不同危险分级胃间质瘤的超声内镜特征分析
栾明亚 徐永红 齐兴四 陈浩 毛涛 房良

[摘要]目的分析不同危險分级胃间质瘤(GISTs)的超声内镜(EUS)特征的差异性及其影响因素。方法收集经EUS检查、并经病理明确诊断为胃GISTs病人142例,分析其一般资料及EUS特征,总结不同危险分级胃GISTs的EUS特征的差异性及其影响因素。结果142例胃GISTs中,71例位于胃底(50.0%),60例位于胃体(42.3%),11例位于胃窦(7.7%)。胃GISTs直径为0.50~8.00 cm,平均(2.42±1.63)cm。根据术后病理肿瘤大小及核分裂象,分为高危组10例(7.0%)、中危组15例(10.6%)、低危组47例(33.1%)、极低危组70例(49.3%)。138例(97.0%)起源于固有肌层,1例起源于黏膜肌层(0.7%),3例起源于黏膜下层(2.0%)。85例表现为回声不均(59.9%),32例存在液化表现(22.5%),18例存在钙化(12.7%),13例病变表面溃疡形成(9.2%)。单因素分析显示,不同危险分级组肿瘤大小(F=7.872,P<0.01)、起源层次(χ2=14.994,P<0.05)、回声异质性(χ2=21.472,P<0.01)、边界不清(χ2=9.970,P<0.05)、表面溃疡(χ2=21.352,P<0.01)、液化(χ2=23.388,P<0.01)发生率差异有统计学意义,而年龄、性别、肿瘤位置、钙化、小叶间隔差异无统计学意义(P>0.05)。将低危、极低危归类为低危组,中危、高危分级归类为较高危险分级GISTs,Logistic多因素回归分析显示,EUS下病变回声不均匀(OR=5.07,95%CI=2.42~10.62,P<0.01)、边界不规则(OR=6.80,95%CI=1.46~31.6,P<0.05)、存在液化(OR=7.80,95%CI=2.79~21.79,P<0.01)的GISTs为较高危险分级的风险增加。结论EUS显示内部回声不均、液化、边界不规则的胃GISTs病人病理较高危险分级的概率增加。
[关键词]胃肠道间质肿瘤;腔内超声检查;危险性评估
[中图分类号]R735;R445.1[文献标志码]A[文章编号]2096-5532(2019)03-0312-05
[ABSTRACT]ObjectiveTo analyze the endoscopic ultrasonographic (EUS) features of gastric stromal tumors (GISTs) with different risk classifications and their influencing factors. MethodsThe clinical data of 142 patients were collected whose GISTs were diagnosed by EUS and further confirmed by pathology. The general data and EUS features were then analyzed, and the differences in EUS features between GISTs with different risk classifications and their influencing factors were summarized as well. ResultsOf the 142 cases of GISTs, 71(50.0%) were located in the gastric fundus, 60(42.3%) in the gastric body, and 11(7.7%) in the gastric antrum. The diameter of GISTs ranged from 0.50 cm to 8.00 cm, with a mean of (2.42±1.63) cm. According to the size and mitotic count of postoperative tumors, 10 patients (7.0%) were assigned to high-risk group, 15(10.6%) to medium-risk group, 47(33.1%) to low-risk group, and 70(49.3%) to extremely low-risk group. Of all the cases, 138(97.0%) originated from the muscularis propria, 1(0.7%) from the muscularis mucosa, and 3(2.0%) from the submucosa. Eighty-five cases (59.9%) showed echo heterogeneity, 32(22.5%) had liquefaction, 18(12.7%) had calcification, and 13(9.2%) had surface ulceration. Univariate analysis showed that there were significant differences regarding tumor size (F=7.872,P<0.001), layer of origin (χ2=14.994,P<0.05), echo heterogeneity (χ2=21.472,P<0.01), unclear boundary (χ2=21.352,P<0.01), surface ulceration (χ2=21.352,P<0.01), and liquefaction (χ2=23.388,P<0.01) in GISTs with different risk classifications. However, there were no significant differences in age, gender, tumor location, calcification, and interlobular septum (P>0.05). The low-, and extremely low-risk GISTs were categorized as lower-risk GISTs, The medium-, and high-risk GISTs were categorized as higher-risk GISTs. Logistic multivariate regression analysis showed that echo heterogeneity (OR=5.07,95% CI=2.42-10.62,P<0.01), irregular boundary (OR=6.80,95% CI=1.46-31.6,P<0.05), and liquefaction (OR=7.80,95% CI=2.79-21.79,P<0.01) were associated with an increased risk of higher risk classification. The differences between their influencing factors were significant. ConclusionEUS showed an increased risk of higher risk classification in GISTs with echo heterogeneity, liquefaction, and irregular boundary.
[KEY WORDS]gastrointestinal stromal tumors; endosonography; risk assessment
胃肠道间质瘤(GISTs)是消化系统常见的间叶源性肿瘤,占消化道肿瘤的0.1%~3.0%[1],最常见于胃(60%)[2],起源于卡哈尔细胞或其前体细胞,其生物学行为可表现为良性及恶性分化,有研究表明20%~30%胃GISTs表现为恶性分化[3]。目前,GISTs侵袭危险性判定主要参照NIH2008(改良版)标准或Fletcher标准[4-6],根据肿瘤大小、核分裂象分为极低、低、中、高危危险,不同危险分级的胃GISTs预后也不同。多数学者认为GISTs具有一定的恶性潜能,以侵袭危险性评估其生物学行为更为合理。NILSSON等[7]研究认为,高度危险分级的GISTs复发率及肿瘤引起的死亡率明显高于中低度危险组。故GISTs的危险性评估对于治疗方式的选择至关重要。超声内镜(EUS)可以观察肿瘤起源层次、回声高低、边界、浸润深度、大小等情况,是目前胃GISTs的主要检查方法。已有研究显示,EUS在胃GISTs的危险性评估中有重要价值[8],但目前国内研究不同侵袭危险性分级胃GISTs的EUS图像特点差异的文献较少。本研究通过回顾性分析142例胃GISTs病人的临床资料、EUS特征与病理危险分级,探讨不同危险分级胃GISTs与EUS特征的相关性及其影响因素。
1资料和方法
1.1病例及其来源
收集2016年8月—2018年6月于我院行EUS检查、并经病理检查确诊为胃GISTs病人142例,年龄29~83岁,平均(58±10)岁。其中78例完成内镜下切除术,其余64例行手术治疗。
1.2EUS检查方法
使用OlympusUM-DP20-25R、GF-UE260或GF-UCT260型EUS进行检查,记录内镜下肿瘤超声特征,包括部位、大小、起源层次、表面有无溃疡、回声特性、边界及有无钙化、液化、小叶间隔等。
1.3相关变量定义
病变部位包括胃底、胃体、胃角及胃窦;起源层次包括固有肌层、黏膜下层、黏膜肌层;胃GISTs的侵袭危险分级参照NIH2008(改良版)标准进行评估,侵袭危险性分为极低、低、中、高危[4-5]。极低危组(A组)为胃GISTs核分裂象≤5/50 HPF,最大径≤2 cm;低危组(B组)胃GISTs核分裂象≤5/50 HPF,2 cm<肿瘤最大径<5 cm;中危组(C组)胃GISTs核分裂象(6~10)/50 HPF,肿瘤最大径≤5 cm;高危组(D组)胃GISTs最大径>5 cm,核分裂象计数不限。
1.4统计学分析
采用SPSS 20.0软件进行统计学分析,计量资料结果以±s形式表示,多组间比较采用单因素ANOVA方差分析;计数资料以百分比表示,组间比较采用卡方检验;多因素分析采用Logistis回归分析。P<0.05为差异有统计学意义。
2结果
2.1胃GISTs病人一般资料与肿瘤危险分级的相关性
胃GISTs病人142例中,极低危险分级70例,低危分级47例,中危分级15例,高危分级10例。男60例,女82例,男女比例为1∶1.36。瘤体位于胃底71例(50.0%),胃体60例(42.3%),胃窦11例(7.7%)。单因素分析显示,胃GISTs病人不同危险分级组间性别、年龄、肿瘤部位差异无统计学意义(P>0.05)。见表1。
2.2EUS检查
所有胃GISTs病人均有胃黏膜下病变,其中138例(97.2%)起源于固有肌层,1例起源于黏膜肌层(0.7%),3例起源于黏膜下层(2.0%)。138例病人(97.2%)病灶呈低回声改变,85例(59.9%)表现为回声不均,32例(22.5%)存在液化表现,18例(12.7%)病灶存在钙化,13例(9.2%)病变表面存在溃疡。
2.3病理检查
本组病例均经术后病理明确危险分级,其中极低危组70例,肿瘤直径0.5~2.0 cm,平均(1.20±0.42)cm;低危组47例,肿瘤直径2.0~5.0 cm,平均(3.08±0.68)cm;中危组47例,肿瘤直径1.7~9.8 cm,平均(4.57±1.85)cm(其中有2例胃GISTs最大径<2 cm,分别为1.7、1.8 cm,均为女性,年龄分别为55、67岁;病变分别位于胃底、胃体,均起源于固有肌层;EUS呈低回声表现,内部回声不均匀,未见明显钙化、液化);高危组10例,肿瘤直径1.8~8.0 cm,平均为(4.80±2.14)cm(其中1例直径为1.8 cm,女性,年龄63岁,病变位于胃底,起源于固有肌层;EUS提示低回声,病变呈类圆形,向腔内外突出,以腔内为主,内部回声尚均匀,边界尚规则,无液化、钙化及表面溃疡形成;彩色多普勒示无血流信号,弹性成像以蓝色为主。
2.4EUS特征与胃GISTs不同危险分级的相关性单因素分析
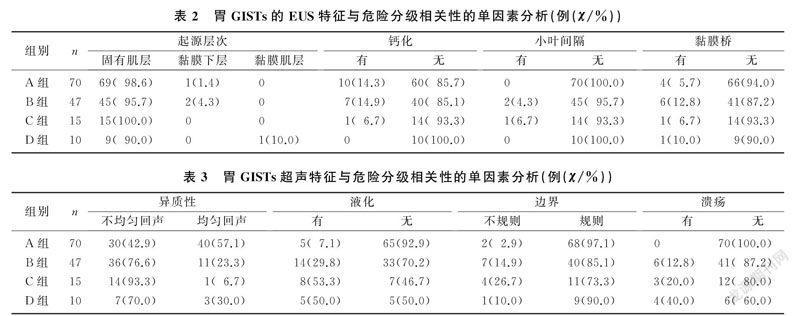
单因素分析结果显示,胃GISTs不同危险分级组间肿瘤大小(F=7.872,P<0.01)、起源层次(χ2=14.994,P<0.05)、回声异质性(χ2=21.472,P<0.01)、边界不清(χ2=9.970,P<0.05)、表面溃疡(χ2=21.352,P<0.01)以及液化发生率(χ2=23.388,P<0.01)差异有统计学意义,而年龄、性别、肿瘤位置、钙化、小叶间隔组间差异无统计学意义(P>0.05)。见表2、3。
2.5EUS特征与胃GISTs不同危险分级相关性的Logistic多因素分析
将胃GISTs极低危、低危分级归类为低危组,中危、高危分级归类为较高危险分级,进一步行Logistic多因素回归分析显示,EUS下病变回声不均匀(OR=5.07,95%CI=2.42~10.62,P<0.01)、边界不规则(OR=6.80,95%CI=1.46~31.60,P<0.05)、存在液化(OR=7.80,95%CI=2.79~21.79,P<0.01)的GISTs為较高危险分级的风险增加。因本组病例中起源于黏膜下层的胃GISTs共3例,术后病理分别为极低、低危分级;起源于黏膜肌层1例,术后病理证实为高危分级,所以未对其起源层次进行Logistic回归分析。因极低危分级胃GISTs中均无溃疡的形成,所以没有对溃疡进行Logistic回归分析。
3討论
胃GISTs是最常见的消化道黏膜下肿瘤,年发病率为2.9%~3.5%[4,7-8],其生物学行为可以表现为良性、恶性潜能或恶性[5]。目前,国际上采用分级标准(NIH2008(改良版))综合肿瘤大小和核分裂象将胃GISTs分为极低危险、低危险、中危险、高危险等4个分级。不同危险分级胃GISTs的预后不同,TATEISHI等[9]研究认为,胃肠道GISTs危险分级越高病死率越高,复发和转移越多见。NCCN指南(2016年第2版)[10]报道,原发性极低危分级胃GISTs转移或肿瘤相关病死率为0,低危分级胃GISTs转移或肿瘤相关病死率<4%,中危分级胃GISTs病死率为4%~16%,高危分级胃GISTs死亡及远处转移发生率可达55%~86%。2017年《中国胃肠间质瘤诊断治疗共识》[11]提出,极低度侵袭危险性胃GISTs可选择内镜下随访,而低度、中度、高度侵袭危险性GISTs则建议内镜下切除或外科手术治疗。因此,术前胃GISTs侵袭危险分级对于治疗方式的选择、疾病预后具有重要指导意义。
胃GISTs病人临床症状无特异性,可表现为腹痛、恶心、嗳气等症状,当肿瘤体积增大或破溃可能出现腹痛加重、呕血、黑便等[12]。随着内镜的发展,越来越多的胃GISTs于内镜检查中被发现,常规内镜下其表现为黏膜下隆起性病变(SMTs)[13],但普通内镜无法观察肿瘤大小、形态等特征,常规活检也无法获取病变组织,EUS逐渐成为诊断胃GISTs的主要检查手段。
EUS对胃GISTs的诊断有独特的优势,能够清晰显示肿瘤的位置、起源层次、大小、边界、回声、边缘形态等特征[14-15]。EUS下,典型的胃GISTs好发于胃底,多数起源于胃固有肌层,少数起源于黏膜肌层[3]。林戴英等[16]对53例胃GISTs进行回顾性研究显示,男女患病比例接近1∶1,平均年龄54.8岁。卢光荣等[17]研究显示,胃GISTs好发于胃底(53.8%)。本研究病人发病平均年龄为(58±10)岁,男女比例为1∶1.36,50.0%胃GISTs发生于胃底,42.3%位于胃体,7.7%发生于胃窦;单因素分析显示不同危险分级胃GISTs病人性别、年龄、肿瘤发生部位差异无统计学意义,说明年龄、性别、肿瘤胃内分布位置与胃GISTs不同危险分级无相关性,与既往研究结果相似[18]。
EUS也可以根据肿瘤大小、边界、液化等超声特征对胃GISTs进行良恶性鉴别,阳性预测值达80%[14-15],对不同危险分级胃GISTs治疗方案的选择具有重要指导意义。有研究对38例胃GISTs病人分析显示,术前危险分级评估与术后病理分级符合率为79%[19]。PALAZZO[20]研究结果显示,肿瘤直径>4 cm、表面溃疡、回声不均匀伴液化、边界不规则等是GISTs的恶性特征。JEON等[21]的研究发现,直径>3 cm、边界不规则、表面溃疡形成均提示较高危险分级。还有研究显示,侵袭危险性高的胃GISTs在EUS下常表现出回声不均匀、液化、溃疡形成、外形不规则、钙化等特点 [17,22-24]。本研究回顾性分析142例胃GISTs的一般资料及EUS特征,结果显示,不同危险分级的胃GISTs病人肿瘤大小、起源层次、回声异质性、表面溃疡、液化发生率等存在差异,EUS诊断为胃GISTs且存在回声不均、液化、边界不规则表现者病理危险分级为较高危险分级的概率增加,因极低危分级胃GISTs中均无溃疡形成,所以未对溃疡行Logistic回归分析。该研究结果与国外研究基本一致。
对于直径≤2 cm的间质瘤称为小间质瘤,其生物学行为多表现为良性或惰性经过,但有极少数病例可表现恶性潜能[25-27]。薛倩等[28]对≤2 cm的胃GISTs病人进行 EUS 随访,结果显示肿瘤直径及面积在短期均有增加趋势。SAWAKI等[29]报道1例胃GISTs病人肿瘤直径在2年内由1.8 cm增大至10.0 cm,且在2年内死亡。本文研究中,肿瘤最大直径<2 cm的胃GISTs病人中有3例术后病理明确诊断为中高危分级,其中1例术后病理提示高危分级,EUS图像呈类圆形,边界欠规则;余2例为中危分级,EUS均表现为内部回声不均匀。提示肿瘤大小并不是判断胃GISTs危险分级的绝对指标。EUS表现为回声不均、液化或溃疡形成,同样提示较高侵袭危险可能,这与相关研究结果一致[30]。
综上所述,不同危险分级胃GISTs病人的年龄、性别、肿瘤位置分布均无差异性,EUS拟诊为胃GISTs且EUS下存在液化、回声不均、边界不规则表现病人病理危险分级为较高危的概率增加,应积极行内镜或手术治疗。对于EUS拟诊胃GISTs但EUS图像无特征性表现、且肿瘤直径≤2 cm的病变应EUS下随访,一旦出现肿瘤直径增大、回声不均、液化、边界不规则等表现应积极干预。EUS在胃GISTs危险性评估中有重要作用,可为临床医师选择治疗方案提供依据。
[参考文献]
[1]荣光宏,刘芝兰,马颖才,等. 819例上消化道黏膜下病变的超声内镜诊断分析[J]. 中华消化内镜杂志, 2017,34(6):437-438.
[2]KIM M N, KANG S J, KIM S G, et al. Prediction of risk of malignancy of gastrointestinal stromal tumors by endoscopic ultrasonography[J]. Gut and Liver, 2013,7(6):642-647.
[3]CROOM K F, PERRY C M. Imatinib mesylate:in the treatment of gastrointestinal stromal tumours[J]. Drugs, 2003,63(5):513-522.
[4]MUENST S, THIES S, WENT P, et al. Frequency, phenotype, and genotype of minute gastrointestinal stromal tumors in the stomach: an autopsy study[J]. Human Pathology, 2011,42(12):1849-1854.
[5]MURPHEY M D. World Health Organization classification of bone and soft tissue tumors: modifications and implications for radiologists[J]. Seminars in Musculoskeletal Radiology, 2007,11(3):201-214.
[6]FLETCHER C D, BERMAN J J, CORLESS C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach[J]. Human Pathology, 2002,33(5):459-465.
[7]NILSSON B, PER BMMING, MEIS-KINDBLOM J M, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in Western Sweden[J]. Cancer, 2005,103(4):821-829.
[8]ROSSI S, MICELI R, MESSERINI L, et al. Natural history of imatinib-naive GISTs: a retrospective analysis of 929 cases with long-term follow-up and development of a survival nomogram based on mitotic index and size as continuous variables[J]. American Journal of Surgical Pathology, 2011,35(11):1646-1656.
[9]TATEISHI U, HASEGAWA T, SATAKE M, et al. Gastrointestinal stromal tumor-correlation of computed tomography findings with tumor grade and mortality[J]. Journal of Computer Assisted Tomography, 2003,27(5):792-798.
[10]VON M M, RANDALL R L, BENJAMIN R S, et al. Soft Tissue Sarcoma, Version 2.2018, NCCN clinical practice guidelines in oncology[J]. Journal of the National Comprehensive Cancer Network: JNCCN, 2018,16(5):536.
[11]中国临床肿瘤学会胃肠间质瘤专家委员会. 中国胃肠间质瘤诊断治疗共识(2017 年版)[J]. 肿瘤综合治疗(电子杂志), 2018,4(1):31-43.
[12]FAULX A L, KOTHARI S, ACOSTA R D, et al. The role of endoscopy in subepithelial lesions of the GI tract[J]. Gastrointestinal Endoscopy, 2017,85(6):1117-1132.
[13]NISHIDA T, KAWAI N, YAMAGUCHI S, et al. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors[J]. Digestive Endoscopy, 2013,25(5):479-489.
[14]JI Feng, WANG Ziwei, WANG Lijun, et al. Clinicopathological characteristics of gastrointestinal mesenchymal tumors and diagnostic value of endoscopic ultrasonography[J]. Journal of Gastroenterology and Hepatology, 2008,23(8 Pt 2):e318-e324.
[15]史良玉,王玉平,任茜,等. 胃腸道间质瘤治疗的研究进展[J]. 医学综述, 2017,23(19):3764-3768.
[16]林黛英,吴先衡,汪丹凤,等. 胃肠道间质瘤CT 表现与肿瘤危险程度的相关性评价[J]. 医学影像学杂志, 2014,24(8):1342-1345.
[17]卢光荣,周羽翙,钟金伟,等. 不同侵袭危险性胃间质瘤的超声内镜图像特点比较及相关危险因素分析[J]. 中国内镜杂志, 2016,22(1):1-4.
[18]HOU Y Y, LU S H, ZHOU Y, et al. Predictive values of clinical and pathological parameters for malignancy of gastrointestinal stromal tumors[J]. Histology & Histopathology, 2009,24(6):737-747.
[19]王晓凡,谭诗云,李明,等. 术前超声内镜检查对胃间质瘤危险性判断及治疗方式选择分析[J]. 中华全科医师杂志, 2014,13(6):452-456.
[20]PALAZZO L. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours[J]. Gut, 2000,46(1):88-92.
[21]JEON S W, PARK Y D, CHUNG Y J, et al. Gastrointestinal stromal tumors of the stomach: endosonographic differentiation in relation to histological risk[J]. Journal of Gastroente-rology and Hepatology, 2007,22(12):2069-2075.
[22]CHEN T H, HSU C M, CHU Y Y, et al. Association of endoscopic ultrasonographic parameters and gastrointestinal stromal tumors (GISTs): can endoscopic ultrasonography be used to screen gastric GISTs for potential malignancy[J]? Scandinavian Journal of Gastroenterology, 2015,51(3):1-4.
[23]郎翠翠,李玉红,董新茜. 超声内镜下胃间质瘤特征与病理诊断相关性研究[J]. 中华消化内镜杂志, 2011,28(6):305-308.
[24]彭春艳,吕瑛,徐桂芳,等. 术前超声内镜对胃间质瘤的诊断及侵袭危险性评估价值研究[J]. 中华消化内镜杂志, 2015,32(6):361-366.
[25]YU Qingxiang, HE Zhankun, WANG Jiang, et al. Clinical presentations of gastric small gastrointestinal stromal tumors mimics functional dyspepsia symptoms[J]. World Journal of Gastroenterology, 2014,20(33):11800-11807.
[26]KIM I H, KWAK S G, CHAE H D. Prognostic factors of patients withgastric gastrointestinal stromal tumor after curative resection:aretrospective analysis of 406 consecutive cases in a multicenterstudy[J]. Eur Surq Res, 2015,55(1/2):12-23.
[27]DEMETRI G D, VON MEHREN M, ANTONESCU C R, et al. NCCN task force report: update on the management of patients with gastrointestinal stromal tumors[J]. Journal of the National Comprehensive Cancer Network: JNCCN, 2010,8(Suppl 2): S1.
[28]薛倩,文娟,王晶桐. 超聲内镜下胃间质瘤的影像特征及随访研究[J]. 中国超声医学杂志, 2015,31(11):991-993.
[29]SAWAKI A, MIZUNO N, TAKAHASHI K, et al. Long-term follow up of patients with small gastrointestinal stromal tumors in the stomach using endoscopic ultrasonography-guided fine-needle aspiration biopsy[J]. Digestive Endoscopy, 2006,18(1):40-44.
[30]SEPE P S, BRUGGE W R. A guide for the diagnosis and ma-nagement of gastrointestinal stromal cell tumors[J]. Nature Reviews Gastroenterology & Hepatology, 2009,6(6):363-371.
(本文编辑 黄建乡)

