Experimental Study of Pratia Extact on Acute Liver Injury in Mice
Junxiu LI Chanling JIANG Lichun ZHAO

Abstract [Objectives] This study was conducted to observe the protective effect of Pratia extract on acute liver injury in mice.
[Methods] Mice were randomly divided into 6 groups according to body weight, 10 in each group: normal control group, ethanol-induced/CCl4 liver injury model, low-dose, middle-dose and high-dose Pratia extract groups, and bifendatatum group. Except the blank group, other groups were given 50% ethanol intragastrically at a dose of 12 ml/kg to cause acute alcoholic liver injury, or intraperitoneally injected with 10% CCl4 soybean oil solution to cause acute CCl4 liver injury. The serum ALT and AST activity were measured as well as liver SOD and MDA concentrations.
[Results] Pratia extract effectively reduced serum ALT and AST, decreased MDA content and increased liver tissue SOD activity.
[Conclusions] Pratia extract has certain protective effect on acute liver injury.
Key words Pratia extact; Alcoholic liver injury; CCl4 liver injury; Protective effect
Liver disease has always been one of the diseases that plague human health. Liver injury is the liver damage caused by the invasion of the liver by external factors. At present, according to liver injury caused by different reasons, hepatitis can be divided into the following types: drug-induced hepatitis, alcoholic hepatitis and viral hepatitis. Liver injury caused by various harmful factors can be manifested as liver necrosis, fatty liver, cholestasis, liver fibrosis, liver cirrhosis, etc., and eventually develop into liver cancer.
As we all know, many Chinese herbal medicines have a certain protective effect on acute liver injury in that they can reduce the damage of exogenous substances to the liver. Pratia, also known as Xiaotongchui, Dikouzi, Tongchuicao and Dishiliu, refers to the dry whole plant of Pratia begonafolia (Wall) Lindl. or P. nummularia (Lam.) A. Br. et Aschers in Campanulaceae, which can be eaten as a kind of edible wild herb. Pratia has the effects of dispelling wind and eliminating dampness, promoting blood circulation and relieving internal heat or fever, and can be used to treat rheumatic pain, bruises, acute mastitis and innominate inflammation of unknown origin.
It has been reported in literatures that it contains flavonoids, polyacetylene, triterpene ester and other ingredients. Its pharmacological activity has rarely been reported by modern pharmacology. So far, no techniques have been used to prepare active extracts from Pratia, and no studies have been conducted on the use of Pratia as the main constituent in drugs or health care products for preventing liver injury.
Materials and Methods
Experimental animals
Male ICR mice, with a weight of (20±2) g, were purchased from Bethune medical university laboratory animal center, Jilin University. They were fed for 1 week before the formal experiment.
Experimental reagents
CCl4, Beijing Chemical Works; edible soybean oil; alanine aminotransferase (ALT) kit and aspartate aminotransferase (AST) kit and SOD kit, MDA kit and Coomassie brilliant blue kit, Nanjing Jiancheng Bioengineering Institute.
Experimental instruments
HC-2517 high-speed centrifuge: Anhui USTC Zonkia Scientific Instruments Co., Ltd.; DK-98-1 electric thermostatic water bath: Tianjin Taisite Instrument Co., Ltd.; continuous-spectrum scanning microplate reader SpectraMaxPlus384: American Molecular Biotechnology Company (Provided by the Engineering Research Center of Bioreactor and Pharmaceutical Development, Ministry of Education, Jilin Agricultural University).
Experimental methods
Preparation of Pratia extract
Pratia plants were collected from Guangxi, and identified as the dry whole plant of P. begonafolia or P. nummularia in Campanulaceae by Professor Zhang from Jilin Agricultural University. The raw material was pulverized to fine powder (200 mesh), which was extracted with ethanol for 3 times, and the extracts were merged and concentrated. The dregs were decocted with water for three times, and the decoctions were merged and concentrated. The two concentrates were merged, vacuum dried and pulverized, giving a Pratia extract.
Effect of CCl4 on acute liver injury in mice
The mice were fed for one week during which they were free to eat and drink. After one week, they were randomly divided into 6 groups according to body weight, 10 in each group: normal control group, CCl4 model group, low-dose (100 mg/(kg·d) of Pratia extract), middle-dose (200 mg/(kg·d)) and high dose groups (400 mg/(kg·d)), and bifendatatum group (positive control group, 150 mg/(kg·d)). All the drugs were dissolved in physiological saline with the assistance of ultrasound. Except for the blank control group and the model group which were administered the same amount of physiological saline by weight, other drug-administered groups were given corresponding drugs according to body weight intragastrically once daily, for 7 d in total. On the 6th d during the drug administration, the mice were fasted for 12 h at night, and 1 h after the normal administration on the next day, except for the blank control group, other groups were intraperitoneally injected with 10% CCl4 soybean oil solution, resulting in acute CCl4 liver injury. After 6 h, blood was taken from the eyeballs, and the serum was centrifuged at 4 200 rpm for 10 min. The supernatant was stored in a refrigerator at 4 ℃. Immediately after taking the blood, the mice were sacrificed by cervical dislocation, and the liver and spleen were taken by dissection. After weighing, the liver surface was cleaned with physiological saline, and the physiological saline on the surface was wiped off. Some livers were soaked with 10% formalin, and some were wrapped in tin foil, and immediately stored in a -80 ℃ refrigerator.
Testing of serological and liver indexes: The centrifuged serum was loaded into a 96-well plate according to the instructions of the ALT and AST kits, and the OD value was measured by a microplate reader to calculate the activity of ALT and AST in the serum of the mice.
A certain weight of the frozen liver was weighed, homogenized according to the weight ratio to cold physiological saline at 1∶9, and centrifuged at 4 200 rpm for 10 min in a refrigerated centrifuge, obtaining 10% liver homogenate. The preparation of the 10% liver homogenate was carried out at a low temperature. A small amount of the 10% liver homogenate was taken and diluted to 1% liver homogenate with cold physiological saline.
According to the instructions of Coomassie brilliant blue kit and the SOD and MDA kits, the samples were loaded in a 96-well plate, and the OD values were determined by a microplate reader. The liver protein concentration in the 1% liver homogenate and the activity of SOD in the livers were calculated, and the concentration of MDA in the 10% liver homogenate was also calculated.
Effect of ethanol-induced acute liver injury in mice
The mice were fed for one week during which they were free to eat and drink. After one week, they were randomly divided into 6 groups according to body weight, 10 in each group: normal control group, ethanol model group, low-dose (100 mg/(kg·d) of Pratia extract), middle-dose (200 mg/(kg·d)) and high dose groups (400 mg/(kg·d)), and bifendatatum group (positive control group, 150 mg/(kg·d)). All the drugs were dissolved in physiological saline with the assistance of ultrasound. Except for the blank control group and the model group which were administered the same amount of physiological saline by weight, other drug-administered groups were given corresponding drugs according to body weight intragastrically once daily, for 7 d in total. On the 6th d during the drug administration, the mice were fasted for 12 h at night, and 1 h after the normal administration on the next day, except for the blank control group, other groups were intragastrically given 50% ethanol at a dose of 12 ml/kg, resulting in ethanol-induced acute liver injury. After 12 h, blood was taken from the eyeballs, and the serum was centrifuged at 4 200 rpm for 10 min. The supernatant was stored in a refrigerator at 4 ℃. Immediately after taking the blood, the mice were sacrificed by cervical dislocation, and the liver and spleen were taken by dissection. After weighing, the liver surface was cleaned with physiological saline, and the physiological saline on the surface was wiped off. Some livers were soaked with 10% formalin, and some were wrapped in tin foil, and immediately stored in a -80 ℃ refrigerator.
Testing of serological and liver indexes: The centrifuged serum was loaded into a 96-well plate according to the instructions of the ALT and AST kits, and the OD value was measured by a microplate reader to calculate the activity of ALT and AST in the serum of the mice.
A certain weight of the frozen liver was weighed, homogenized according to the weight ratio to cold physiological saline at 1∶9, and centrifuged at 4 200 rpm for 10 min in a refrigerated centrifuge, obtaining 10% liver homogenate. The preparation of the 10% liver homogenate was carried out at a low temperature. A small amount of the 10% liver homogenate was taken and diluted to 1% liver homogenate with cold physiological saline.
According to the instructions of Coomassie brilliant blue kit and the SOD and MDA kits, the samples were loaded in a 96-well plate, and the OD values were determined by a microplate reader. The liver protein concentration in the 1% liver homogenate and the activity of SOD in the livers were calculated, and the concentration of MDA in the 10% liver homogenate was also calculated.
Results and Analysis
Effect of CCl4 on acute liver injury in mice
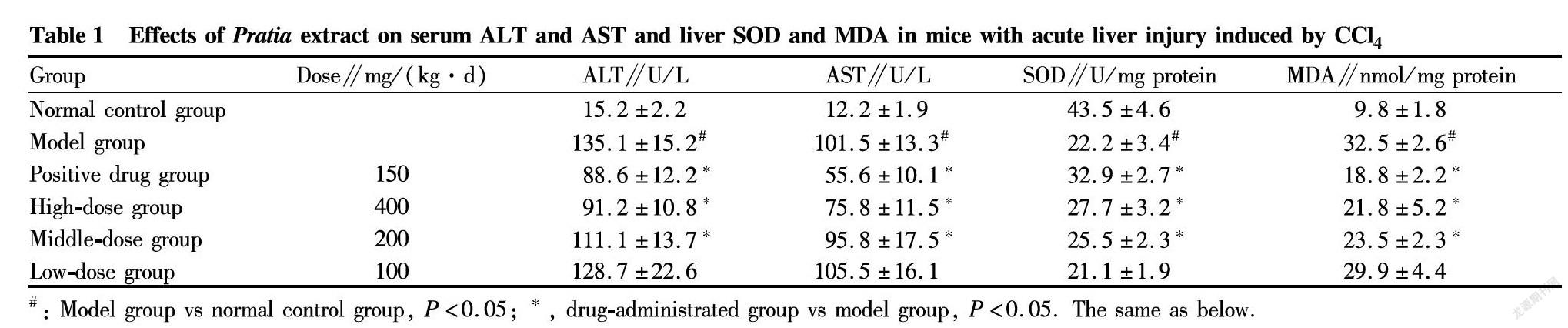
The results of this experiment showed that after CCl4 injection, the ALT and AST of the model group were significantly higher than the normal control group, suggesting successful modeling (P<0.05). Compared with the model group, the high-dose and middle-dose groups both can reduce the activity of increased ALT and AST in serum, exhibiting significant difference (P<0.05). Meanwhile, the high- and middle-dose Pratia extract groups can effectively reduce the MDA level of liver tissue and increase the SOD level of liver tissue, and the differences were significant (P<0.05). The low-dose group had a decreasing trend, which was not statistically significant (P>0.05). The specific results are shown in Table 1.
Effect of ethanol-induced acute liver injury in mice
The results of this study showed that after oral administration of 50% ethanol, the ALT and AST of the model group were significantly higher than the normal control group, suggesting successful modeling (P<0.05). Compared with the model group, the high- and middle-dose Pratia extract groups can reduce the activity of increased ALT and AST in serum, with significant differences (P<0.05). Meanwhile, the high- and middle-dose Pratia extract groups can effectively reduce the MDA level of liver tissue and increase the SOD level of liver tissue, with significant differences (P<0.05). The low dose group had a decreasing trend, which was not statistically significant (P>0.05). The specific results are shown in Table 2.
Conclusions and Discussion
The extract had a protective effect on mice with acute liver injury caused by CCl4/ethanol, which effectively reduced serum alanine aminotransferase and aspartate aminotransferase, decreased malondialdehyde content and increased liver tissue SOD activity.
It is proposed that Pratia extract can significantly reduce serum ALT and AST activity and liver tissue MDA content in CCl4/ethanol-induced liver injury model mice, and enhance liver SOD activity, indicating that the active extract has an obvious protective effect on chemical liver injury and alcoholic liver injury.
References
[1]Jiangsu New Medical College. Chinese medicine dictionary[M]. 1992. (in Chinese)
[2]LIU XK, QIU MH, LI ZR. Two new triterpene ester from Pratia begonafolia[J]. Natural Product Research and Development, 1999, 10(3): 20-23. (in Chinese)
[3]KANJI ISHIMARUA, MAIKO OSABEA, LI YANA. Plyacetylene glycosides from Pratia nummularia cultures[J]. Phytochemistry, 2003, (62): 643–646.
[4]ISHIMARU KANJI, MATSUURA ETSUKO. Flavonoids and a polyacetylene in Pratia nummularia-chemistry, bioactivity analysis and biotechnology[J]. Foods & Food Ingredients Journal of Japan, 2000, 186: 33-44.
- 农业生物技术(英文版)的其它文章
- Analysis of the Southern China Tilapia Production and Economic Benefits of Different Breeding Patterns in 2018
- Dynamic Monitoring and Control Measures of Spodoptera frugiperda (J.E.Smmith) in Low Latitude Plateau Sugarcane Areas
- Control Effects of a New Sex Pheromone Trap and Biological Agents on Sesamia inferens Walker and Argyroploce schistaceana (Snellen)
- Comparative Study on Grain Cadmium Content and Yield in Different Rice Varieties
- Simulation Experiment of Air Temperature Variation in Multi-film Covering at Night
- Identification of Growth-promoting Bacteria from Rhizosphere of Pastures and Their Effects on Growth of Lotus corniculatus L.

