Validation for Microbial Limit Test Method of Compound Yu E Nose Drops
Renhui YANG Peixue CAO Zhen ZENG Xiangling QU


Abstract [Objectives] This study was conducted to establish a method for microbial limit test of Compound Yu E Nose Drops.
[Methods] According to the Chinese Pharmacopoei (General Rules in the fourth part of the 2015 edition), the microbial limit test method for Compound Yu E Nose Drops was verified.
[Results] Compound Yu E Nose Drops has a strong inhibitory effect on Staphylococcus aureus and Bacillus subtilis, and the inhibitory activity was significantly eliminated after increasing the diluent (1∶20). The recoveries were all in the range of 0.5-2.0 when the total quantities of aerobic microbes were determined by the dilution method (1∶20). When the total quantities of mould and yeast were determined by the conventional method, the recoveries were both in the range of 0.5-2.0. When examining control bacteria, Escherichia coli, S. aureus and Pseudomonas aeruginosa can all be detected in the test groups by the test liquid dilution method.
[Conclusions] For Compound Yu E Nose Drops, the total quantities of aerobic microbe can be counted by the dilution method; the quantities of mould and yeast can be examined by the conventional plate method; and the conventional method can be used for control microbe examination.
Key words Compound Yu E Nose Drops; Microbial limit test; Dilution method
Compound Yu E Nose Drops is a traditional Chinese medicine prescription preparation prepared by The Second Affiliated Hospital of Guiyang College of Traditional Chinese Medicine. The prescription is sourced from Department of Otorhinolaryngology of the hospital, which has the effects of clearing away heat and toxic materials, dehumidifying, and dredging the orifices with aromatics, and is used for treating acute and chronic rhinitis, sinusitis (nasosinusitis) and allergic rhinitis[1]. In order to improve the quality of preparations in the hospital, according to Chinese Pharmacopoei (General Rules in the fourth part of the 2015 edition), methodological validation was performed to the applicability of the microbial limit test method for the preparation, and the microbial limit test method of the preparation was finally established, so as to ensure the accuracy and effectiveness of test results and improve drug safety[2].
Materials and Methods
Experiment materials
Samples
Compound Yu E Nose Drops, specification: 10 ml/bottle, lot number: 20181001, 20181002, 20181003, provided by The Second Affiliated Hospital of Guiyang College of Traditional Chinese Medicine.
Instruments
High-pressure sterilizer; purification operation room; clean bench; biosafety cabinet; oscillator; electro-heating thermostatic water bath; electro-heating standing-temperature cultivator.
Medium
The used media included sterile sodium chloride-peptone buffer solution (pH 7.0), trypticase soy agar medium, trypticase soy broth medium, Sabouraud dextrose agar medium, MacConkey broth medium, Maconkey agar medium, cetrimide agar medium and mannitol and sodium chloride agar medium. The media were all prepared and sterilized according to Ch.P2015 version by Beijing Land Bridge Technology Co., Ltd.
Strains
The strains were all of the third generation, including Pseudomonas aeruginosa [CMCC(B)10104], Staphylococcus aureus[CMCC(B)26003], Bacillus subtilis[CMCC(B)63501], Candida albicans[CMCC(F)98001], Aspergillus niger[CMCC(F)98003], and Escherichia coli[CMCC(B)44102]. All the strains were provided by National Institutes for Food and Drug Control.
Experiment methods
The experiment was carried out in a bioclean room attained the criteria of 104-grade cleanliness with unidirectional flow of air attained the criteria of 102-grade cleanliness, satisfying the requirements for "microbial limit test" in general rules in Chinese Pharmacopoeia (2015 edition)[3].
Preparation method of microbial liquids
(1) Each of S. aureus, P. aeruginosa, B. subtilis, and E. coli was cultured on trypticase soy broth medium at 34 ℃ for 24 h. Each culture was diluted with sterile sodium chloride-peptone buffer solution with a pH of 7.0 into a bacterial suspension with a bacterial concentration not higher than 10 000 cfu/ml.
(2) C. albicans was cultured in Sabouraud dextrose agar medium at 24 ℃ for 72 h. The culture was diluted with 0.9% sterile sodium chloride to a fungal concentration not higher than 10 000 cfu/ml by the 10-fold dilution method.
(3) One bottle of A. niger was cultured on Sabouraud dextrose agar slant at 24 ℃ for 7 d. The culture was added with 5 ml of 0.9% sterile sodium chloride solution containing 0.05% polysorbate 80, to wash off A. niger spores, which were sucked to a sterile test tube. The fungal suspension was diluted with 0.9% sterile sodium chloride solution containing 0.05% polysorbate 80 to a fungal suspension with a spore concentration not higher than 10 000 cfu/ml by the 10-fold dilution method.
Preparation of test liquid
A certain amount of Compound Yu E Nose Drops (10 ml) was added with sterile sodium chloride-peptone buffer solution (pH 7.0) to 100 ml. The mixture was oscillated in a water bath at 45 ℃, obtaining a 1∶10 test liquid for later use. In addition, the 1∶10 testing solution was also diluted with sterile sodium chloride-peptone buffer solution (pH 7.0) to 1∶20 and 1∶40 test liquids, to screen the method for eliminating the antimicrobial activity.
Control microbe examination
Method validation was performed according to "control microbe examination method" in General Rules 1106 in Chinese Pharmacopoeia (2015 edition).
Results and Analysis
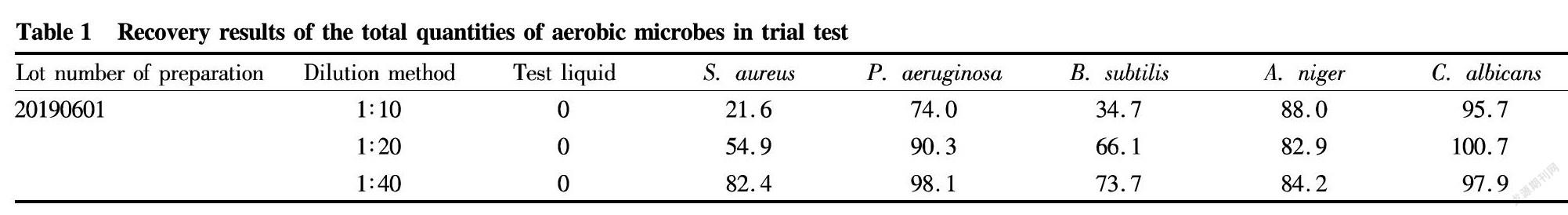
Total quantities of aerobic microbes
A certain amount of the 1∶10, 1∶20 and 1∶40 test liquids (10 ml) were added into sterilized test tubes, respectively, and then, 0.1 ml of corresponding testing microorganism was added when ensuring the final concentration not higher than 100 cfu/ml. After mixing well, 1 ml of corresponding microbial liquid was taken from its test tube and added into a plate, followed by solidification and culture at 30-35 ℃. Two plates were prepared for each strain in parallel. The number of colonies was determined in the test groups, microbial liquid groups and control groups of the test liquids by the same method. The recovery of each strain was calculated according to following formula: Recovery=(Average number of colonies in test group-Average number of colonies in the control group of test liquid/Average number of colonies in the control group of microbial liquid. The results are shown in Table 1.
The results showed that when performing aerobic microbes counting of this product by the conventional method (1∶10 test liquid), the recoveries of S. aureus and B. subtilis were all smaller than 0.5. Therefore, the conventional method is not suitable for the aerobic microbe counting of this product. Verification was performed by increasing dilution times (1∶20 and 1∶40), and the recoveries of above five kinds of aerobic microbes were in the range of 0.5-2.0.
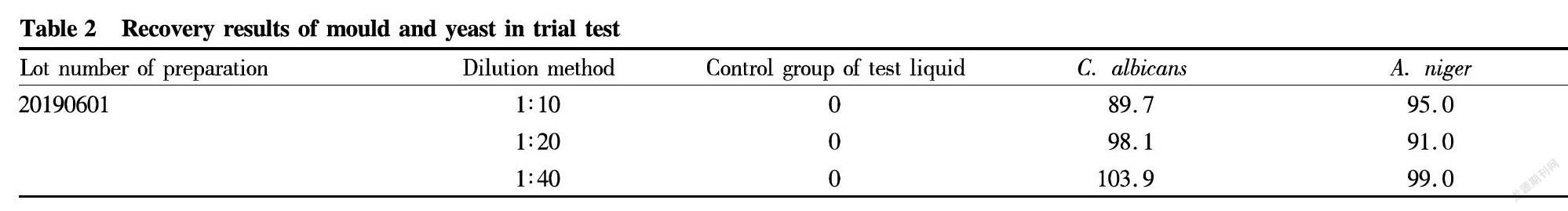
Total quantities of mould and yeast
A certain amount of the 1∶10, 1∶20 and 1∶40 test liquids (10 ml) were added into sterilized test tubes, respectively, and then, 0.1 ml of corresponding testing microorganism was added when ensuring the final concentration not higher than 100 cfu/ml. After mixing well, 1 ml of corresponding microbial liquid was taken from its test tube and added into a plate, followed by solidification and culture at 20-25 ℃. Two plates were prepared for each strain in parallel. The number of colonies was determined in the test groups, microbial liquid groups and control groups of the test liquids by the same method. The recovery of each strain was calculated according to following formula: Recovery=(Average number of colonies in test group-Average number of colonies in the control group of test liquid/Average number of colonies in the control group of microbial liquid. The results are shown in Table 2.
The results showed that when performing mould and yeast counting of this product by the conventional method (1∶10 testing solution), the recoveries of C. albicans were all larger than 0.5. Therefore, the conventional method is suitable for the aerobic microbe counting of this product.
Microbe examination
E. coli
The test group: At first, 10 ml of the 1∶10 test liquid prepared in "Preparation of test liquid" was inoculated to 100 ml of trypticase soy broth medium. Then, 0.1 ml of prepared E. coli and S. aureus suspensions were added, respectively. After mixing well, the bacteria were cultured at 34 ℃ for 18-24 h. Next, 1 ml of the culture was inoculated to 100 ml of MacConkey broth medium and then cultured at 44 ℃ for 48 h. The MacConkey liquid culture was streak-inoculated onto Maconkey agar medium plates, and cultured at 34 ℃ for 24-72 h.
The test liquid group: Similarly, 10 ml of the 1∶10 test liquid was inoculated to 100 ml of trypticase soy broth medium. The culture condition and the examination method were the same as the test group.
The negative group: As negative control, 10 ml of sterile sodium chloride-peptone buffer solution (pH 7.0) was added into 100 ml of trypticase soy broth medium. The culture condition and the examination method were the same as the test group.
Validation results: The test group was tested to be positive, and the colonies on the plates were separated, and confirmed as E. coli by pure culture and Gram stain microscopy. Both the test liquid group and the negative control group were negative. Therefore, the E. coli examination of this product can be operated by the conventional method. The results are shown in Table 3.
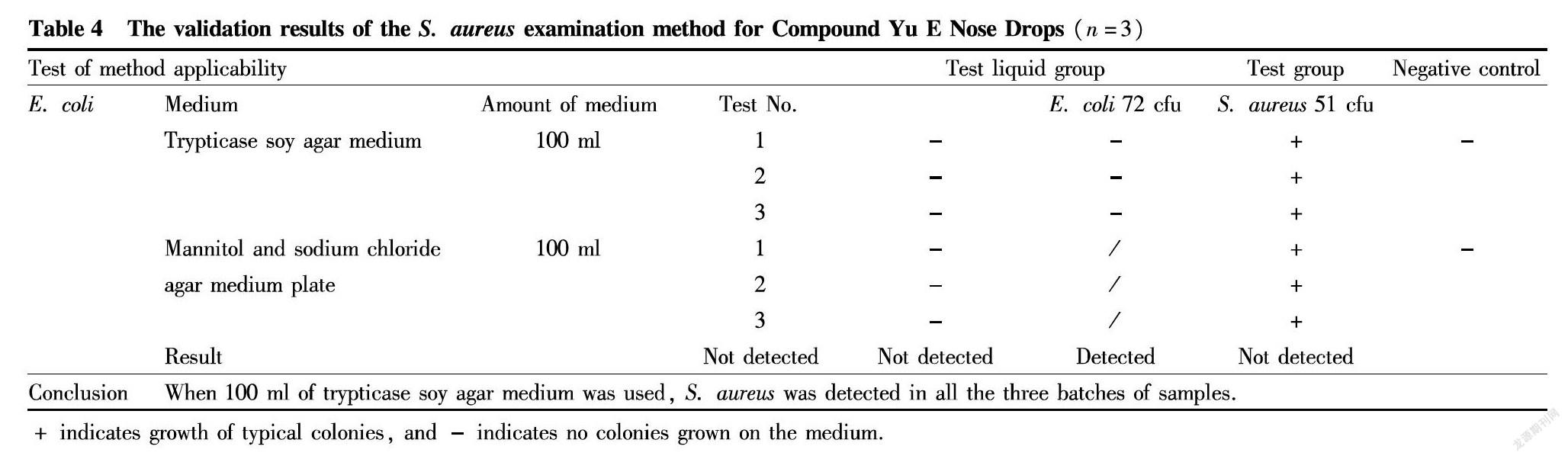
S. aureus
The test group: At first, 10 ml of the 1∶10 test liquid prepared in "Preparation of test liquid" was inoculated to 100 ml of trypticase soy broth medium. Then, 0.1 ml of prepared E. coli and S. aureus suspensions were added, respectively. After mixing well, the bacteria were cultured at 34 ℃ for 18-24 h. Finally, the culture was streak-inoculated to mannitol and sodium chloride agar medium plates and then cultured at 34 ℃ for 18-72 h.
The test liquid group: Similarly, 10 ml of the 1∶10 test liquid was inoculated to 100 ml of trypticase soy broth medium. The culture condition and the examination method were the same as the test group.
The negative group: As negative control, 10 ml of sterile sodium chloride-peptone buffer solution (pH 7.0) was added into 100 ml of trypticase soy broth medium. The culture condition and the examination method were the same as the test group.
Validation results: The test group was tested to be positive, and the colonies on the plates were separated, and confirmed as S. aureus by pure culture and Gram stain microscopy. Both the test liquid group and the negative control group were negative. Therefore, the S. aureus examination of this product can be operated by the conventional method. The results are shown in Table 4.
P. aeruginosa
The test group: At first, 10 ml of the 1∶10 test liquid prepared in "Preparation of test liquid" was inoculated to 100 ml of trypticase soy broth medium. Then, 0.1 ml of prepared E. coli and P. aeruginosa suspensions were added, respectively. After mixing well, the bacteria were cultured at 34 ℃ for 18-24 h. Finally, the culture was streak-inoculated to cetrimide agar medium plates and then cultured at 34 ℃ for 18-72 h.
The test liquid group: Similarly, 10 ml of the 1∶10 test liquid was inoculated to 100 ml of trypticase soy broth medium. The culture condition and the examination method were the same as the test group.
The negative group: As negative control, 10 ml of sterile sodium chloride-peptone buffer solution (pH 7.0) was added into 100 ml of trypticase soy broth medium. The culture condition and the examination method were the same as the test group.
Validation results: The test group was tested to be positive, and the oxidase test was positive. The colonies on the plates were separated, and confirmed as P. aeruginosa by pure culture and Gram stain microscopy. Both the test liquid group and the negative control group were negative. Therefore, the P. aeruginosa examination of this product can be operated by the conventional method. The results are shown in Table 5.
Conclusions and Discussion
In this study, method validation tests were carried out by the method of diluting testing solution. The data showed that during the aerobic microbe counting test, Compound Yu E Nose Drops contained antimicrobial active ingredients and showed a stronger inhibitory effect on S. aureus and B. subtilis, the recovery of which did not exceed 50%. Therefore, the dilution method should be adopted, and finally, for the 1∶20 test liquid, the recoveries of aerobic microbes were all in the range of 0.5-2.0. During the mould and yeast counting test, the recoveries of C. albicans and A. niger both exceeded 50%, so the product has no inhibitory effect on both mould and yeast. Therefore, when performing mould and yeast counting in the 1∶10 test liquid by the conventional method, the recoveries of the mould and the yeast were both in the range of 0.5-2.0. Compound Yu E Nose Drops inhibits beta hemolytic streptococcus, Salmonella typhi, S. aureus, Staphylococcus albus, Pseudomonas aeruginosa, E. coli, Proteus species, Fs dysentery bacillus and C. albicans[4]. When preparing Chinese herba preparations, Chinese herba preparations have the characteristics of diverse and complicated chemical components, and the antimicrobial effect of any component might affect the accuracy of microbial limit test. Therefore, the problem whether there are antimicrobial components in the preparation to be prepared should be discussed at first, and validation of the test method should then be performed under the premise of excluding the interference of antimicrobial components. In this way, we would not waste time in experiments or face the condition of finding no suitable methods. Therefore, it is necessary to verify the microbial test methods of traditional Chinese medicine preparations through validation tests. It can be seen from the control microbe examination results of Compound Yu E Nose Drops that positive bacteria grew well in test groups, indicating that the product has no inhibitory effects on E. coli, S. aureus and P. aeruginosa. The control microbe examination was carried out according to the conventional method in Chinese pharmacopoeia (2015 version), and the experimental results were feasible.
It could be seen from the validation results that by the method of increasing diluent (1∶20 test liquid), the three batches of Compound Yu E Nose Drops showed the recoveries of S. aureus, P. aeruginosa, B. subtilis, C. albicans (TSA culture) and A. niger (TSA culture) all in the range of 0.5-2.0, and by the conventional method (1∶10 testing solution), Compound Yu E Nose Drops showed the recoveries of A. niger (SDA culture) and C. albicans (SDA culture) both in the range of 0.5-2.0 in mould and yeast counting. The product only has an antimicrobial effect on S. aureus. Therefore, this product has a strong inhibitory effect on total quantities of aerobic microbes. When using the conventional method for the method validation test of control microbes, the results showed that the positive bacteria grew well, indicating that the product has weak inhibitory activity against E. coli, S. aureus and P. aeruginosa. The methods for eliminating the antimicrobial effect of tested products recorded in Chinese Pharmacopeia (2015 edition) include increasing diluent or medium volume, adding suitable neutralizer[5]or inactivator, performing membrane filtration or combining above methods[6]. The results showed that the test samples have strong antimicrobial activity against the test strains such as S. aureus and B. subtilis, and the counting validation of total quantities of aerobic microbes by increasing diluent (1∶20 test liquid) and that of total quantities of mould and yeast by the conventional test showed that the recoveries of various prescribed strains all reached the pharmacopoeia requirements. The method is accurate and reliable, and can be used for quality control of Compound Yu E Nose Drops.
References
[1]WANG X, HUA J. Anti-inflammatory effect of hospital preparation Compound Yu E Nose Drops[J]. Journal of Practical Traditional Chinese Internal Medicine, 2012, 9(26): 7-8.
[2]TENG Y, XU H, ZOU L, et al. Discussion on applicability of microbial limit test method for Chonglou Jiedu Tincture[J]. Chinese Traditional Patent Medicine, 2019, 4(41): 951-953.
[3]Chinese Pharmacopoeia Commission. Chinese Pharmacopeia (four part, 2015 edition)[S]. Beijing: China Medical Science Press, 2015: 1105-1107.
[4]HAN JN, QING SY. Experimental study on antibacterial activity of Compound Yu E Nose Drops in vitro[J]. Journal of Chinese Medicine, 2014, 189(29): 235-236.
[5]Chinese Pharmacopoeia Commission. Chinese pharmacopoeia analytical testing technical guide[S]. Beijing: China Medical Science and Technology Press, 2017: 567.
[6]Chinese Pharmacopoeia Commission. Chinese pharmacopeia (four part, 2015 edition)[S]. Beijing: China Medical Science Press, 2015: 142.
- 农业生物技术(英文版)的其它文章
- Analysis of the Southern China Tilapia Production and Economic Benefits of Different Breeding Patterns in 2018
- Dynamic Monitoring and Control Measures of Spodoptera frugiperda (J.E.Smmith) in Low Latitude Plateau Sugarcane Areas
- Control Effects of a New Sex Pheromone Trap and Biological Agents on Sesamia inferens Walker and Argyroploce schistaceana (Snellen)
- Comparative Study on Grain Cadmium Content and Yield in Different Rice Varieties
- Simulation Experiment of Air Temperature Variation in Multi-film Covering at Night
- Identification of Growth-promoting Bacteria from Rhizosphere of Pastures and Their Effects on Growth of Lotus corniculatus L.

