Natural products as a crucial source of anti-inflammatory drugs: recent trends and advancements
Yan-Hang Wang, Ke-Wu Zeng*
Natural products as a crucial source of anti-inflammatory drugs: recent trends and advancements
Yan-Hang Wang1, Ke-Wu Zeng1*
1State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, China.
Natural active molecules are key sources of modern innovative drugs. Particularly, a great amount of natural active molecules have been reported to possess promising therapeutic effects on inflammatory diseases, including asthma, rheumatoid arthritis, hepatitis, enteritis, metabolic disorders and neurodegenerative diseases. However, these natural active molecules with various molecular structures usually exert anti-inflammatory effects through diversiform pharmacological mechanisms, which is necessary to be summarized systematically. In this review, we introduced the current major anti-inflammatory natural active molecules based on their chemical structures, and discussed their pharmacological mechanisms including anti-inflammatory molecular signaling pathways and potential target proteins, which providing a referential significance on the development of novel anti-inflammatory drugs, and also revealing new therapeutic strategies for inflammatory diseases.
Natural products, Anti-inflammation, Traditional Chinese medicine, Mechanism of action, Drug target.
This review introduced the current major anti-inflammatory natural active molecules based on their chemical structures, and discussed their pharmacological mechanisms.
Compared with non-steroidal anti-inflammatory drugs and glucocorticoids, natural anti-inflammatory compounds from plants may have more advantages due to less side effects and toxic reactions.
Background
Inflammation is a kind of active defense reaction of the organisms responding to external stimulations, and also a pivotal risk factor for a great amount of human diseases, including arthritis, allergy, infection, cancer, arteriosclerosis, metabolic disorders, and neurodegenerative diseases. Thus, the development of anti-inflammatory agents is a key research field attaching great concern in recent years.
Natural products mainly refer to the secondary metabolites synthesized in various organisms for billions of years. Natural products possess novel chemical structures beyond imagination, making them a key source of lead compounds for critical diseases [1]. In recent years, a great amount of natural products, especially from plants, have been reported to exhibit obvious anti-inflammatory effects bothand[2]. In the light of molecular structure type, natural products from plants with anti-inflammation effects mainly include monoterpene, diterpene, triterpene, phenylpropanoid, lignanoid, coumarin, flavonoid, anthraquinone, alkaloid, and polyphenol. These natural products exert significant anti-inflammatory effects via acting on different drug targets and cell signaling pathways.
Currently, inflammation therapy is mainly based on chemical medicine including non-steroidal anti-inflammatory drugs and glucocorticoids, which possess various side effects such as cardiotoxicity, hepatotoxicity and immunological dysfunction. Thus, naturalanti-inflammatory compounds from plants have attracted the attention of many researchers for treatment of enteritis, arthritis, skin inflammation and so on.
In this review, we summarized the distinctive pharmacological mechanisms as well as targets for a series of promising bioactive natural products, which are also called star-molecules, further providing significance for the development of novel anti-inflammatory agents in clinical trials.
Monoterpene and diterpene
Peoniflorin
Paeoniflorin (PF) is a main bioactive component isolated from the root of herb Shaoyao (), which was used to control pain recorded in thein the third century A.D(Han Dynasty of China)(Fig 1a). PF possesses a variety of pharmacological activities such as anti-inflammatory, anti-oxidative and immune-regulatory effects [3]. Currently, the anti-inflammatory effect of PF has been widely demonstratedandstudies. For example, PF effectively decreases proteinuria and ameliorates creatinine clearance rate in/mice, and blocks nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation and macrophage recruitment, together with the suppression on inflammatory cytokines and chemokines [4]. Moreover, AMPK and p38 MAPK pathways are also involved in PF-dependent anti-inflammatory effects [5].
Ginkolide B
Ginkgolide B (GB) is a predominant bioactive constituent derived from Chinese herb Yinxing (), which has protective effects against cough recorded in the(Fig 1b). Recent studies reveal GB exerts neuroprotective property by anti-inflammation mechanism. GB inhibits I/R-induced microglia activation and pro-inflammatory cytokines production via inactivating inhibitor of NF-κB (IκB)kinase/IκB-α/NF-κB pathway, resulting in an attenuation of NF-κB-mediated apoptosis [6]. GB also suppresses toll-like receptors/myeloid differentiation primary response gene 88 (MyD88)/NF-κB-mediated inflammatory response in oxygen-glucose deprivation and reoxygenation-induced microglia cells [7], contributing to lessen neuronal apoptosis in traumatic and hemorrhagic brain injury [8]. Additionally, GB suppresses intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 expression via blocking NF-κB activation in human vascular endothelial cells stimulated by oxidized low density lipoprotein, indicating a benefit in the treatment of atherosclerosis [9]. Further, GB is a known inhibitor of platelet activating factor as well, which plays an important role in the pathogenesis of asthma and extrinsic allergic alveolitis by inactivating macrophages and lymphocytes via ERK/ MAPK pathway [10].
Andrographolide
Andrographolide (Andro) is a major active ingredient from Chinese herb Chuanxinlian (), which has heat-clearing and detoxifying effects recorded in the(Fig 1c). Andro exerts various biological functions including anti-inflammatory, anti-oxidative, antiviral, anti-cancer, lipid-decreasing and hypoglycemic activities. Specially, Andro shows significant anti-inflammatory effect on several inflammatory models, suggesting a desirable therapeutic value in asthma, sepsis, rheumatoid arthritis, colitis, psoriasis, Alzheimer’s disease, bone loss, non-alcoholic steatohepatitis and liver injury. Besides, Andro can reduce pro-inflammatory cytokines production and inhibits leucocyte migration and macrophage activation, alleviating pro-inflammatory cytokines- associated cytotoxicity. Mechanism studies reveal that Andro suppresses IKK/IκBα/NF-κB and MAPK pathways, which may be associated with MyD88 degradation [11]. Moreover, Andro is demonstrated to inhibit nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome activation by inhibiting NLRP3/caspase 1 complex assembly andinterleukin (IL)-1 secretion [12]. Andro also suppresses nuclear erythroid 2-related factor 2 (Nrf2)-related inflammatory response via activating Keap1/Nrf2/ARE/ HO-1 pathway [13]. Further, PI3K/AKT/mTOR pathway is reported to account for Andro-induced anti-inflammatory effects [14].
Triptolide
Triptolide is a major pharmacological compound from Chinese herb Chuanxinlian (Hook.f.), which shows significant therapeutic effect in arthritis found in the(Fig 1d). Accumulating evidence show that triptolide can exert various bioactive activities including immunosuppression, anti-cancer, anti-diabetic, anti-oxidant, reproductive inhibition and anti-mutagenic effects. Particularly, triptolide exerts anti-inflammatory effects on a variety of inflammatory diseases such as rheumatic arthritis, diabetic nephropathy, cardiomyocyte hypertrophy/ myocardial fibrosis, pulmonary fibrosis, ultraviolet radiation B-mediated inflammation, and spinal cord injury. Moreover, triptolide exerts anti-inflammatory activity via several inflammation-associated signal transduction pathways. NF-κB is the most widely investigated target which is markedly down-regulated by regulating toll-like receptor (TLR) 4 [15]. Moreover, triptolide also phosphorylates and stabilizes p53 to block NF-κB inflammation signal [16]. Furthermore, triptolide exerts anti-inflammatory effect through acting on some other targets such as miR-225-3p [15],CXC chemokine receptor (CXCR) 2 [17],and mitogen-activated protein kinases (MAPKs) [18].
Ligustilide
Ligustilide is a natural compound from Chinese herb Chuanxiong (Franch.), which shows obvious therapeutic effect on headache recorded in the(Fig 1e). Ligustilide has been demonstrated to prevent the progresses of a variety of inflammatory diseases, including inflammatory pain, cardiovascular, cerebrovascular diseases and ultraviolet radiation-induced skin inflammation [19]. Ligustilide may exert anti-inflammatory activities by inhibiting tumor necrosis factor-α (TNF-α), IL-1, IL-10 and prostaglandin E2 (PGE2) expressions by specially preventing NF-κB activation and down-regulating cyclooxygenase-2 (COX-2) andinducible nitric oxide synthase (iNOS) [20]. Moreover, ligustilide inhibits NF-κB pathway via suppressing IкB-α degradation, which is a key regulatory node upstream of NF-κB [19]. Besides, several other inflammatory pathways are also involved in ligustilide-associated inflammation inhibition such as MAPK/AP-1 [21], Prx/TLR4 pathway [22] and Nrf2/HO-1 pathway [19], suggesting a complicated immunoregulatory network.
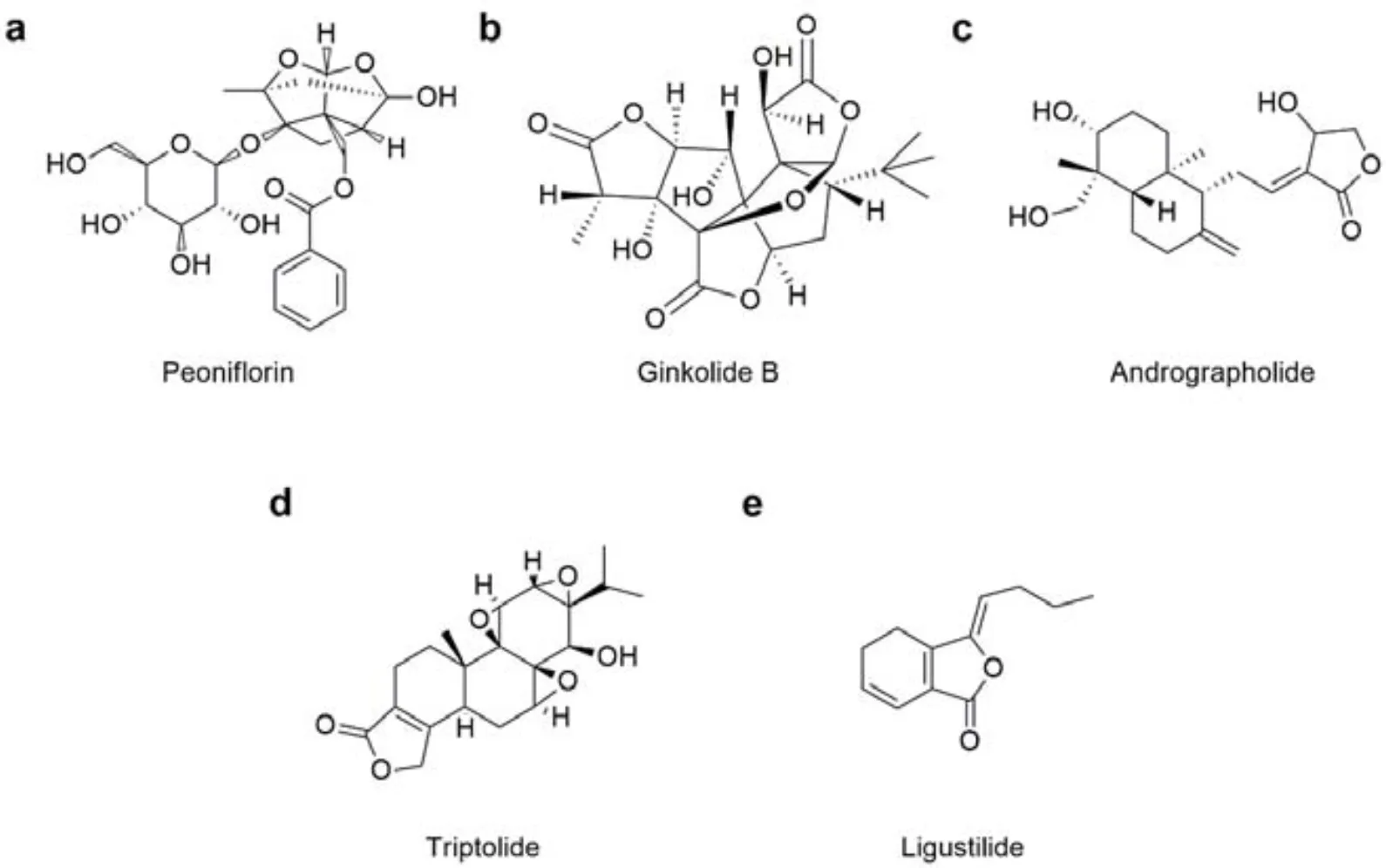
Figure 1 Chemical structures of Peoniflorin, Ginkolide B, Andrographolide, Triptolide and Ligustilide
Figure 2 Chemical structures of Celastrol, Ginsenoside Rb1, Asiaticoside and Ursolic acid
Triterpene
Celastrol
Celastrol is an active compound from Leigongteng (Hook.f.) with curative effects in cancer and inflammation (Fig 2a). The anti-inflammatory effects of celastrol have been demonstrated in animal models including arthritis, Alzheimer’s disease, asthma, and systemic lupus erythematosus. Celastrol is reported to regulate TNF-α, NF-κB, COX-2, vascular endothelial growth factor (VEGF), protein kinase B (Akt), and CXCR4 [22]. For example, celastrol inhibits inhibitor of nuclear factor kappa-B kinase (IκK) α/βactivity and IκBα degradation [24]. Besides, celastrol promotes ubiquitinated Nur77 migration to mitochondria, which interacts with p62/SQSTM1, leading to mitophagy and inflammation suppression [25].
Ginsenoside Rb1
The tetracyclic triterpenoid ginsenoside Rb1 is a major bioactive saponin from Chinese herb Renshen (C. A. Mey), which is famous for tonic properties in the(Fig 2b). Rb1 provides diverse benefits against inflammation, tumor, ischemia-reperfusion injury, fatigue and oxidative stress. Rb1 attenuates inflammatory injuries induced by 2, 4, 6-trinitrobenzene sulfuric acid, lipopolysaccharide, carbon tetrachloride, and TNF-α. Further studies reveal that Rb1 regulates the various inflammatory cytokines through Nrf2-antioxidant response element and NF-κB inflammatory pathways. Additionally, Rb1 improves formalin-induced inflammatory nociception by inhibiting ERK-MAPK pathway [26]. Rb1 also shows a suppressive effect on endoplasmic reticulum stress by dephosphorylating inositol-requiring enzyme 1α and PERK (protein kinase R-like endoplasmic reticulum kinase), thereby reducing thioredoxin-interacting protein -associated NLRP3 inflammasome activation in adipose tissue [27].
Asiaticoside
Asiaticoside, a triterpenoid from Chinese herb Jixuecao ((L.) Urban) recorded in thein 1578 A.D(Ming Dynasty of China), has been described to possess various biological activities such as wound healing, neuroprotective, immunomodulatory,antioxidant and anti-inflammatory activities (Fig 2c). Particularly, asiaticoside suppresses inflammation response by inhibiting pro-inflammatory mediators including TNF-α, IL-1β, IL-6 and PGE2. Mechanism study shows asiaticoside may inhibit MAPKs and NF-κB activations, and increase peroxisome proliferator- activated receptor (PPAR)-γ expression [28-30]. Besides, other studies reveal that asiaticoside exerts anti-inflammatory effect by increasing IL-10, thereby up-regulating the level of heme oxygenase-1 [31].
Ursolic acid
Ursolic acid (UA) is a pentacyclic triterpenoid with antibacterial, antiprotozoal, anti-inflammatory, and antitumor activities (Fig 2d). Specially, UA shows significant therapeutic effect in several inflammatory diseases such as pleurisy, atherosclerosis, allergic asthma, arthritis, cerebral ischemia and liver and kidneys inflammation. UA effectively reduces pro-inflammatory cytokines such as TNF-α, interferon-γ and IL-2 [32]. Additionally, UA suppresses the formation of advanced glycation end productsand reactive oxygen species (ROS) [33]. As for the potential mechanism, NF-κB is considered as a major target which UA affects. Moreover, UA inactivates transcriptional factors such as nuclear factor of activated T cells and activator protein 1 [34] with inhibiting STAT3 and PI3K/AKT/mTOR signaling pathways [35].
Phenylpropanoid
Ferulic acid
The major pharmacological effects of ferulic acid (FA) are antioxidation and anti-inflammation (Fig 3a). FA ameliorates aortic inflammation, ulcerative colitis, and neuroinflammation in transgenic mouse model of Alzheimer’s disease. Moreover, FA exerts anti-inflammatory effect by decreasing pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, or by increasing anti-inflammatory cytokines [36]. Mechanism studies reveal FA regulates oxidative stress and inflammatory response by scavenging ROS production via targeting ERK/JNK/p38/MAPKs pathway [37, 38]. In addition, FA also inhibits inflammatory response through NF-κB as well as Nrf2-antioxidant response element (ARE)pathways [39].
Salvianic acid
Salvianolic acids are from Chinese herb Danshen (Bge.), which are widely used to treat cardiovascular diseases in the. Salvianolic acid A and B show strong pharmaceutical activity for cardiocerebral vascular diseases (Fig 3b, 3c). Due to their polyphenolic structures, salvianolic acids are thought to be free radical scavengers, which show potential anti-inflammatory applications in clinical trials [40, 41]. Additionally, some reports show that salvianolic acids alleviate myocardial damage by suppressing NF-κB activity to reduce TNF-α expression and abate inflammatory cell infiltration [42, 43]. It is also demonstrated that Salvianolic acid B effectively attenuates vascular cell adhesion molecule-1 (VCAM-1) and intercellular cell adhesion molecule-1 (ICAM-1) in TNF-α-treated human amniotic epithelial cells. Collectively, these findings are highly associated with the anti-inflammatory properties of salvianolic acids through inhibiting NF-κB activation [44], making salvianolic acids excellent candidates for future development of cardiac-cerebral vascular protective agents.
Lignanoid and coumarin
Obovatol
Obovatol, a phenolic compound from the bark of Magnolia (Desr) used to reduce fever recorded in the, has been reported to show antioxidant, neuroprotective, anti-inflammatory, anti-thrombotic, anti-tumour, anti-gastriculcer, anti- allergic and anti-bacterial effects (Fig 3d). Obovatol relieves the progresses of inflammatory diseases like Alzheimer's disease, cardiovascular disease, bone disorders, and atherosclerosis. Moreover, obovatol inhibits NO production as well as the expressions of iNOS and COX-2. Furthermore, obovatol exerts anti-inflammatory effect via inactivation of NF-κB/JNK and ERK signal pathways through suppressing NF-κB nuclear translocation [45]. Besides, obovatol markedly enhances ROS-scavenging activity of peroxiredoxin 2 (Prx2) [46].
Schisandrin B
Schisandrin B is a kind of dibenzocyclooctadiene lignans from Chinese herb Wuweizi () discoveried in the, showing hepatoprotective, neuroprotective, anti-inflammatory, anti-tumor, anti-oxidant, and anti-bacterial properties (Fig 3e). Schisandra B prevents the occurrence of various inflammatory diseases including neuroinflammation, hepatitis, enteritis, and pneumonia. In addition, schisandrin B inhibits the expression of various inflammatory mediators such as COX-2, IL-6, IL-8, TNF-α and iNOS. Mechanism studies reveal schisandra B induces nuclear translocation of Nrf2 and inhibits NF-κB activity by suppressing IκB degradation [47]. Moreover, schisandrin B attenuates the inflammatory response by inhibiting p53 pathway and up-regulating heat shock protein/Beclin expression [48]. Furthermore, some other signaling pathways are also directly or indirectly involved in schisandrin B-associated inflammation inhibition, such as PPAR-γ signaling pathway [49].
Anthraquinone
Shikonin
Shikonin is a natural compound from Chinese herb Zicao () recorded in the, which has anti-cancer, anti-inflammatory and antibacterial activities (Fig 4a). Recent reports have shown shikonin shows effective anti-inflammatory effects in allergic airway remodeling and osteoarthritis. Shikonin inhibits the productions of IL-6 and IL-8, as well as chemokine C-C motif ligand 20 in human periodontal ligament cells [50]. Shikonin also down-regulates the expressions of HMGB1(high mobility group box-1 protein) and interferon-β. In the meantime, Shikonin decreases the ratio of nuclear to cytoplasm for NF-κB [51]. What’s more, Shikonin influences inflammatory gene expressions such as CYBA, GSK3B and EIF4E in macrophages [52].
Flavonoid
Quercetin
Natural resource of quercetin is abundant, which can be separated from many kinds of vegetables, fruits and Chinese herbal medicine. Quercetin processes remarkable anti-cancer, anti-oxidative and anti-inflammatory properties (Fig 4b). Different inflammatory models have been carried out to prove quercetin’s anti-inflammatory effects including enteritis, arthritis, skin inflammation and inflammation caused by hypoxia. Quercetin decreases TNF-α, IL-1β, IL-17 and monocyte chemoattractant protein (MCP)-1 in C57BL/6 mice [53]. Quercetin mediates the phosphorylation levels of p38, ERK, JNK and MAPKs in LPS-induced RAW264.7 macrophages [54]. Moreover, quercetin inhibits TLR2 mRNA expression and suppresses NF-κB activity by influencing p65 and p50 nuclear translocation [55].
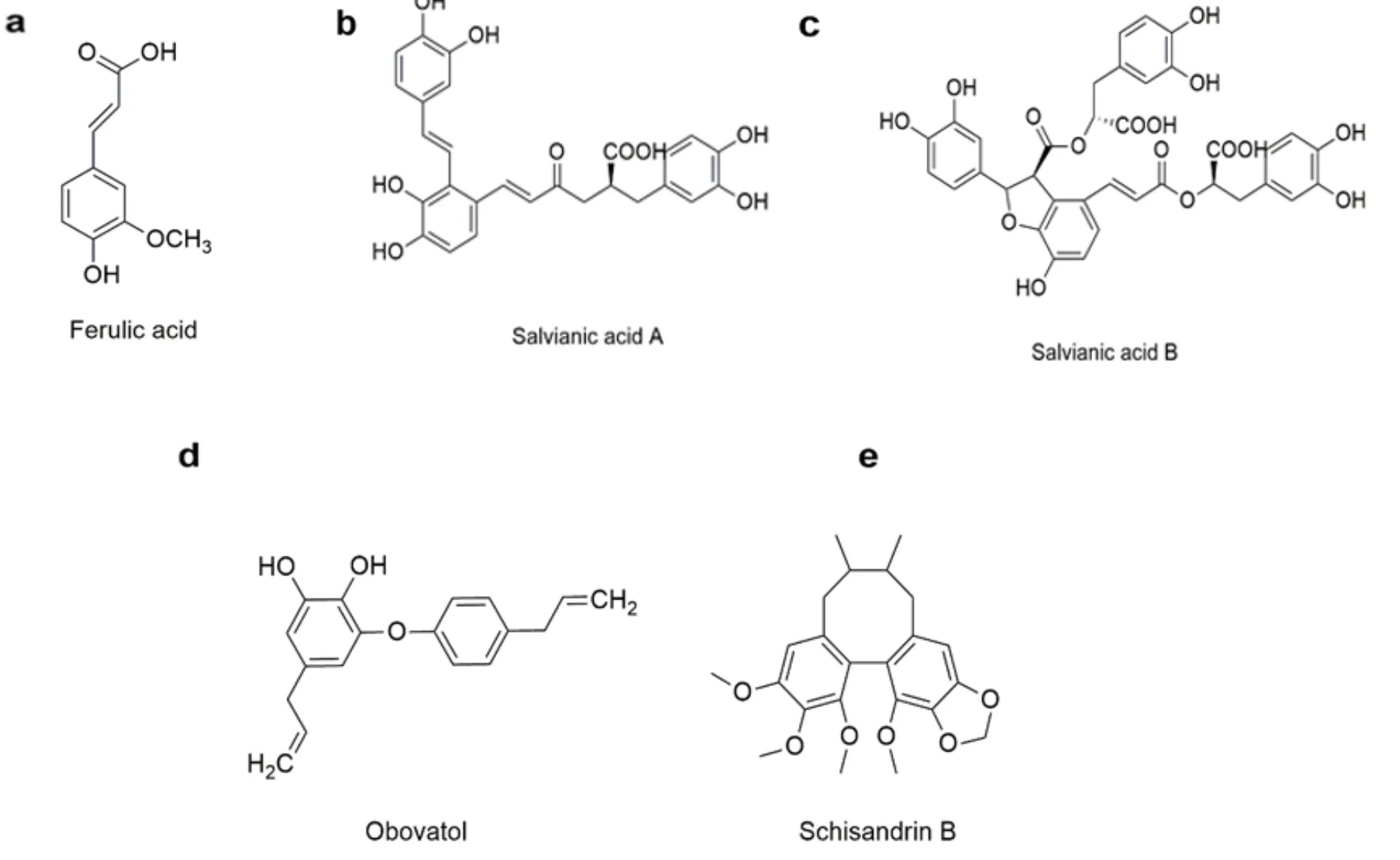
Figure 3 Chemical structures of Ferulic acid, Obovatol, Schisandrin B, Salvianic acid A and Salvianic acid B
Figure 4 Chemical structures of Shikonin, Quercetin andLuteolin
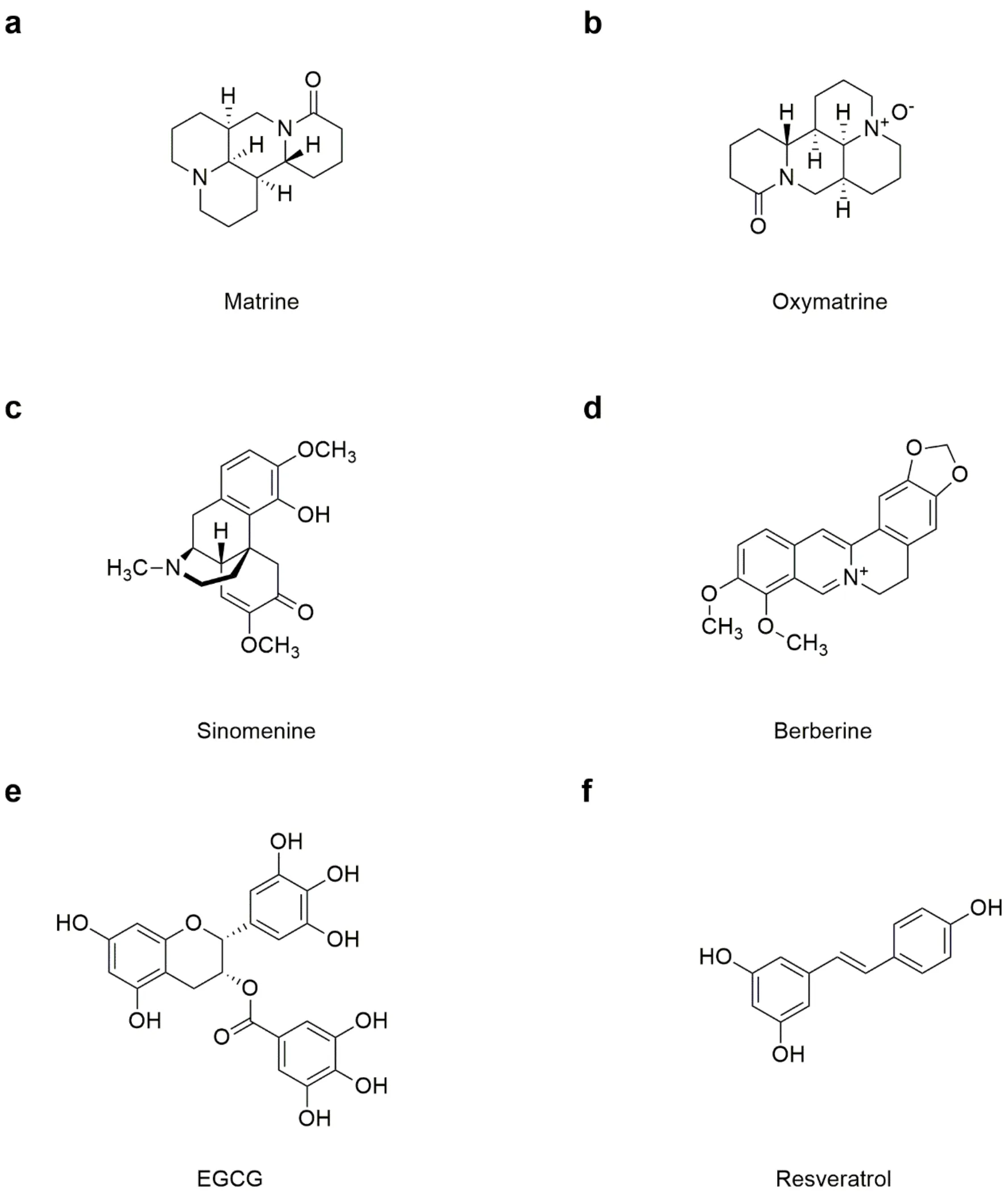
Figure 5 Chemical structures of Matrine, Oxymatrine, Sinomenine, Berberine, EGCG and Resveratrol
Luteolin
Luteolin possesses anti-oxidant, anti-cancer, anti-inflammatory, and neuroprotective effects (Fig 4c). Recently, luteolin is reported to show anti-inflammatory activities on pancreatitis, colitis, neuroinflammation and high glucose-induced inflammation. Meanwhile, luteolin decreases the protein expressions of major histocompatibility complex-Ⅱ, iNOS, COX-2, IL-1β, IL-8 and IL-6 [56, 57]. Genes related with inflammation are also inhibited, such as IL-6, IL-1β, NF-κB1, chemokine ligand (Ccl)2 [58]. It is worth mentioning that luteolin suppresses the nuclear translocation of NF-κB via blockade of ATP binding in Src and Syk [59]. Furthermore, luteolin down-regulates inflammatory mediators by regulating heme oxygenase-1 level [60].
Alkaloid
Matrine and oxymatrine
Matrine and oxymatrine are mainly from the roots of Chinese herb Kushen (Ait), which is traditionally used for the treatment of pain in thein 341 A.D. (Dongjin Dynasty of China) (Fig 5a, 5b). In recent years, the anti-inflammatory effects of matrine and oxymatrine have been confirmed by a large number of evidence bothand, including airway inflammation, hepatitis, enteritis, rheumatoid arthritis and the inflammatory response in the central nervous system like experimental autoimmune encephalomyelitis [61]. Mechanism studies reveal that matrine down-regulates inflammatory mediators including TNF-α, IL-1β, IL-6, MCP-1 and ROS, by suppressing NF-κB/IκBphosphorylations and increasing IκB expression [62]. Moreover, matrine also inhibitsphospholipase A2 and protein kinase Cactivations [63, 64].
Sinomenine
Sinomenine is an isoquinoline alkaloid with analgesic, anti-osteolysis, anti-inflammatory, and immuno- suppressory effects from Chinese herb Qingfengteng (), which is mentioned in(Fig 5c). In clinical trials, sinomenine is extensively used for the treatment of rheumatic arthritis. Mechanism study reveal sinomenine inhibits the phosphorylations of ERK and p38 MAPKs, which are two key players for inflammation progress [65]. Secondary, sinomenine inactivates NADPH oxidase which is a key enzyme for extracellular ROS production, and antagonizes dopaminergic neurotoxicity mediated by activated microglia [66]. Moreover, sinomenine blocks NF-κB through suppressing IκB degradation [67]. Another observation suggests that sinomenine also targets α7 nicotinic acetylcholine receptor to inhibit NF-κB pathway with decreasing inflammatory mediators including TNF-α and IL-6 [68]. Furthermore, sinomenine decreases MyD88 to exert an effective therapeutic effect for inflammation-induced joint destructive progression [69]. More recently, sinomenine has been reported to show inhibition on inflammatory pain by regulating GluN2B receptor and mTOR pathway [70].
Berberine
Berberine is an isoquinoline alkaloid from Chinese herb Huanglian (Franch.), which is famous as cathartics in thepublished in the third century A.D.(Fig 5d). Berberine is found to reduce airway inflammation induced by cigarette smoke, which is a key pathogenic feature of chronic airway diseases. Berberine also shows efficacy in experimental colitis, gouty arthritis and the inflammatory response in atherosclerosis. Notably, berberine alleviates postoperative cognitive dysfunction by suppressing microglia-mediated TNF-α, IL-1β, and IL-6 productions in aged mice [71]. Furthermore, berberine exerts protective effect against myocardial ischemia/reperfusion injury by inhibiting TNF-α, IL-6, superoxide generation, gp91 phox expressions [72]. Mechanism studies suggest that berberine inhibits TLR4/MyD88-dependent NF-κB pathway [73]. Moreover, AMPK-dependent activating transcription factor-3 pathway is targeted by berberine to block MAPK phosphorylation and suppress inflammatory mediator productions [74]. Berberine also exerts anti-inflammatory effect by activating Nrf2 anti-oxidant pathway as well as AMPK-dependent autophagy [75].
Polyphenols
EGCG
Epigallocatechin-3-gallate (EGCG) is a strong antioxidant compound from green tea (Fig 5e). EGCG prevents the progresses of various inflammatory diseases such as rheumatic arthritis, infection, atherosclerosis, and allergies. Currently, IKK/IкB/NF-κB has been reported to be the main inflammation pathway associated with EGCG [76]. Besides, some other inflammatory pathways are also involved, such as MAPKs [77], STATs (signal transducers and activators) of transcription [78], and matrix degrading enzymes [79]. We speculate that EGCG may exert its anti-inflammatory effect by mediating several different inflammatory pathways. Besides, these pathways interact with each other to form an immunoregulatory network.
Resveratrol
Resveratrol is a phytoalexin produced by numerous plants, which was firstly isolated from the root of Chinese herb Maoyelilu () in 1940’s (Fig 5f).experiments have indicated that resveratrol shows significant anti-inflammatory effect in various cell types including macrophages, T3T preadipocytes, endothelial cells, smooth muscle cells, chondrocytes, and microglial cells. Currently, resveratrol is reported to regulate COX-1/2 [80], Leukotriene A4 hydrolase [81], estrogen receptor [82],death-associated protein kinase1 [83], suggesting that resveratrol may exert anti-inflammatory effect via acting on multiple cell targets. Moreover, animal studies based on arthritis, inflammatory bowel disease, asthma and obesity models also confirm the anti-inflammatory effects of resveratrol observed.
Conclusion
Currently, numerous natural products show markedly inhibitory effects on inflammatory responses. Particularly, natural products can provide abundant molecular skeletons as well as pharmacophores; therefore, rational structure modification is an important strategy for new anti-inflammatory drug development. Thus, it is instructive for us today to take a look at the key roles of natural products in new drug development again. Natural products could help to alleviate inflammatory symptoms such as fever and pain. Such nutraceutical compounds may reduce infection risk, prevent inflammatory disease development and protect against other diseases in clinics.
Though dramatic advances have been made in discovering novel anti-inflammatory drugs from natural source, the detailed pharmacological mechanisms and drug targets involved are still unknown and largely unexplored. Therefore, a great amount of continuous efforts combined with chemical biology, cell biology as well as molecular pharmacology are still needed for clarifying these problems in the future.
1. Karlowicz-Bodalska K, Han S, Freier J,. Curcuma longa as medicinal herb in the treatment of diabetic complications. Acta Poloniae Pharmaceutica 2017, 74: 605-610.
2. Miranda AS, Brant F, Rocha NP.. Further evidence for an anti-inflammatory role of artesunate in experimental cerebral malaria. Malaria J 2013, 12: 388.
3. Jiang D, Chen Y, Hou X,. Influence of Paeonia lactiflora roots extract on cAMP-phosphodiesterase activity and related anti-inflammatory action. J Ethnopharmacol 2011, 137: 914-920.
4. Niu Y, Dong Q, Li R. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-κB signaling. Cell Biol Int 2017, 41: 611-621.
5. Yu J, Xiao Z, Zhao R,. Paeoniflorin suppressed IL-22 via p38 MAPK pathway and exerts anti-psoriatic effect. Life Sci 2017, 180: 17-22.
6. Gu JH, Ge JB, Li M,. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm Sci 2012, 47: 652-660.
7. Zhou JM, Gu SS, Mei WH,. Ginkgolides and bilobalide protect BV2 microglia cells against OGD/reoxygenation injury by inhibiting TLR2/4 signaling pathways. Cell Stress Chaperones 2016, 21: 1037-1053.
8. Yu WH, Dong XQ, Hu YY,. Ginkgolide B reduces neuronal cell apoptosis in the traumatic rat brain: possible involvement of toll-like receptor 4 and nuclear factor kappa B pathway. Phytother Res 2012, 26: 1838-1844.
9. Li R, Chen B, Wu W,. Ginkgolide B suppresses intercellular adhesion molecule-1 expression via blocking nuclear factor-κB activation in human vascular endothelial cells stimulated by oxidized low-density lipoprotein. J Pharmacol Sci 2009, 110: 362-369.
10. Chu X, Ci X, He J,. A novel anti-inflammatory role for ginkgolide B in asthma via inhibition of the ERK/MAPK signaling pathway. Molecules 2011, 16: 7634-7648.
11. Shao F, Tan T, Tan Y,. Andrographolide alleviates imiquimod-induced psoriasis in mice via inducing autophagic proteolysis of MyD88. Biochem Pharmacol 2016, 115: 94-103.
12. Shao F, Tan T, Tan Y,. Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Biochem Pharmacol 2016, 115: 94-103.
13. Seo JY, Pyo E, An JP,. Andrographolide Activates Keap1/Nrf2/ARE/HO-1 Pathway in HT22 Cells and Suppresses Microglial Activation by Aβ42 through Nrf2-Related Inflammatory Response. Mediators Inflamm 2017, 2017: 5906189.
14. Chen HW, Lin AH, Chu HC,. Inhibition of TNF-α-Induced Inflammation by Andrographolide via Down-Regulation of the PI3K/Akt Signaling Pathway. J Nat Prod 2011, 74: 2408-2413.
15. Sun B, Hu S, Li Y,. Triptolide contributes to decrease in TLR4 expression by upregulating miR-224-3p to inhibit the inflammatory reaction in diabetic nephropathy. Oncotarget 2016, 7: 85675.
16. Zheng L, Jia J, Dai H. Triptolide-Assisted Phosphorylation of p53 Suppresses Inflammation- Induced NF-κB Survival Pathways in Cancer Cells. Mol Cell Biol 2017, 37: e00149-17.
17. Chen C, Yang S, Zhang M,. Triptolide mitigates radiation-induced pneumonitis via inhibition of alveolar macrophages and related inflammatory molecules. Oncotarget 2017, 8: 45133-45142.
18. Yang Y, Ye Y, Qiu Q,. Triptolide inhibits the migration and invasion of rheumatoid fibroblast-like synoviocytes by blocking the activation of the JNK MAPK pathway. Int Immunopharmacol 2016, 41: 8-16.
19. Wu Z, Uchi H, Morino-Koga S,. Z-ligustilide ameliorated ultraviolet B-induced oxidative stress and inflammatory cytokine production in human keratinocytes through upregulation of Nrf2/HO-1 and suppression of NF-κB pathway. Exp Dermatol 2015, 24: 703-708.
20. Wang J, Du JR, Wang Y,, Z-ligustilide attenuates lipopolysaccharide-induced pro- inflammatory response via inhibiting NF-kappaB pathway in primary rat microglia. Acta Pharmacol Sin 2010, 31: 791-797.
21. Su YW, Chiou WF, Chao SH,. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol 2011, 11: 1166-1172.
22. Zhao LX, Du JR, Zhou HJ. Differences in Proinflammatory Property of Six Subtypes of Peroxiredoxins and Anti-Inflammatory Effect of Ligustilide in Macrophages. PLoS One 2016, 11: e0164586.
23. Kannaiyan R, Shanmugam MK, Sethi G. Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett 2011, 303: 9-20.
24. Lee JH, Koo TH, Yoon H,. Inhibition of NF-κB activation through targeting IκB kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol 2006, 72: 1311-1321.
25. Hu M, Luo Q, Alitongbieke G,. Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol Cell 2017, 66: 141-153.
26. Hu M, Luo Q, Alitongbieke G,. Ginsenoside Rb1 attenuates acute inflammatory nociception by inhibition of neuronal ERK phosphorylation by regulation of the Nrf2 and NF-kappaB pathways. Mol Cell 2017, 66: 141-153.
27. Chen W, Wang J, Luo Y,. Ginsenoside Rb1 and compound K improve insulin signaling and inhibit ER stress-associated NLRP3 inflammasome activation in adipose tissue. J Ginseng Res 2016, 40: 351-358.
28. Luo Y, Fu C, Wang Z,. Asiaticoside attenuates the effects of spinal cord injury through antioxidant and anti‑inflammatory effects, and inhibition of the p38‑MAPK mechanism. Mol Med Rep 2015, 12: 8294-8300
29. Chen S, Yin ZJ, Jiang C,. Asiaticoside attenuates memory impairment induced by transient cerebral ischemia-reperfusion in mice through anti-inflammatory mechanism. Pharmacol Biochem Behav 2014, 122: 7-15.
30. Fong LY, Ng CT, Zakaria ZA,. Asiaticoside inhibits TNF‐α‐induced endothelial hyper- permeability of human aortic endothelial cells. Phytother Res 2015, 29: 1501-1508.
31. Wan J, Gong X, Jiang R,. Antipyretic and anti-inflammatory effects of asiaticoside in lipopolysaccharide-treated rat through up-regulation of heme oxygenase-1. Phytother Res 2013, 27: 1136-1142.
32. Ahmad SF, Khan B, Bani S,. Amelioration of adjuvant-induced arthritis by ursolic acid through altered Th1/Th2 cytokine production. Pharmacol Res 2006, 53: 233-240.
33. Lu J, Wu DM, Zheng YL,. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb Cortex 2010, 20: 2540-2548.
34. Lu J, Wu DM, Zheng YL,. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. Cereb Cortex 2010, 20: 2540-2548.
35. Ma JQ, Ding J, Xiao ZH,. Ursolic acid ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney by inhibiting the STAT3 and NF-κB activities. Int Immunopharmacol 2014, 21: 389-395.
36. Lampiasi N, Montana G. The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated Raw 264.7 cells. Immunobiology 2016, 221: 486-493.
37. Almeida A, Delgado-Esteban M, Bolaños JP,. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J Neurochem 2002, 81: 207-217.
38. Lin WC, Peng YF, Hou CW. Ferulic acid protects PC12 neurons against hypoxia by inhibiting the p-MAPKs and COX-2 pathways. Iran J Basic Med Sci 2015, 18: 478-484.
39. Song Y, Wen L, Sun J.. Cytoprotective mechanism of ferulic acid against high glucose-induced oxidative stress in cardiomyocytes and hepatocytes. Food Nutr Res 2016, 60: 30323.
40. Sun Y, Zhu H, Wang J,. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J Chromatogr B Analyt Technol Biomed Life Sci 2009, 877(8-9): 733-737.
41. Zhao GR, Zhang HM, Ye TX,. Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem Toxicol 2008, 46: 73-81.
42. Yang FG, Chen ZY, Lian ZX,. Inhibitory effects of Salvianolic acid B on myocardial cellular nuclear transfer of NF-κB p65 and the expression of TNF-α during ischemia-reperfusion in rabbits hearts in vivo. Chin J Mod Med 2009, 19: 672-675, 679.
43. Zhang Y, Wang J, Wu GJ,. Salvianolic Acid Binhibits TLR4-NFκB-TNFα Pathway and Attenuates Neonatal rat Cardiomyocytes Damage Induced by Hypoxia. Liaoning J Tradit Chin Med 2012, 39: 20-22.
44. Chen YH, Lin SJ, Ku HH,. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem 2001, 82: 512-521.
45. Choi MS, Lee SH, Cho HS,. Inhibitory effect of obovatol on nitric oxide production and activation of NF-κB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells. Eur J Pharmacol 2007, 556(1-3): 181-189.
46. Ock J, Han HS, Hong SH,. Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Br J Pharmacol 2010, 159: 1646-1662.
47. Checker R, Patwardhan RS, Sharma D,. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-κB. Free Radic Biol Med 2012, 53: 1421-1430.
48. Giridharan VV, Thandavarayan RA, Arumugam S,. Schisandrin B ameliorates icv-infused amyloid β induced oxidative stress and neuronal dysfunction through inhibiting RAGE/NF-κB/MAPK and up-regulating HSP/Beclin expression. PLoS One 2015, 10: e0142483.
49. Liu N, Zheng JX, Zhuang YS,. Anti-inflammatory effects of schisandrin B on LPS-Stimulated BV2 microglia via activating PPAR-γ. Inflammation 2017, 40: 1006-1011.
50. Shindo S, Hosokawa Y, Hosokawa I,Shikonin inhibits inflammatory cytokine production in human periodontal ligament cells. Inflammation 2016, 39: 1124-1129.
51. Yang Y, Wang J, Yang Q,. Shikonin inhibits the lipopolysaccharide-induced release of HMGB1 in RAW264.7 cells via IFN and NF-kappaB signaling pathways. Int Immunopharmacol 2014, 19: 81-87.
52. Yoshida LS, Kakegawa T, Yuda Y. Shikonin changes the lipopolysaccharide-induced expression of inflammation-related genes in macrophages. J Nat Med 2017, 71: 723-734.
53. Haleagrahara N, Miranda-Hernandez S, Alim MA,. Therapeutic effect of quercetin in collagen-induced arthritis. Biomed Pharmacother 2017, 90: 38-46.
54. Cho YH, Kim NH, Khan I,. Anti-inflammatory potential of Quercetin-3-O-beta-D-("2"-galloyl)- glucopyranoside and quercetin isolated from diospyros kaki calyx via suppression of MAP signaling molecules in LPS-induced RAW 264.7 macrophages. J Food Sci 2016, 81: 2447-2456.
55. Tang Y, Li J, Gao C,. Hepatoprotective effect of quercetin on endoplasmic reticulum stress and inflammation after intense exercise in mice through phosphoinositide 3-Kinase and Nuclear Factor-Kappa B. Oxid Med Cell Longev 2016, 2016: 8696587.
56. Hytti M, Szabó D, Piippo N,. Two dietary polyphenols, fisetin and luteolin, reduce inflammation but augment DNA damage-induced toxicity in human RPE cells. J Nutr Biochem 2017, 42: 37-42.
57. Funaro A, Wu X, Song M,. Enhanced anti-inflammatory activities by the combination of luteolin and tangeretin. J Food Sci 2016, 81: H1320-1327.
58. Kure A, Nakagawa K, Kondo M,. Metabolic fate of luteolin in rats: its relationship to anti-inflammatory effect. J Agric Food Chem 2016, 64: 4246-4254.
59. Lee JO, Jeong D, Kim MY,. ATP-binding pocket-targeted suppression of src and syk by luteolin contributes to its anti-inflammatory action. Mediators Inflamm 2015, 2015: 967053.
60. Sung J, Lee J. Anti-inflammatory activity of butein and luteolin through suppression of NFkappaB activation and induction of heme oxygenase-1. J Med Food 2015, 18: 557-564.
61. Kan QC, Lv P, Zhang XJ,. Matrine protects neuro-axon from CNS inflammation-induced injury. Exp Mol Pathol 2015, 98: 124-130.
62. Sun D, Wang J, Yang N,. Matrine suppresses airway inflammation by downregulating SOCS3 expression via inhibition of NF-κB signaling in airway epithelial cells and asthmatic mice. Biochem Biophys Res Commun 2016, 477: 83-90.
63. Qiu G, Tu ZG, Li XW,. Effect of matrine on PLA_2 activity of LPS-induced inflammatory rats and its mechanism. Chin Tradit Herb Drugs 2002, 33: 630-632.
64. Hu ZL, Tan YX, Zhang JP,. Effects of inhibitor of protein kinase C on brain edema formation evoked by experimental cerebral ischemia in gerbils and rats. Acta pharmaceutica Sinica 1996, 31: 886.
65. Oh YC, Kang OH, Kim SB,. Anti-inflammatory effect of sinomenine by inhibition of pro-inflammatory mediators in PMA plus A23187-stimulated HMC-1 Cells. Eur Rev Med Pharmacol Sci 2012, 16: 1184-1191.
66. Qian L, Xu Z, Zhang W,. Sinomenine, a natural dextrorotatory morphinan analog, is anti- inflammatory and neuroprotective through inhibition of microglial NADPH oxidase. J Neuroinflammation 2007, 4: 23.
67. Teng P, Liu HL, Zhang L,. Synthesis and biological evaluation of novel sinomenine derivatives as anti-inflammatory agents. Eur J Med Chem 2012, 50: 63-74.
68. Yi L, Luo JF, Xie BB,. Alpha7 nicotinic acetylcholine receptor is a novel mediator of sinomenine anti-inflammation effect in macrophages stimulated by lipopolysaccharide. Shock 2015, 44: 188-195 .
69. Mu H, Yao RB, Zhao LJ,. Sinomenine decreases MyD88 expression and improves inflammation-induced joint damage progression and symptoms in rat adjuvant-induced arthritis. Inflammation 2013, 36: 1136-1144.
70. Li S, Han J, Wang DS,. Sinomenine attenuates chronic inflammatory pain in mice. Metab Brain Dis 2016, 32: 1-9.
71. Zhang Z, Li X, Li F. Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int Immunopharmacol 2016, 38: 426-433.
72. Yu L, Li Q, Yu B.. Berberine attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation response: role of silent information regulator 1. Oxid Med Cell Longev 2016, 2016: 1689602.
73. Gu L, Li N, Gong J,. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J Infect Dis 2011, 203: 1602-1612.
74. Bae YA, Cheon HG. Activating transcription factor-3 induction is involved in the anti-inflammatory action of berberine in RAW264.7 murine macrophages. Korean J Physiol Pharmacol 2016, 20: 415-424.
75. Zhou H, Feng L, Xu F,. Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: A new mechanism linking berberine to insulin resistance improvement. Biomed Pharmacother 2017, 89: 864-874.
76. Singh R, Ahmed S, Islam N,. Epigallocatechin-3-gallate inhibits interleukin-1beta- induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum 2002, 46: 2079-2086.
77. Bae JY, Choi JS, Choi YJ,. (−)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: Involvement of mitogen-activated protein kinase. Food Chem Toxicol 2008, 46: 1298-1307.
78. Tedeschi E, Suzuki H, Menegazzi M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann N Y Acad Sci 2002, 973: 435-437.
79. Ahmed S, Pakozdi A, Koch AE. Regulation of interleukin-1β-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 2006, 54: 2393-2401.
80. Annabi B, Lord-Dufour S, Vézina A,. Resveratrol targeting of carcinogen-induced brain endothelial cell inflammation biomarkers MMP-9 and COX-2 is sirt1-independent. Drug Target Insights 2012, 6: 1-11.
81. Oi N, Jeong CH, Nadas J,. Resveratrol, a red wine polyphenol, suppresses pancreatic cancer by inhibiting leukotriene A₄hydrolase. Cancer Res 2010, 70: 9755-9764.
82. Bowers JL, Tyulmenkov VV, Jernigan SC,. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 2000, 141: 3657-3667.
83. Mukhopadhyay R, Ray PS, Arif A,. DAPK-ZIPK-L13a axis constitutes a negative- feedback module regulating inflammatory gene expression. Mol Cell 2008, 32: 371-382.
:
This work was supported by grants from the National Key Technology R & D Program “New Drug Innovation” of China (No. 2017ZX09101003-008-003) and the Natural Science Foundation of China (No. 81773932).
:
PF, Paeoniflorin; GB, Ginkgolide B; Andro, Andrographolide; UA, Ursolic acid; FA, Ferulic acid; EGCG, Epigallocatechin-3-gallate; NF-κB, Nuclear factor kappa-light-chain-enhancer of activated B cells; IκB, Inhibitor of NF-κB; MyD88, Myeloid differentiation primary response gene 88; NLRP3, Nucleotide-binding oligomerization domain-like receptor protein 3; IL, Interleukin; Nrf2, Nuclear erythroid 2-related factor 2; MAPKs, Mitogen-activated protein kinases; COX-2, Cyclooxygenase-2; TNF-α, Tumor necrosis factor-α; VEGF, Vascular endothelial growth factor; Akt, Protein kinase B; IκK, Inhibitor of nuclear factor kappa-B kinase; PGE2, Prostaglandin E2; iNOS, Inducible nitric oxide synthase; CXCR, CXC chemokine receptor; PPAR, Peroxisome proliferator-activated receptor; ROS, Reactive oxygen species; Prx2, Peroxiredoxin 2; TLR, Toll-like receptor; Ccl, Chemokine ligand; MCP, Monocyte chemoattractant protein.
:
The authors declare that they have no conflict of interest.
:
Yan-Hang Wang, Ke-Wu Zeng. Natural products as a crucial source of anti-inflammatory drugs: recent trends and advancements. Traditional Medicine Research 2019, 4 (5): 257-268.
:Cui-Hong Zhu.
: 27 October 2018,
26 November 2018,
: 11 December 2018.
10.12032/TMR20190831133
Ke-Wu Zeng, State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing, China. E-mail: ZKW@bjmu.edu.cn.
 Traditional Medicine Research2019年5期
Traditional Medicine Research2019年5期
- Traditional Medicine Research的其它文章
- Assessment of microwave assisted and hydrodistllation extraction on Echinops persicus essential oils chemical composition and evaluation of its biological activity
- Quantitation of phytochemical constituents of Fumaria vaillantii L. with different extract methods
- Prescribing Chinese patent medicines without traditional Chinese medicine training is now banned in China
- Immunomodulatory effect of schisandrae oil in mouse model of autoimmune hepatitis induced by concanavalin A
- Plant distribution and pharmacological activity of flavonoids
- Antitumor applications of nano-traditional Chinese medicine
