Tissue Culture of Red Sandalwood (Pterocarpus santalinus)
Siting CHEN Yiqing LI Chengxiang XU Xiaonan HUANG Tingting LIU Bilin PENG Jianfang PAN Minting LIN Yuanbei LIAO








Abstract [Objectives] This study was conducted to develop a rapid propagation method for red sandalwood (Pterocarpus santalinus) by tissue culture.
[Methods] The well-grown red sandalwood seed embryos were inoculated into three kinds of culture media after aseptic treatment, and the aseptic explants were obtained and inoculated into six kinds of media for light culture.
[Results] In the best disinfection schemes of red sandalwood, disinfecting with HgCl2 for 8 min achieved the highest germination and survival rates; when the medium for inducing red sandalwood explants was MS+0.2 mg/L IBA, the induction rate reached a maximum value; and when the culture medium for inducing stem segments of aseptic red sandalwood plantlets was MS+3.0 mg/L 6-BA+0.3 mg/L IBA, the growth of the stem segments achieved the best effect. The optimal medium for inducing red sandalwood explants was MS+IBA 0.2 mg/L, and the optimal medium for inducing stem segments of red sandalwood was MS+3.0 mg/L 6-BA+0.3 mg/L IBA.
[Conclusions] The results of this study have a large reproductive coefficient, simple process and low cost, which have outstanding value for promoting the breeding and promotion of red sandalwood seedlings.
Key words Pterocarpus santalinus; Tissue culture; Induction of explants; Culture of stem segments
Precious wood resources are a symbol of the countrys international status and an important strategic resource. They are mainly used to make high-end furniture, sculptures, musical instruments and arts and crafts, with dark stripes and natural textures, which are well received by the public. At present, more than 98% of Chinas Hongmu timber is imported. There are 29 Hongmu tree species worldwide. Their textures and values vary greatly from each other, but the basic characteristics of wood are hard, heavy, fine, red/black. One of the most precious and second-to-none timber is red sandalwood (Pterocarpus santalinus). Red sandalwood, commonly known as Xiaoyezitan, belonging to Pterocarpus in Papilionaceae. Its heartwood is the wood that is truly called "red sandalwood" and ranks first among Hongmu. However, red sandalwood is naturally distributed in tropical virgin forests. The main producing areas are Mysore and Myanmar in India. For some countries and regions, not only the restrictions on the export of red sandalwood are very strict and the sanctions are severe, but also the control of its seeds is stricter.
China is not the origin of red sandalwood, and there is no natural distribution of red sandalwood. It is only introduced to China and has not received much attention for a long time. At present, China has only a small amount of red sandalwood cultivated in Hainan Island, and adult plants are extremely rare. In addition, the phenomenon of "embryo abortion" is serious during seed development. The annual seed yield is very small, and the field germination rate is very low and even 0 in case of a typhoon attack. Therefore, red sandalwood seeds and seedlings are very scarce. Breaking through the cultivation technology of red sandalwood seedlings and developing a modern biological seedling raising technology is an important way.
Tissue culture is an important part of biotechnology, which has the advantages of short breeding cycle and no restrictions by seasons and climate. Furthermore, the obtained plantlets can well inherit excellent genes from the parent population, maintain the excellent traits of the varieties, and grow neatly. So far, no reports have been made on the tissue culture of red sandalwood plantlets.
In view of the precious, rare and urgent market demand of red sandalwood seedlings, a tissue-culture method for raising red sandalwood plantlets, which can produce red sandalwood plantlets with good quality quickly, efficiently and at a low cost, was developed after years of experiments, and reported below.
Materials and Methods
Based on the previous experimental study on red sandalwood, seeds were selected as the explants because the stem segments as explants were poorly propagated. The seeds were purchased from the Jianfengling Tropical Forestry Experiment Station of the Chinese Academy of Forestry. They were packed in paper bags and brought back to the laboratory. Seeds, which were relatively well grown and contained enough moisture, were selected as explants.
With MS medium as the basic medium, sucrose 30g/L and carrageenan 6-8 g/L (regulated to the appropriate ratio as required) were added, and the mixture was adjusted to pH 5.4- 5.8. After subpackaging, the mixture was placed in an autoclave and sterilized at the temperature of 121 ℃ for 23 min. The culture temperature was (25±1) ℃, and the light with an intensity of 20-40 μmol/(m2·s2) was provided 12 h per day.
The seeds were first cleaned with water. Then, the outer shell (pericarp and seed coat) was removed with scissors, and the embryos were removed carefully without damaging the protective layer of the embryo. The embryos were placed in bottles and immersed with water slightly over the embryos for 4-5 h. Then, they were rinsed with water for 6-8 min, soaked with a detergent solution for 8-10 min and finally rinsed with tap water. On a clean bench, the seeds were soaked for 3-5 min with 75% ethanol, rinsed for 3-4 times with sterile water, then treated with 0.1% HgCl solution for 2, 5, 8 and 10 min, and finally rinsed for 3-5 times with sterile water.
The above-mentioned sterile well-grown full red sandalwood seed embryos were inoculated into following three media: MS minimal medium without any growth hormone, MS+IBA 0.1 mg/L and MS+IBA 0.2 mg/L, when strictly following the laboratory rules and tissue culture and inoculation operations. After inoculation, the embryos were transferred to a culture chamber for light culture.
After 3 weeks of induction, the cultured red sandalwood plantlets were cut into 1 or 2 stem segments (2-4 cm) on a clean bench, which were inoculated in following 6 media for light culture in a culture chamber: 2/3 MS medium, MS+IBA 0.1 mg/L+6-BA 2.0 mg/L medium, MS+IBA 0.1 mg/L+6-BA 1.0 mg/L medium, MS+IBA 0.2 mg/L+6-BA 2.0 mg/L medium, MS+IBA 0.3 mg/L medium and MS+6-BA 3.0 mg/L medium.
Results and Analysis
Effect of HgCl2 treatment time on sterilization of explants
The starting medium was composed of MS medium as basic medium, sucrose 30 g/L and carrageenan 6 g/L. The pH medium was 5.4-5.8, and no growth regulator was added. Experiments showed that, although selected, the germination rate of red sandalwood was still smaller than 40%; and the disinfection of seeds with 0.1% HgCl2 was an effective measure to maintain a higher germination rate after sowing of red sandalwood seeds. The treatment time was preferably 8 min. When the treatment time was prolonged to 10 min, the germination rate was reduced, and damage was caused by a longer time (Table 1). It can be seen from Table 1 that when the disinfection time (0.1% HgCl2 solution) was 8-10 min, the contamination rate of explants was the lowest; and after 10 min of disinfection, the survival rate of explants was the highest, and the disinfection effect was the best. However, when the disinfection time reached 10 min, the survival rate was reduced to the minimum, indicating that the disinfection of explants in 0.1% HgCl2 solution for a long time made the contamination rate of explants lower, but inhibited seed germination, resulting in the condition that some explants lost vitality and the survival rate of explants decreased.
The inoculation was performed in the very year of harvesting.
Before the disinfection, the seeds were soaked for 24 h; and the medium for inoculation was MS medium (the basic medium) containing 30 g/L sucrose, 6 g/L carrageenan and no growth regulator. The inoculation was performed according to 1 seed/bottle.
Effects of storage method and sowing date on survival rate of explants (seeds)
After harvesting, if stored at room temperature, the germination rate of red sandalwood seeds after aseptic sowing decreased over time, and the decline was significant after more than 6 months. After 12 months, the germination rate was only 1/3 of the highest germination rate. However, if stored at a low temperature (8 ℃) for 14 months, the viability can still be well maintained and the germination rate was not reduced.
Agricultural Biotechnology2019
Effect of growth regulator concentration on subculture of explants
0.1-0.3 mg/L IBA can promote the induction rate of explants. The induction rate of explants treated with 0.1 mg/L IBA was lower, and the addition of 0.2-0.3 mg/L IBA can achieve a higher induction rate of explants. The induction rate of explants was relatively low in media 1, 2 and 6 without the addition of any hormone. The induction rates of explants in media 1 and 2 were close, and the induction rates of explants in medium 6 was quite different. It can be seen that the medium supplemented with 0.3 mg/L IBA hormone promoted the induction of explants and promoted the growth of stem buds, and the induction rate was up to 15%, so the optimal medium for the induction of red sandalwood explants was medium MS+0.3 mg/L IBA.
After the successful induction of red sandalwood explants in the medium supplemented with 0.3 mg/L IBA, the healthy growing explants showed no obvious change on the 3rd d after light culture, and no germ grew out; until the 6th to the 7th d, the cotyledons unfolded, the embryos broke through the seed coat and showed the buds which gradually grew, and the root primordia grew out; on the 10th to the 15th d, the explants further grew, showing 3.0-5.5 cm roots, 2.0-4.0 cm stems and a few green leaves; from the 18th to the 20th d, the stems of the explants gradually elongated to 6.0-8.0 cm when the roots grew to 7.0-9.0 cm, the lateral branches sprouted and exhibited lateral roots, and the number of lateral roots increased; and on the 25th d, the lateral branches gradually increased in length and number, the color of the leaves changed from light green to dark green, and the stems gradually grew and became thick and strong, giving healthy aseptic plantlets.
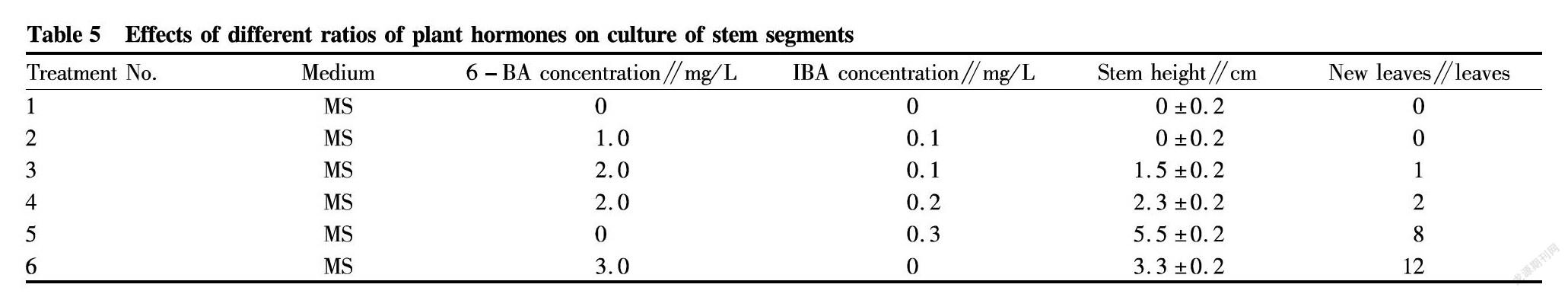
In the medium without the addition of growth regulators, the stem segments of red sandalwood hardly grew, showing no change, and there were no new leaves. Different concentrations of 6-BA and IBA promoted the proliferation of explants, and the higher the concentrations were, the more obvious the stem segments grew. Low concentrations of auxin and cytokinin can promote stem segment growth and root cell division, but the effects were not obvious. When the concentration of IBA reached 0.3 mg/L, the growth of the stem segments reached the maximum in that the stem grew rapidly, became relatively thick and hard, and showed green tender leaves, the number of leaves increased, and new leaves grew out 20 d later. Overall, the stem segments grew stably. When the concentration of 6-BA reached 3.0 mg/L, the newly transferred stem segments produced significantly more branches, and there were most new leaves. Overall, the stem segments grew stably. It can be seen that when the concentration of the two hormones reached a suitable ratio, the growth of the stem segments can be maximally promoted, and the optimal medium for the cultivation of the stem segments of red sandalwood was MS+6-BA 3.0 mg/L+0.3 mg/L IBA.
The stem segments of red sandalwood treated with MS+0.3 mg/L IAA first germinated on the 5th d under light culture. The leafbuds slightly sprouted at first, showing slightly enlarged base, and a small amount of calli appeared in the roots. After further culture to the 15th d, the leafbuds sprouted, and elongated slightly, and a small amount of calli appeared. On the 25th d of continuous cultivation, the stems obviously elongated, by (3.7±0.2) cm, new green leaves grew out, and the lateral buds sprouted. On the 35th d, the stems grew rapidly, and elongated by (5.5±0.2) cm, and many green leaves appeared. Overall, the growth was stable.
For the stem segments cultured in medium MS+3.0 mg/L 6-BA, the leafbuds began to sprout on the 5th d of light culture, and a small amount of calli appeared but showed on obvious growth. On the 15th d of continuous culture, the stems elongated by (1.5±0.2) cm; there were 3 to 4 small branches; and leafbuds sprouted. On the 25th d of continuous cultivation, the stems elongated by (2.3±0.2) cm; there were 5-6 branches; the leafbuds increased, and a few green leaves appeared. After 35 d of cultivation, a large number of calli were formed; the stem elongation was not obvious; and the leaves increased in number, having a color changing from emerald green to dark green, and the stretch was stable.
Effect of medium composition on rooting culture
The obtained subculture plantlets were transferred to a rooting medium for cultivation. The experimental results showed that the suitable rooting medium was 1/2 MS medium containing sucrose 30 g/L, carrageenan 6 g/L, 0.05 mg/L 6-BA, 1.0 mg/L indole-3-butyric acid (IBA) and 0.25 mg/L IAA. After 30-35 d of cultivation, the plants showed a height up to 6-7 cm and had 5-6 leaves thereon; the rooting rate was over 95%; and there were 2-3 white roots with a length of 3-4 cm. In the net coverage area where 50% of sunshine was shaded, most plantlets were trained for 1 d, and some plantlets were trained for 2 d. The tissue culture plantlets were taken out, washed with warm water to remove attached medium, and finally transplanted to the greenhouse nursery, and each plant was irrigated with 15 ml of 1/2MS nutrient solution. After 60 d of cultivation, new roots can be formed in a large quantity and transplanted into field nursery for cultivation. The transplanting substrate was a loose permeable well-drained matrix, which was composed of fine perlite and peat soil mixed in equal proportion (V/V).
Conclusions and Discussion
In this study, after treating with HgCl2 for the best disinfection time, there was still a serious contamination phenomenon, which might be due to seasonal reasons. When the temperature is suitable and the air humidity is large, bacteria and fungi may be more likely to grow, resulting in easy contamination of media and higher contamination rate of induced explants. Therefore, it is better to carried out experiments in August-September, and meanwhile, penicillin can be added to the media[6], to effectively inhibit the growth and reproduction of some bacteria and reduce the contamination rate. The disinfection treatment of laboratories and operating tables should be strict, and the aseptic operation rooms should be sterilized by ultraviolet ray before experiments. During experiments, experimenters should be qualified to reduce contamination caused by human.
Some of the successfully induced red sandalwood explants were neither contaminated nor germinated. One of the possible reasons was that some seeds were not full enough and had poor vitality, and failed to reach their growth conditions. The second reason may be that the dormant period of red sandalwood is relatively long, so after peeling off the seeds, they can be immersed in water for a suitable period of time before experimenting. Because the red sandalwood seed material is limited, and most of the seeds have low viability and are not easy to be induced successfully, this study only designed a comparative experiment of adding IBA phytohormone to explore the optimal IBA concentration for explant growth. It is suggested that in the condition of sufficient material, it is better to design a combination of 6-BA and IBA to promote the induction of explants, so as to explore a medium that promotes the induction of red sandalwood explants.
The aseptic red sandalwood plantlets successful cultured were used to perform culture of stem segments. Most of the stem segments do not continue to grow after the appearance of a large number of calli. The calli differed in type and color, and some of the calli were darker and larger, while some were smaller and loose. This might be due to the hormone in the medium and its proportion, which caused more calli and inhibited the growth of the stem segments. The medium MS+0.2 mg/L IBA+2.0mg/L 6-BA showed almost stagnant growth when the stem segments grew to 2.5 cm, and very few leaves were observed, which might be due to less water in the MS medium, or the hormone ratio, which caused the nutrients and moisture provided by the medium to be insufficient. Some stem segments were browned, which might be due to the oxidation of polyphenol oxidase. Therefore, attention should be paid to the disinfection of used tools during operation.
References
[1]China General Administration of Quality Supervision, Inspection and Quarantine. GB/T 18107–2017 Hongmu[S]. Beijing: China Standard Press, 2017. (in Chinese)
[2]XU CX, ZENG J, CUI TC, et al. Introduction,growth performance and ecological adaptability of Hongmu tree species (Pterocarpus Spp.) in China[J]. Journal of Tropical Forest Science, 2016, 28(3): 260-267. (in Chinese)
[3]MENG Q, LUO XJ, LIU Y, et al. Trade Dynamics of the CITES- listed Timber Species[J]. World Forestry research, 2017, 30(2): 73-76. (in Chinese)
[4]LI YK. Eighteen Hongmu tree species are listed in the CITES control list,how should Chinese Hongmu enterprises respond[N]. China Green Times, 2016, November/2/003. (in Chinese)
[5]JIANG XM, YIN JP, YIN YF. Gradually decreasing Hongmu resources[J]. Life World, 2012, (7): 4-13. (in Chinese)
[6]LI W. Seed dormancy mechanism and its elimination method[J]. Chinese Agricultural Science Bulletin, 2013(3): 49. (in Chinese)
[7]CHEN BH, YAO QD, LI QZ, et al. Study on tissue culture techniques of Dalbergia odorifera[J]. Wuyi Science Journal, 2010, 26: 48-51. (in Chinese)
[8]ZHANG FQ, WANG JZ, JIN F. Effect of 6-BA, IBA and IAA on subculture of jujube cv. Mingshandazao plantlets in vitro[J]. Journal of Gansu Agricultural University, 1995, 30(4): 289-292. (in Chinese)
- 农业生物技术(英文版)的其它文章
- Analysis of the Southern China Tilapia Production and Economic Benefits of Different Breeding Patterns in 2018
- Dynamic Monitoring and Control Measures of Spodoptera frugiperda (J.E.Smmith) in Low Latitude Plateau Sugarcane Areas
- Control Effects of a New Sex Pheromone Trap and Biological Agents on Sesamia inferens Walker and Argyroploce schistaceana (Snellen)
- Comparative Study on Grain Cadmium Content and Yield in Different Rice Varieties
- Simulation Experiment of Air Temperature Variation in Multi-film Covering at Night
- Identification of Growth-promoting Bacteria from Rhizosphere of Pastures and Their Effects on Growth of Lotus corniculatus L.

