S100β、IL-1β与IL-6联合检测对青壮年颅脑损伤诊断及预后评估的价值
张鹏 徐志明 左安俊 刘维生 赵鹏 丁婷
[摘要] 目的 探討血清和脑脊液中S100蛋白质β(S100β)、白细胞介素1β(IL-1β)、白细胞介素6(IL-6)联合检测对青壮年创伤性颅脑损伤(TBI)诊断和预后评估的价值。方法 选取青岛市市立医院神经外科术后转入ICU治疗的青壮年TBI病人132例作为观察组(轻型35例,中型55例,重型42例),以同期需行脑脊液穿刺检查的非TBI住院青壮年病人50例作为对照组。采用电化学发光法检测两组病人血清和脑脊液中S100β、IL-1β、IL-6的含量。应用受试者工作特征曲线(ROC曲线)分析S100β、IL-1β、IL-6联合检测对TBI的诊断价值,分析血清S100β、IL-1β、IL-6水平与TBI病人预后的相关性。结果 观察组重型病人血清和脑脊液中S100β、IL-1β、IL-6含量均显著高于对照组(F=8.351~8.967,P<0.05)。观察组血清中S100β、IL-1β、IL-6的含量与脑脊液中的含量均呈正相关关系(r=0.83~0.89,P<0.05)。血清S100β、IL-1β、IL-6检测及三者联合检测诊断TBI的ROC曲线下面积分别为0.810、0.758、0.703和0.922。观察组血清和脑脊液中S100β、IL-1β、IL-6的表达水平与青壮年TBI预后呈负相关关系(F=8.671~9.371,P<0.05)。结论 血清和脑脊液中S100β、IL-1β、IL-6水平与青壮年TBI病人病情严重程度和预后密切相关,且三者联合检测诊断效率高于单独检测。
[关键词] 颅脑损伤;S100蛋白质类;白细胞介素1β;白细胞介素6;诊断;预后
[中图分类号] R446.1;R651 [文献标志码] A [文章编号] 2096-5532(2019)04-0461-05
[ABSTRACT] Objective To investigate the value of combined measurement of S100β, interleukin-1β (IL-1β), and interleukin-6 (IL-6) in serum and cerebrospinal fluid in the diagnosis and prognostic evaluation of young adults with traumatic brain injury (TBI). Methods A total of 132 young adult patients with TBI who underwent surgery and were then transferred to the intensive care unit in Department of Neurosurgery in Qingdao Municipal Hospital were enrolled as observation group, among whom 35 had mild TBI, 55 had moderate TBI, and 42 had severe TBI; 50 young adult patients without TBI who were hospitalized and underwent cerebrospinal fluid puncture were enrolled as control group. Electrochemical luminescence was used to measure the levels of S100β, IL-1β, and IL-6 in serum and cerebrospinal fluid. The receiver operating characteristic (ROC) curve was used to observe the value of combined measurement of S100β, IL-1β, and IL-6 in the diagnosis of TBI, and the correlation of serum S100β, IL-1β, and IL-6 with the prognosis of TBI patients was analyzed. Results The patients with severe TBI had significantly higher levels of S100β, IL-1β, and IL-6 in serum and cerebrospinal fluid than those in the control group (F=8.351-8.967,P<0.05). In the observation group, the levels of S100β, IL-1β, and IL-6 in serum were positively correlated with their levels in cerebrospinal fluid (r=0.83-0.89,P<0.05). The measurement of serum S100β, IL-1β, or IL-6 alone had an area under the ROC curve of 0.810, 0.758, and 0.703, respectively, while combined measurement of serum S100β, IL-1β, and IL-6 had an area of 0.922. In the observation group, the expression levels of S100β, IL-1β, and IL-6 in serum and cerebrospinal fluid were negatively correlated with the prognosis of young adults with TBI (F=8.671-9.371,P<0.05). Conclusion The levels of S100β, IL-1β, and IL-6 in serum and cerebrospinal fluid are closely associated with disease severity and prognosis of young adult patients with TBI, and combined measurement of S100β, IL-1β, and IL-6 has a higher diagnostic efficiency than the measurement of S100β, IL-1β, or IL-6 alone.
[KEY WORDS] craniocerebral trauma; S100 proteins; interleukin-1beta; interleukin-6; diagnosis; prognosis
创伤性颅脑损伤(TBI)是神经外科常见的急症,颅脑外伤是青壮年的首要死亡原因[1]。因此, TBI的及时诊疗及其预后的精准判断就显得尤为重要。近年来的研究发现,TBI发生后机体产生级联应激性炎症反应和免疫应答,进而引起神经细胞损伤、凋亡[2-3]。很多生物标志物与TBI密切相关,如 S100蛋白质β(S100β)、白细胞介素1β(IL-1β)、白细胞介素6(IL-6)等[4-6]。目前,我国诊断TBI的主要方法是影像学检查,且临床中用以辅助判断的实验室指标大多为S100β[7],比较单一。本研究测定了青壮年TBI病人血清和脑脊液中S100β、IL-1β、IL-6的表达水平,采用受试者工作特征曲线(ROC曲线)[8]评估三者联合检测对TBI的诊断价值,并探讨上述指标对评估TBI预后的临床意义。
1 资料与方法
1.1 一般资料
2017年5月—2018年5月,选取青岛市市立医院神经外科术后转入ICU治疗的青壮年TBI病人132例作为观察组,其中男性77例,女性55例;年龄28~55岁,平均(40.5±11.2)岁。TBI病人均经临床确诊,均符合TBI诊疗指南的标准[9]。根据格拉斯哥昏迷评分(GCS评分)的标准[10],轻型35例(GCS评分13~15分),中型55例(GCS评分9~12分),重型42例(GCS评分3~8分)。以我院同期需行脑脊液穿刺检查的非TBI住院青壮年病人50例作为对照组,其中男33例,女17例;年龄 24~56 岁,平均(38.9±12.7)岁。两组病人的性别、年龄差异无统计学意义。两组病人采血和脑脊液前均未用药物,均无器官功能衰竭、肿瘤等严重疾病;排除消化道出血、血液病、颅内感染等影响因素;符合脑脊液采集的适应证。本研究经青岛市市立医院伦理委员会批准,病人及家属均知情同意。
1.2 研究方法
1.2.1 标本采集 TBI病人伤后12 h内于生化促凝管中采集静脉血5 mL,腰椎穿刺留取3 mL脑脊液。对照组病人因病情需要于入院后12 h内于生化促凝管中采集静脉血5 mL,腰椎穿刺留取脑脊液3 mL。所有标本均送我院检验科检查。
1.2.2 检测方法 应用ROCHE Cobase 602电化学发光仪(瑞士罗氏公司)及原装配套试剂盒,采用电化学发光法测定血和脑脊液中S100β、IL-1β、IL-6含量,操作严格按照说明书进行。
1.2.3 随访 本组病人均获得随访。采取电话或门诊随访的形式,由工作人员在病人出院3个月及6个月后分别进行随访。根据Glasgow预后分级(GOS)[10]评价病人目前的预后状态,4~5分为预后良好,2~3分为预后不良,1分为死亡。
1.3 统计学处理
采用SPSS 18.0软件进行统计学处理。符合正态分布计量资料结果以±s表示,组间比较采用方差分析;相关性分析采用Pearson 相关分析;绘制ROC曲线,并计算ROC曲线下面积(AUC),分析S100β、IL-1β、IL-6联合检测对TBI的诊断价值。以P<0.05为差异有统计学意义。
2 结 果
2.1 各组血清S100β、IL-1β、IL-6水平的比较
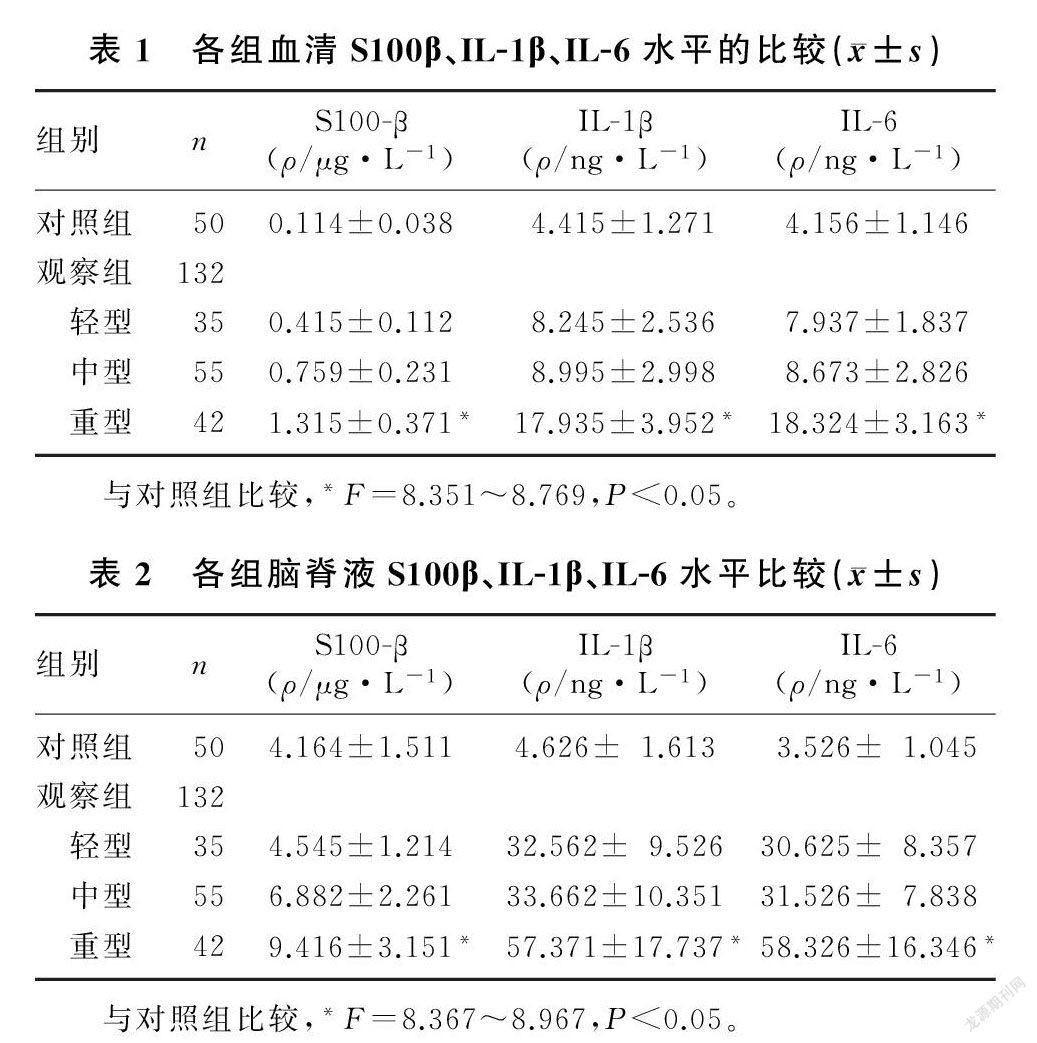
重型TBI病人血清S100β、IL-1β、IL-6水平均明显高于对照组病人,差异有统计学意义(F=8.351~8.769,P<0.05);轻型和中型TBI病人血清S100β、IL-1β、IL-6水平与对照组相比,差异均无统计学意义(P>0.05)。见表1。
2.2 各组脑脊液S100β、IL-1β、IL-6水平的比较
重型TBI病人脑脊液S100β、IL-1β、IL-6水平均明显高于对照组病人,差异有统计学意义(F=8.367~8.967,P<0.05);轻型和中型病人脑脊液S100β、IL-1β、IL-6水平与对照组相比,差异均无统计学意义(P>0.05)。见表2。
TBI病人血清中S100β、IL-1β、IL-6的含量与脑脊液中的含量均呈正相关关系(r=0.83~0.89,
2.4 血清S100β、IL-1β和IL-6联合检测对TBI的诊断价值
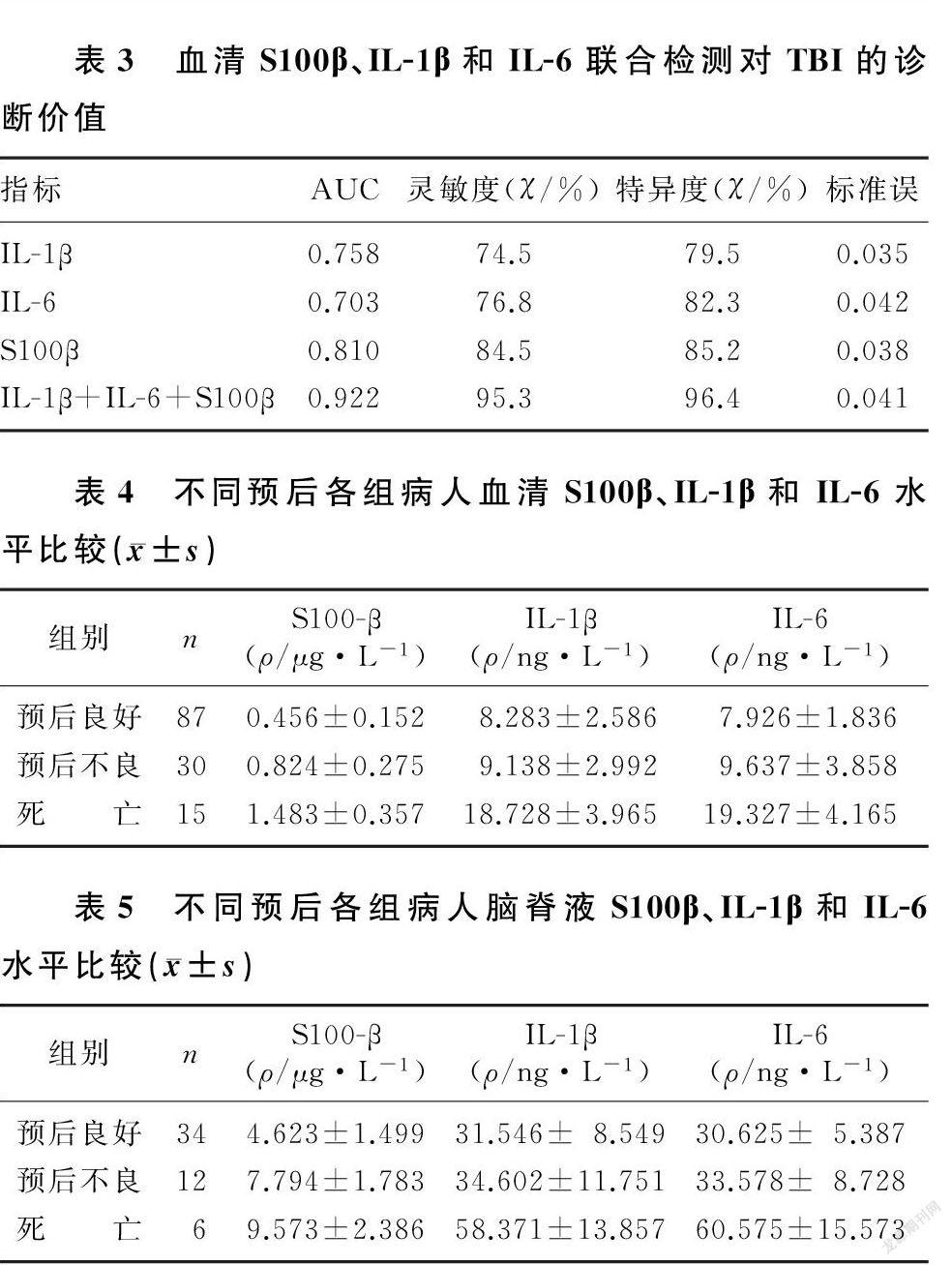
ROC曲线分析显示,血清S100β、IL-1β、IL-6检测及三者联合检测诊断TBI的AUC分别为0.810、0.758、0.703和0.922,以联合检测的灵敏度和特异度最高。见表3。
2.5 血清S100β、IL-1β和IL-6水平与TBI预后的相关性
随访6个月,获得之前采集血液标本病人的预后信息:死亡15例,预后不良30例,预后良好87例。不同预后各组病人血清S100β、IL-1β和IL-6表达水平比较差异均有统计学意义(F=8.671~8.849,P<0.05)。见表4。Spearman相关分析显示,血清S100β、IL-1β和IL-6水平均与GOS评分呈负相关关系(r=-0.426~-0.366,P<0.05),提示血清S100β、IL-1β和IL-6水平越高,GOS評分越低,病人预后越差。
2.6 脑脊液S100β、IL-1β和IL-6水平与TBI预后的相关性
随访6个月,获得之前采集脑脊液标本病人的预后信息:死亡6例,预后不良12例,预后良好34例。不同预后各组病人脑脊液S100β、IL-1β和IL-6水平比较差异均有统计学意义(F=8.948~9.371,P<0.05)。见表5。Spearman相关分析显示,脑脊液S100β、IL-1β和IL-6水平与GOS评分呈负相关关系(r=-0.372~-0.252,P<0.05),提示脑脊液S100β、IL-1β和IL-6水平越高,GOS评分越低,病人预后越差。
3 讨 论
TBI在外伤中仅次于四肢骨折,位居第2位,且主要患病人群为青壮年[11]。随着医疗水平的提高和先进医疗设备的引进,即使加以严密的护理和治疗,TBI病人仍有42.50%的病死率和15.00%的致残率[12]。TBI病人入院时通常情况紧急,大多临床医生只能依靠神经学检查和CT检查来进行即时诊断[13-14]。到目前为止,虽然还未有TBI诊断及预后评估的明确方法,但是有关生物标志物已成为TBI诊断及预后判断的首选[15]。生物标志物特指能够客观反映正常生理过程、致病过程的指示物,甚至能够反映治疗的药理学反应[16-18]。最新的研究显示,S100β以及炎症因子是具有研究价值的诊断TBI及
S100β属于Ca2+结合蛋白超家族,该超家族的蛋白质常作为重要的实验室生物标志物用于成人和儿科检验医学中[20-21]。脑损伤发生后,病人出现急性炎症反应,同时S100β的合成和分泌增加,以利于损伤部位的修复。TBI发生后,血-脑脊液屏障通透性增加,通过该屏障S100β于外周释放,引起外周血中S100β含量升高。本研究结果表明,血和脑脊液中S100β的含量与脑损伤的严重程度以及预后相关,这一结论与有关研究的观点一致[22]。
病人发生TBI后,大脑神经元因应激反应发生以炎症和免疫反应为主的损伤,进而导致脑组织死亡。有研究表明,細胞因子IL-1β和IL-6可促进炎症因子的聚集,从而导致血-脑脊液屏障受损[23-24]。生理状态下脑组织中的IL-1β和IL-6含量极少,缺血低氧状态下其含量会迅速增加,引起嗜酸性粒细胞、单核细胞以及中性粒细胞等大量炎症细胞聚集和激活,导致脑组织继发性水肿,同时也改变内环境稳态,使血管内外渗透压差增大,血管结构发生变化,进而破坏血-脑脊液屏障,引发脑组织继发性损伤[25]。本研究结果显示,IL-1β和IL-6的含量与脑损伤的严重程度以及预后相关,颅脑损伤越严重,血清及脑脊液中IL-1β和IL-6的含量越高,病人预后
TBI的年发病率为(180~250)/10万,青壮年是TBI的主要群体。TBI在青壮年死亡原因中占第1位,致死率高。因此,本研究选取28~55岁年龄段的病人为研究对象,探究TBI诊断及预后评估的敏感方法。本文研究结果显示,轻型和中型TBI病人S100β、IL-1β和IL-6水平与对照组比较差异均无统计学意义。这可能是由于轻型和中型TBI病人的神经细胞未被破坏,血-脑脊液屏障没有明显损伤性改变[2,26]。重型TBI病人S100β、IL-1β和IL-6水平与对照组相比较,差异具有统计学意义,且Spearman相关性分析显示三者的含量与GOS评分均呈负相关关系,提示TBI病人的S100β、IL-1β和IL-6水平与疾病严重程度相关,并且其含量变化与预后具有密切联系。同时,本研究采用ROC曲线分析,客观评价血清S100β、IL-1β和IL-6检测及三者联合检测对TBI的诊断价值。结果显示,血清S100β、IL-1β和IL-6水平诊断TBI的AUC分别为0.810、0.758和0.703,按照SWEETS的判断标准(AUC在0.7~0.9表明诊断试验具有相当的准确性,AUC大于0.9则代表准确性较高),三者可作为TBI的诊断标志物,并且可用于评判病情及指导预后。而S100β、IL-1β和IL-6联合检测的AUC为0.922,相较于单独检测某一指标,三者联合检测对TBI的诊断价值更高。
综上所述,血清和脑脊液中S100β、IL-1β、IL-6水平与青壮年TBI病人病情严重程度和预后密切相关,三者均可作为评价颅脑创伤程度和判断预后的辅助实验室指标,且三者联合检测诊断效率高于单独检测。
[参考文献]
[1] 张硕,王峰,孙奎胜,等. 重型颅脑损伤患者肺部感染发生率与吸烟的关系[J]. 中华神经外科疾病研究杂志, 2013,12(2):175-176.
[2] LASKOWSKI R A, CREED J A, RAGHUPATHI R. Pathophysiology of mild TBI:implications for altered signaling pathways[J]. Journal of Neuroinflammation, 2015,12(1):120-122.
[3] GRIFFIN G D. The injured brain:TBI, mTBI, the immune system, and infection:connecting the dots[J]. Military Medicine, 2011,176(4):364-368.
[4] HUIE J R, DIAZ-ARRASTIA R, YUE J K, et al. Testing a multivariate proteomic panel for traumatic brain injury biomarker discovery:a TRACK-TBI pilot study[J]. Journal of Neurotrauma, 2019,36(1):100-110.
[5] HASHIZAKI T, NISHIMURA Y, TERAMURA K A, et al. Differences in serum IL-6 response after 1 degrees C rise in core body temperature in individuals with spinal cord injury and cervical spinal cord injury during local heat stress[J]. International Journal of Hyperthermia, 2019,35(1):541-547.
[6] ORIS C, PEREIRA B, DURIF J, et al. The biomarker S100B and mild traumatic brain injury:a meta-analysis[J]. Pediatrics, 2018,141(6):e20180037.
[7] HOOSHMAND M, SOROUSHMEHR S M R, WILLIAMSON C, et al. Automatic midline shift detection in traumatic brain injury [J]. Conference Proceedings, 2018,2018:131-134.
[8] 鄒莉玲,余小金,闵捷,等. ROC曲线在医学诊断中的应用与进展[J]. 东南大学学报(医学版), 2003,22(1):67-70.
[9] CARNEY N, TOTTEN A M, O’REILLY C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition[J]. Neurosurgery, 2016,80(1):6-15.
[10] WANG Xiaogang, GAO Ding, LI Tao, et al. The correlation analysis of prehospital GCS score of brain injury patients and prognosis[J]. Chinese Journal for Clinicians, 2015,87(1):23-25.
[11] DEKOSKY S T, ASKEN B M. Injury cascades in TBI-related neurodegeneration[J]. Brain Injury, 2017,31(9):1177-1182.
[12] IORIO-MORIN C, FORTIN D, BLANCHARD J. TBI prognosis calculator:a mobile application to estimate mortality and morbidity following traumatic brain injury[J]. Clinical Neuro-logy and Neurosurgery, 2016,142:48-53.
[13] CROKE L. Mild TBI in children:guidance from the CDC for diagnosis and treatment[J]. American Family Physician, 2019,99(7):462-464.
[14] PIKSTRA A R A, METTING Z, FOCK J M, et al. The juvenile head trauma syndrome-deterioration after mild TBI:diagnosis and clinical presentation at the Emergency Department[J]. European Journal of Paediatric Neurology, 2017,21(2):344-349.
[15] THOMPSON W H, THELIN E P, LILJA A A, et al. Functional resting-state fMRI connectivity correlates with serum levels of the S100B protein in the acute phase of traumatic brain injury[J]. NeuroImage Clinical, 2016,12:1004-1012.
[16] BOGOSLOVSKY T, GILL J, JEROMIN A, et al. Fluid biomarkers of traumatic brain injury and intended context of use[J]. Diagnostics, 2016,6(4):37.
[17] AGOSTON D V, SHUTES-DAVID A, PESKIND E R. Bio-fluid biomarkers of traumatic brain injury[J]. Brain Injury, 2017,31(9):1195-1203.
[18] WANG K K, YANG Z H, ZHU T, et al. An update on diagnostic and prognostic biomarkers for traumatic brain injury[J]. Expert Review of Molecular Diagnostics, 2018,18(2):165-180.
[19] PARK S H, HWANG S K. Prognostic value of serum levels of S100 calcium-binding protein B,g neuron-specific enolase, and interleukin-6 in pediatric patients with traumatic brain injury[J]. World Neurosurgery, 2018,118:e534-e542.
[20] HEIZMANN C W. S100 proteins:diagnostic and prognostic biomarkers in laboratory medicine[J]. Biochimica et Biophysica Acta-Molecular Cell Research, 2019,1866(7):1197-1206.
[21] MICHETTI F, D’AMBROSI N, TOESCA A, et al. The S100B story:from biomarker to active factor in neural injury[J]. Journal of Neurochemistry, 2019,148(2):168-187.
[22] WELCH R D, AYAZ S I, LEWIS L M, et al. Ability of se-rum glial fibrillary acidic protein, ubiquitin C-terminal hydrolase-L1, and S100B to differentiate normal and abnormal head computed tomography findings in patients with suspected mild or moderate traumatic brain injury[J]. Journal of Neurotrauma, 2016,33(2):203-214.
[23] HELMY A, GUILFOYLE M R, CARPENTER K L, et al. Recombinant human interleukin-1 receptor antagonist promotes M1 microglia biased cytokines and chemokines following human traumatic brain injury[J]. Journal of Cerebral Blood Flow and Metabolism, 2016,36(8):1434-1448.
[24] WEBSTER K M, SUN M J, CRACK P, et al. Inflammation in epileptogenesis after traumatic brain injury[J]. Journal of Neuroinflammation, 2017,14(1):10-12.
[25] HELMY A, CARPENTER K L, MENON D K, et al. The cytokine response to human traumatic brain injury:temporal profiles and evidence for cerebral parenchymal production[J]. Journal of Cerebral Blood Flow and Metabolism, 2011,31(2):658-670.
(本文編辑 马伟平)

