 7 consecutive days ahead of ConA injection. 10 hours later, the levels of serum ALT (A), AST (B) and LDH (C) were measured by the assay kits. Error bars represent mean ± SEM. SCO, Schisandra oil; AIH, Autoimmune hepatitis; ConA, Concanavalin A; ALT, Alanine transaminase; AST, Aspartate transaminase; LDH, Lactate dehydrogenase.
SCO reduces the levels of inflammatory cytokines in the liver
As we know, the main reason for ConA-induced liver injury is T cell activation and cytokine release. To further analyze the influence of SCO on inflammatory microenvironment in liver, a series of cytokines were evaluated by qPCR. It is observed that the expression of IFN-γ, IL-4, IL-17, TNF-α, IL-1β and IL-6 are all upregulated in the livers of mice in model group (< 0.001,= 0.001,< 0.001,= 0.004,= 0.003 and< 0.001, respectively, Figure 2A to F), while all these cytokine levels are reduced with SCO pretreatment (< 0.001,= 0.005,= 0.002,= 0.015,= 0.007 and< 0.001, respectively, Figure 2A to F), revealing that the liver protection of SCO is achieved by the downregulation of inflammatory cytokine expression.
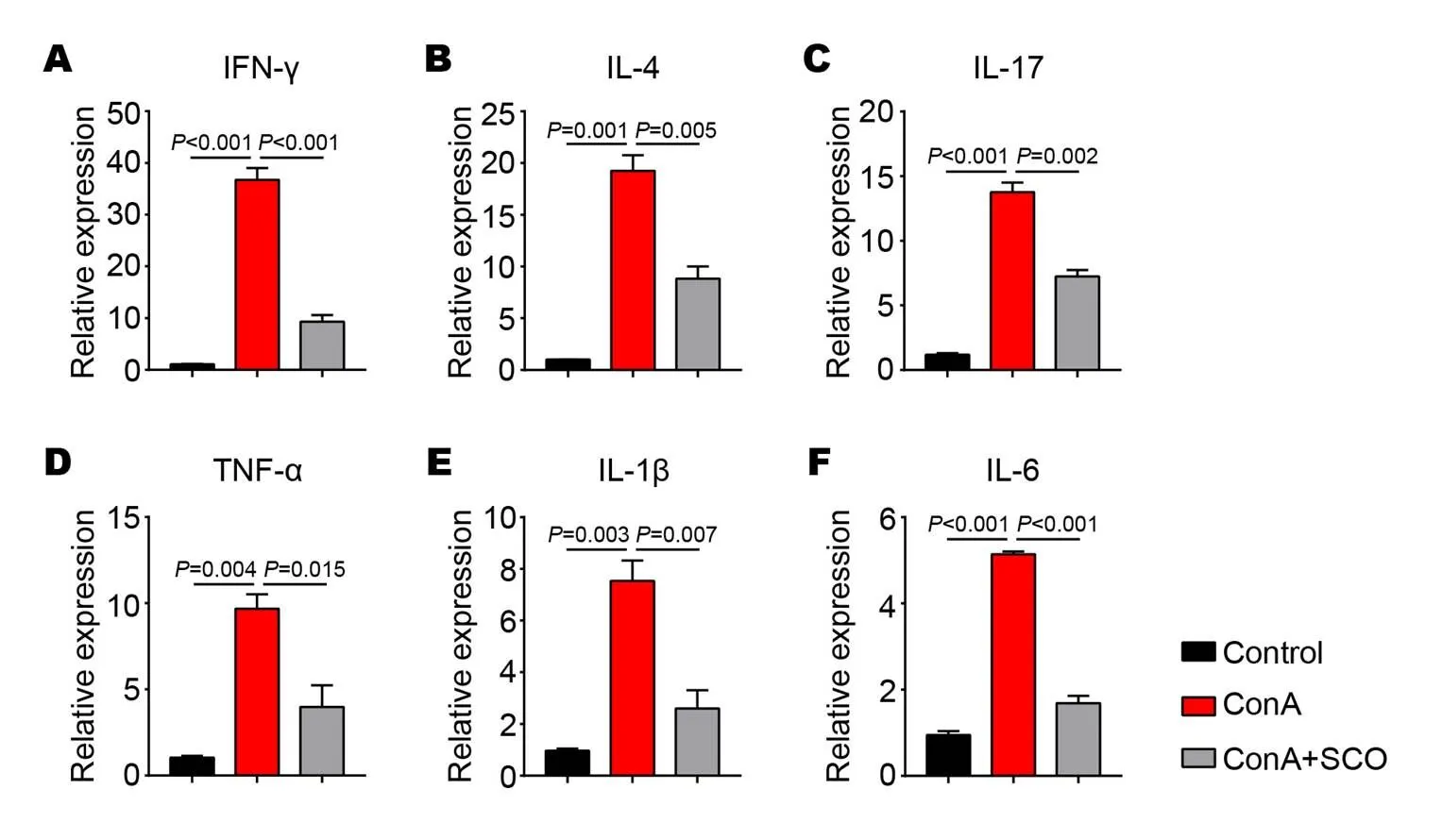
Figure 2 SCO reduces the levels of inflammatory cytokines in the liver
(A to F) C57BL/6 mice were divided into control group, model group and SCO group (n=6 per group). Mice in SCO group were treated i.g. with SCO for 7 consecutive days ahead of ConA injection. 10 hours later, the liver tissues from each group were evaluated for the expression of inflammatory cytokines IFN-γ (A), IL-4 (B), IL-17 (C), TNF-α (D), IL-1β (E) and IL-6 (F) by qPCR. Error bars represent mean ± SEM. SCO, Schisandra oil; ConA, Concanavalin A; IFN-γ, Interferon-γ; IL-4, Interleukin-4; IL-17, Interleukin-17; IL-1β, Interleukin-1β; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor-α.
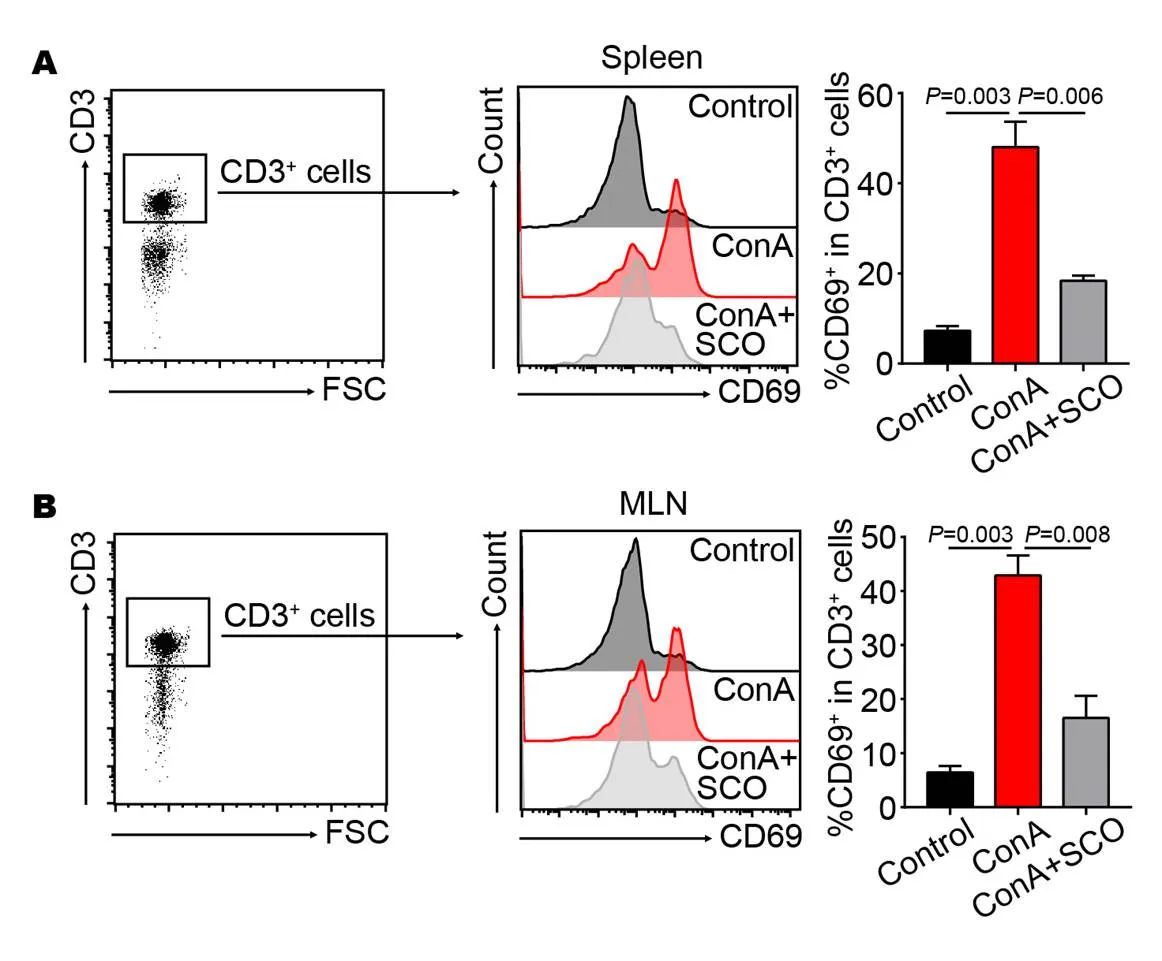
Figure 3 ConA-induced T cells activation is inhibited by SCO pretreatment
(A, B) C57BL/6 mice were divided into control group, model group and SCO group (n = 6 per group). Mice in SCO group were treated i.g. with SCO for 7 consecutive days ahead of ConA injection. 10 hours later, the percentages of CD69+ T cells in spleen (A) and MLN (B) were tested by flow cytometry. CD3+ cells were gated. Error bars represent mean ± SEM. SCO, Schisandra oil; ConA, Concanavalin A; MLN, Mesenteric lymph node.
ConA-induced T cells activation is inhibited by SCO pretreatment
Due to the fact that peripheral T cells can be activated after ConA injection, we further analyze the effect of SCO on T cell activation, which is characterized by a surface marker CD69. SCO was given to mice through oral route, so T cells in spleen and MLN were tested at the same time. As a result, the percentages of CD69+T cells are increased in both spleen (= 0.003) and MLN (= 0.003) after ConA injection (Figure 3A and B), whereas SCO pretreatment significantly decreases the frequencies of activated T cells (= 0.006 and= 0.008, Figure 3A and B), as evaluated by flow cytometry. This data indicated that ConA-induced T cell activation could be inhibited by oral administration of SCO.
The effect of SCO treatment on IFN-γ-producing T cells
Next, we tried to investigate whether SCO treatment had an influence on T cell subsets in the ConA-induced AIH model. The result of flow cytometry shows that IFN-γ expression of CD8+and CD4+T cells from spleen is strikingly up-regulated in the model group (both= 0.002, Figure 4A and B), whereas the percentages of IFN-γ+CD8+and IFN-γ+CD4+T cells significantly decrease in the SCO group (= 0.007 and= 0.021, Figure 4A and B). Simultaneously, the same results are observed in MLN (Figure 4A and B). Together, these data suggested that SCO pretreatment might exert a suppressive effect on CD8+and Th1 cells in ConA-induced T cell activation and differentiation.
The effect of SCO treatment on other CD4+ T cell subsets
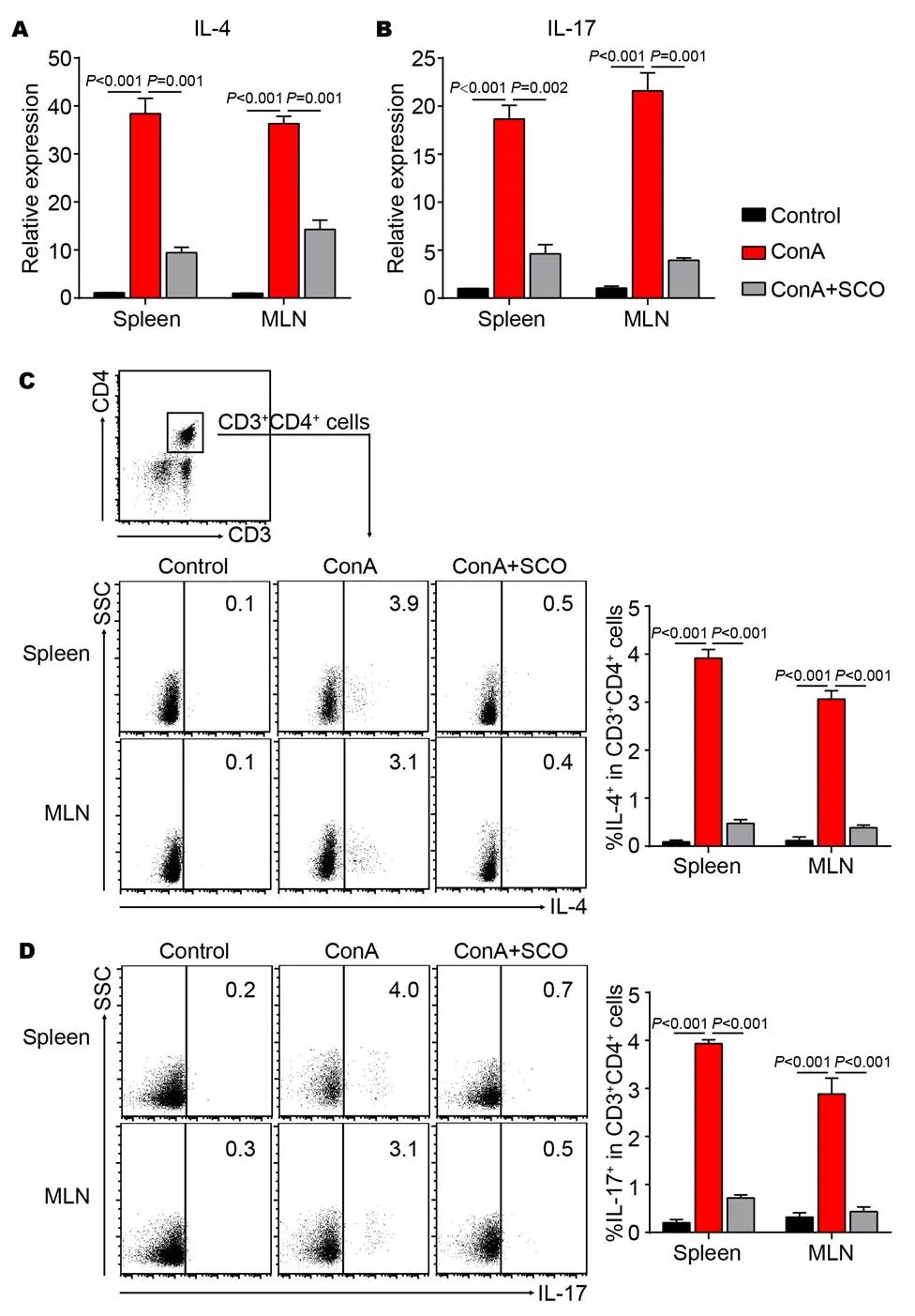
The above data indicated that the frequency of Th1 cells was decreased by SCO pretreatment in the AIH model. To further analyze the effect of SCO on other CD4+T cell subsets, the expression of IL-4 and IL-17 was evaluated by real-time quantitative PCR. It is observed that SCO pretreatment down-regulates the expression of IL-4 and IL-17 in both spleen and MLN (Figure 5A and B). In addition, flow cytometric analysis shows that the percentages of IL-4+and IL-17+cells in CD4+T cells are increased by ConA injection, while SCO treatment decreases the frequency in spleen and MLN (Figure 5C and D). All these suggested that the differentiation of CD4+T into Th2 and Th17 cells could be inhibited by SCO in the ConA-induced AIH model.
Discussion
Clinically, there are two common causes of liver injury: firstly, hepatotoxic substances such as heavy metals or chemical drugs cause direct damage to hepatocytes; Secondly, liver immune cell activation and inflammatory factor overexpression indirectly damage hepatocytes. It's worth mentioning that activation of immune cells leading to liver injury is an important feature of autoimmune hepatitis. ConA is a kind of plant lectin which causes T cell-induced hepatotoxicity as a T cell mitogen [21]. So, ConA-mediated mouse liver injury is a commonly used model of AIH, which closely mimics the pathogenesis mechanisms and pathological changes of patients. In the ConA model, peripheral T cells undergo activation, proliferation and differentiation [22-23], accompanied by cytokine release, like IFN-γ, IL-4, IL-17 and so on [24-28], which lay the foundation of inflammatory microenvironment. In addition to stimulating T cells, ConA is able to activate liver macrophages, mainly resident Kupffer cells and infiltrating monocytes, to produce TNF-α, IL-6 and IL-1β, which are key inflammatory factors that cause liver injury [29-30]. Furtherly, Kupffer cells can promote the immune response of Th1, thereby contributing to hepatotoxicity caused by ConA [31]. Altogether, ConA-stimulated T cells interact with other immune cells, triggering a series of inflammatory responses, subsequently resulting in hepatocellular injury.
In this study, we took advantages of ConA-induced hepatitis model to investigate the immunomodulatory effect of SCO and surprisingly found that SCO pretreatment could reduce the levels of serum ALT, AST and LDH, which are typical indicators of liver injury [32]. We can not help but wonder why does SCO have the function of liver protection. So further research was made to explore the effect of SCO on inflammatory microenvironment. Interestingly, it was observed that with oral administration of SCO, ConA-mediated T cell activation in spleen and MLN was inhibited, Th1, Th2, Th17 cells as well as CD8+T cells were suppressed and the expression of inflammatory cytokines in liver was decreased. Therefore, SCO is capable of alleviating liver inflammation by intervening immune cell activation, so as to achieve liver protection. However, the specific mechanism of immunomodulatory effect of SCO on T cells remains further study.
Conclusion
As stated above, SCO has a protective effect on immune liver injury by inhibiting the activation and differentiation of T cells and reducing the expression of inflammatory cytokines, which reflects that SCO plays a role in the immunomodulation of AIH, indicating that SCO is of great significance for the maintenance of autoimmune homeostasis.
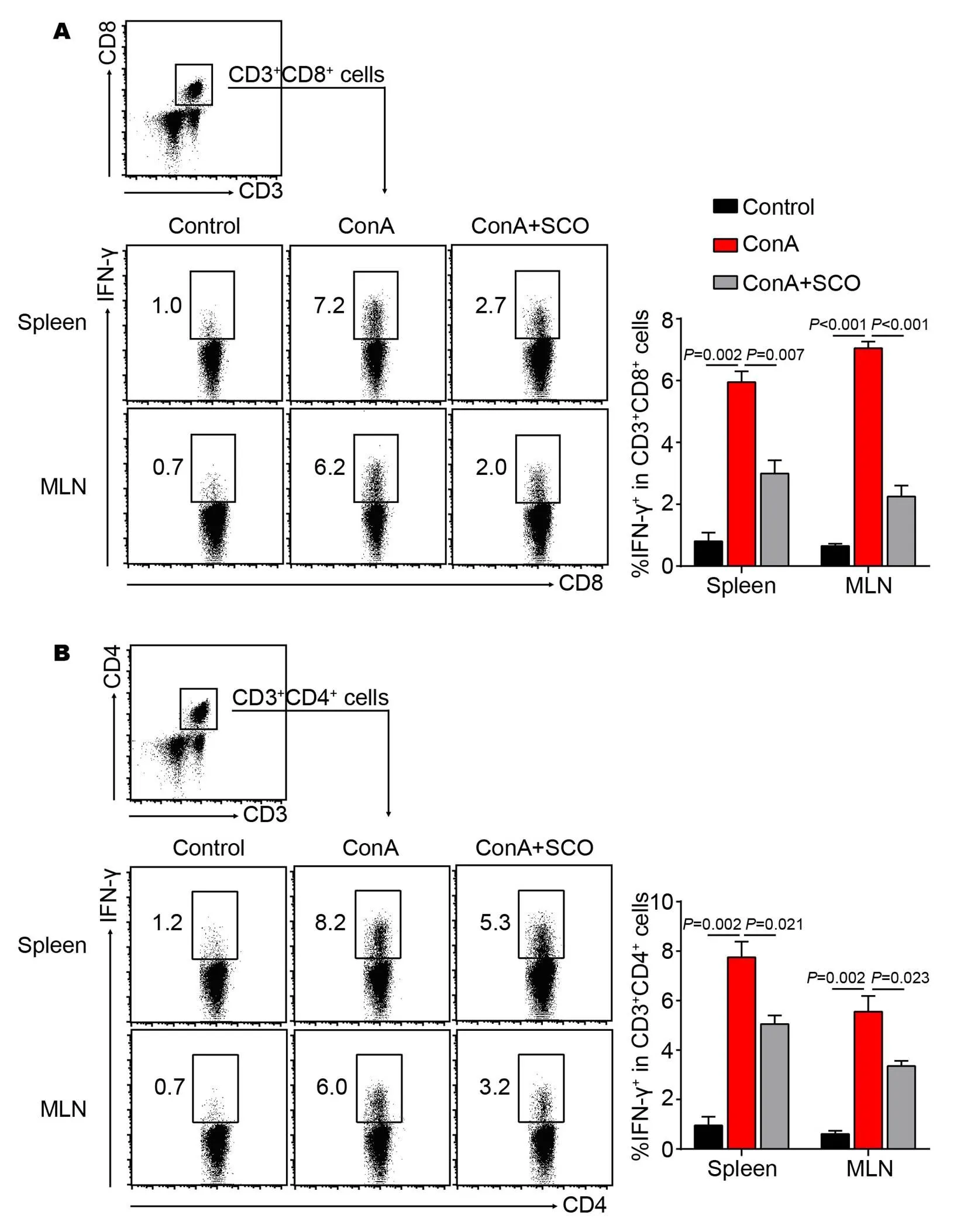
Figure 4 The effect of SCO treatment on IFN-γ-secreting T cells
(A, B) C57BL/6 mice were divided into control group, model group and SCO group (n = 6 per group). Mice in SCO group were treated i.g. with SCO for 7 consecutive days ahead of ConA injection. 10 hours later, lymphocytes from spleen and MLN were isolated and cultured in vitro for another 5 hours in presence of PMA (80 nM), ionomycin (1.3 μM), and Brefeldin A (5 mg/mL), followed by flow cytometric analysis. The percentages of IFN-γ+cells in both CD8+and CD4+T cells were shown. CD3+CD8+or CD3+CD4+cells were gated. Error bars represent mean ± SEM. SCO, Schisandra oil; ConA, Concanavalin A; IFN-γ, Interferon-γ; MLN, Mesenteric lymph node.
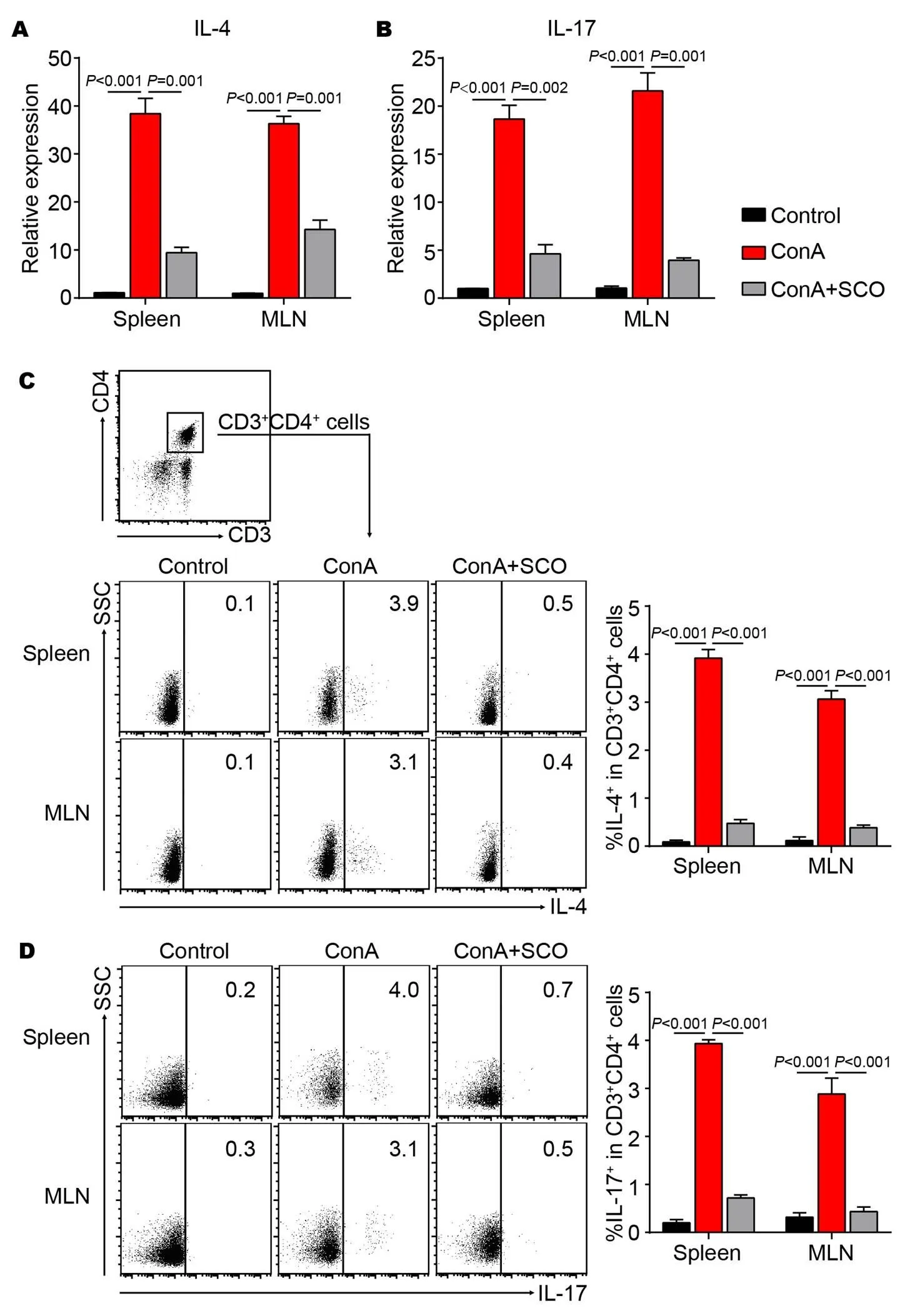
Figure 5 The effect of SCO treatment on other CD4+T cell subsets
C57BL/6 mice were divided into control group, model group and SCO group (n = 6 per group). Mice in SCO group were treated i.g. with SCO for 7 consecutive days ahead of ConA injection. 10 hours later, lymphocytes from spleen and MLN were evaluated for the expression of IL-4 (A) and IL-17 (B) by qPCR. At the same time, lymphocytes from spleen and MLN were isolated and cultured in vitro for another 5 hours in presence of PMA (80 nM), ionomycin (1.3 μM), and Brefeldin A (5 mg/mL), followed by flow cytometric analysis. The percentages of IL-4+(C) and IL-17+(D) cells in CD4+T cells were shown. CD3+CD4+cells were gated. Error bars represent mean ± SEM. SCO, Schisandra oil; Con A, Concanavalin A; MLN, Mesenteric lymph nodes; qPCR, Real-time quantitative PCR; IFN-γ, Interferon-γ; IL-4, Interleukin-4; IL-17, Interleukin-17; PMA, Phorbol-12-myristate-13-acetate.
1. Szopa A, Kokotkiewicz A, Bednarz M,Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD-HPLC method. Phytochem Lett 2017, 20: 462-469.
2. Szopa A, Ekiert R, Ekiert H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: a review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem Rev 2017, 16: 195-218.
3. Mocan A, Zengin G, Crişan G,Enzymatic assays and molecular modeling studies of Schisandra chinensis lignans and phenolics from fruit and leaf extracts. J Enzym Inhib Med Chem 2016, 31(S4): 200-210.
4. Ekiert RJ, Szopa A, Ekiert H,Analysis of lignans in Schisandra chinensis fruits, leaves, biomasses from in vitro cultures and food supplements. J Funct Foods 2013, 5: 1576-1581.
5. Kortesoja M, Karhu E, Olafsdottir ES,Impact of dibenzocyclooctadiene lignans from Schisandra chinensis on the redox status and activation of human innate immune system cells. Free Radical Bio Med 2019, 131: 309-317.
6. Su LL, Li P, Lu TL,Protective effect of Schisandra chinensis total lignans on acute alcoholic-induced liver injury related to inhibiting CYP2E1 activation and activating the Nrf2/ARE signaling pathway. Rev Bras Farmcogn 2019, 29: 198-205.
7. Waiwut P, Shin MS, Yokoyama S,Gomisin A enhances tumor necrosis factor-α-induced G1 cell cycle arrest via signal transducer and activator of transcription 1-mediated phosphorylation of retinoblastoma protein. Biol Pharm Bull 2012, 35: 1997-2003.
8. Jeong EJ, Lee HK, Lee KY,The effects of lignan-riched extract of Shisandra chinensis on amyloid-β-induced cognitive impairment and neurotoxicity in the cortex and hippocampus of mouse. J Ethnopharm 2013, 146: 347-354.
9. Casarin E, Dall’Acqua S, Smejkal K,Molecular mechanisms of antiproliferative effects induced by Schisandra-derived dibenzocyclooctadiene lignans (+)- deoxyschisandrin and (-)-gomisin N in human tumour cell lines. Fitoterapia 2014, 98: 241-247.
10. Thandavarayan RA, Giridharan VV, Arumugam S,Schisandrin B prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of MAPK/p53 signaling. PLoS One 2015, 10: e0119214.
11. Jiang Y, Fan X, Wang Y,Hepato-protective effects of six schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chem Biol Interact 2015, 231: 83-89.
12. Xu L, Grandi N, Del Vecchio C,From the traditional Chinese medicine plant Schisandra chinensis new scaffolds effective on HIV-1 reverse transcriptase resistant to non-nucleoside inhibitors. J Microbiol 2015, 53: 288-293.
13. Li W, Qu XN, Han Y,Ameliorative effects of 5-hydroxymethyl-2-furfural (5-HMF) from Schisandra chinensis on alcoholic liver oxidative injury in mice. Int J Mol Sci 2015, 16: 2446-2457.
14. Wang O, Cheng Q, Liu J,Hepatoprotective effect of Schisandra chinensis (Turcz.) Baill. lignans and its formula with Rubus idaeus on chronic alcohol-induced liver injury in mice. Food Funct 2014, 5: 3018-3025.
15. Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail: an overview of Russian research and uses in medicine. J Ethnopharm 2008, 118: 183-212.
16. Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol 2016, 13: 316-327.
17. Heymann F, Hamesch K, Weiskirchen R,The concanavalin A model of acute hepatitis in mice. Lab Anim 2015, 49(S1): 12-20.
18. Krawitt EL. Autoimmune Hepatitis. N Engl J Med 2006, 354: 54-66.
19. Johnson P, McFarlane IG, Williams R. Azathioprine for long-term maintenance of remission in autoimmune hepatitis. N Engl J Med 1995, 333: 958-963.
20. Langley PG, Underhill J, Tredger JM,Thiopurine methyltransferase phenotype and genotype in relation to azathioprine therapy in autoimmune hepatitis. J Hepatol 2002, 37: 441-447.
21. Soares PA, Nascimento CO, Porto TS,Purification of a lectin from Canavalia ensiformis using PEG-citrate aqueous two-phase system. J Chromatogr B Analyt Technol Biomed Life Sci 2011, 879: 457-460.
22. Zhang X, Wei HX, Rui S,Opposite effects of high and low doses of interleukin-2 on T cell-mediated hepatitis in mice (interleukin-2 on hepatitis). Hepatol Int 2010, 4: 641-648.
23. Varthaman A, Khallou-Laschet J, Clement M,Control of T cell reactivation by regulatory Qa-1-restricted CD8 T cells. J Immunol 2010, 184: 6585-6591.
24. Gove ME, Rhodes DH, Pini M,Role of leptin receptor-induced STAT3 signaling in modulation of intestinal and hepatic inflammation in mice. J Leukoc Biol 2009, 85: 491-496.
25. Takahashi K, Murakami M, Kikuchi H,Derivatives of Dictyostelium differentiation - inducing factors promote mitogen-activated IL-2 production via AP-1 in Jurkat cells. Life Sci 2011, 88: 480-485.
26. Miller ML, Sun Y, Fu YX. Cutting edge: B and T lymphocyte attenuator signaling on NKT cells inhibits cytokine release and tissue injury in early immune responses. J Immunol 2009, 183: 32-36.
27. Küsters S, Gantner F, Künstle G,Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology 1996, 111: 462-471.
28. Sass G, Heinlein S, Agli A,Cytokine expression in three mouse models of experimental hepatitis. Cytokine 2002, 19: 115-120.
29. Morita A, Itoh Y, Toyama T,Activated Kupffer cells play an important role in intra-hepatic Th1-associated necro-inflammation in Concanavalin A-induced hepatic injury in mice. Hepatol Res 2003, 27: 143-150.
30. Schumann J, Wolf D, Pahl A,Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol 2000, 157: 1671-1683.
31. Chen L, Xie XJ, Ye YF,Kupffer cells contribute to concanavalin A-induced hepatic injury through a Th1 but not Th17 type response-dependent pathway in mice. Hepatobiliary Pancreat Dis Int 2011, 10: 171-178.
32. Burke MD. Liver function: test selection and interpretation of results. Clin Lab Med 2002, 22: 377-390.
This study was supported by Province Science and Technology in the Anhui Offends Pass Item (1604a0802072).
SCO, Schisandra oil; Con A, Concanavalin A; AIH, Autoimmune hepatitis; MLN, Mesenteric lymph nodes; PBS, Phosphate buffered saline; ALT, Alanine transaminase; AST, Aspartate transaminase; LDH, Lactate dehydrogenase; qPCR, Real-time quantitative PCR; IFN-γ, Interferon-γ; IL-4, Interleukin-4; IL-17, Interleukin-17; IL-1β, Interleukin-1β; IL-6, Interleukin-6; TNF-α, Tumor necrosis factor-α; i.g., Intragastric administration; i.v., Intravenous.
The authors declare that they have no conflict of interest.
Wen-Qian Dong, Peng Luo, Da-Peng Lu,. Immunomodulatory effect of schisandrae oil in mouse model of autoimmune hepatitis induced by concanavalin A. Traditional Medicine Research, 2019, 4 (5): 227-236.
Jing Liang.
:30 March 2019,
17 July 2019,
:19 July 2019.
10.12032/TMR20190717124
Bao-Long Wang, Department of Clinical Laboratory, The First Affiliated Hospital of USTC, 17 Lujiang road, Luyang District, Hefei 230001, China; Division of Life Sciences and Medicine, University of Science and Technology of China, 96 Jinzhai road, Baohe District, Hefei 230001, China. E-mail: wbl196555@163.com.
 Traditional Medicine Research2019年5期
Traditional Medicine Research2019年5期
- Traditional Medicine Research的其它文章
- Plant distribution and pharmacological activity of flavonoids
- Natural products as a crucial source of anti-inflammatory drugs: recent trends and advancements
- Prescribing Chinese patent medicines without traditional Chinese medicine training is now banned in China
- Quantitation of phytochemical constituents of Fumaria vaillantii L. with different extract methods
- Assessment of microwave assisted and hydrodistllation extraction on Echinops persicus essential oils chemical composition and evaluation of its biological activity
- Antitumor applications of nano-traditional Chinese medicine
