Metastatic potential and prognostic significance of SOX2:A metaanalysis
Arslaan Javaeed, Sanniya Khan Ghauri
Abstract
Key words: Tumor progression; SOX2; Cancer; Cancer stem cell markers; Lymphatic metastasis
INTRODUCTION
The mechanisms by which the metastatic cascade is instigated and developed have merited the attention of the researchers worldwide. Tumor metastasis involves a sequential process, comprising cell invasion through the basement membrane and extracellular matrix followed by intravasation to access the vascular or lymphatic circulation, extravasation out of the circulatory system, and eventually distant localization and proliferation[1]. Failure to manage distant dissemination of cancer cells causes a significant mortality burden, with approximately 1500 cancer spreadrelated deaths reported daily[2]. A plethora of studies has been conducted on animal models to outline the metastatic phenotype. These studies usually employ intravenous injections for metastatic initiation. However, such an approach lacks the appropriate characterization of the origin of metastasis and, thus, multiple triggering factors are either still hypothesized or their contribution in metastasis is not fully delineated.
Of these factors, the transcription factor sex determining region Y-box 2 (SOX2) has been recently integrated in cancer biology[3]. SOX2 is one of the SOX proteins that has≥ 50% similarity in the amino acid structure to a specific domain (HMG domain) of the sex-determining region located on the Y chromosome (Sry)[4]. The encoding gene was initially discovered in 1994 in human, being located on chromosome 3q26.3-q27[5]. Heterozygous mutations of theSOX2gene result in the development of anophthalmia syndrome, which includes an aberrant development of endodermal and ectodermal tissues[6]. SOX2 is an important regulator of pluripotent cellular transcription and is a crucial factor implicated in the development and maintenance of undifferentiated embryonic stem cells. Besides, it contributes to somatic cell reprogramming back towards pluripotent features and this process was shown to be co-induced by ectopic expression ofcMyc,Klf4, andOct4[3].
Intriguingly, this discovery highlighted the role of SOX2 expression in different types of cancers.SOX2gene amplification or increased expression has been demonstrated in malignancy, yet its involvement in the most important aspects of patient survival and metastasis remains relatively unclear. Herein, we provide analytic insight into the association between metastasis and SOX2 expression in cancer patients, along with its relation to the prognostic profile.
MATERIALS AND METHODS
A meta-analysis was conducted on studies that investigated the relationship between development of a metastatic phenotype as well as the prognostic significance of such expression. The general outline of the study was based on the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (commonly known as PRISMA)[7].
Eligibility criteria
The included studies investigated at least the association of SOX2 expression,reported as “low” or “high” expression levels, and either lymph node metastasis(LNM) or distant metastasis (DM). When appropriate, patients' prognosis should have been evaluated using survival analysis, where the overall survival (OS) and/or disease-free survival (DFS) were reported. Only peer-reviewed studies conducted on male or female human patients were considered. The cancer patients should have been diagnosed using pathological examinations. Studies were excluded if they provided insufficient data to be extracted or had employed animal subjects solely.Furthermore, non-English articles, case reports, reviews and cell experiments were omitted.
Information sources
The following scientific databases were used for the search process:MEDLINE,Embase, Cochrane Library, and Google Scholar. Additionally, the bibliographies of the screened articles were thoroughly searched for eligible studies.
Search strategy
Two authors performed the search process using the following key words:(“transcription factor sex determining region Y-box 2” OR “SOX2”) AND (“cancer”OR “metastasis”) AND (“prognosis” OR “survival” OR “hazard”). The publication dates of the included studies were limited to those articles published between 2010 and 2018, to attain the most recent evidence. Screening of the titles and abstracts was performed and the relevant articles were obtained for subsequent assessment of the full-texts. Any disagreement was resolved by discussion.
Data collection
A specifically-designated form was used in Microsoft Excel spreadsheet software(version 2016) to extract data. The extracted data for each study were categorized into four main categories:(1) Study and patients' characteristics, including the name of the first author, publication date, methods of detection of SOX2 expression, method of survival analysis, reported outcomes, number of samples, type of primary cancer, sex of patients, frequency of SOX2 expression (low or high), and clinical stage (I and II or III and IV); (2) Primary outcomes, including the frequency of reported samples with low or high SOX2 expression in the regional lymph nodes (i.e. LNM) or distant organs(i.e. DM); (3) Clinicopathological parameters, including SOX2 expression levels according to patients' age, sex, clinical stage, and tumor size and differentiation; (4)Prognostic data, including direct extraction of the hazard ratios (HRs) and their respective 95% confidence intervals (CIs) of the OS and/or DFS. When both univariate and multivariate analyses were used for survival analysis, multivariate tests were preferentially used.
Quality assessment
A specialized scale for quality assessment was used [i.e.,. Newcastle-Ottawa scale(commonly known as NOS)][8], which employs a scoring system comprising nine items and yielding a total score of 0 to 9 for low-quality to high-quality articles,respectively. The assignment of such scores is based on the selection, comparability,and outcomes of the groups under investigation. A score of ≥ 6 indicated a highquality study.
Statistical analysis
The following formula was used for calculation of the prevalence rate of high SOX2 expression:(number of samples with high SOX2 expression/total number of samples)× 100; the standard error and 95%CIs were calculated as described previously[9]. The RevMan 5.3 software was used for statistical analysis (Review Manager, the Cochrane Collaboration, Oxford, United Kingdom). For dichotomous data (e.g., LNM, DM and clinicopathological data), odds ratios (ORs) and their respective 95%CIs were applied.Regarding prognostic markers (OS and DFS), HRs and their respective 95%CIs were integrated in the meta-analysis. Statistical heterogeneity was assessed using theI2statistical test, which was interpreted as a significant heterogeneity atI2> 50%. In such instance, a random effects model was applied. On the other hand, a fixed effects model was used when the heterogeneity was insignificant. Subgroup analyses were performed according to sample size, the type of primary cancer, and quality score.
RESULTS
Results of the search process
Figure 1 shows the outcomes of the employed search process. The initial search yielded a total of 1120 records, from which 45 records were considered duplicates,while an additional 5 records were identified from the bibliographies of the resultant records. Therefore, 1080 records were screened for eligibility through their titles and abstracts. Subsequently, the full-text versions of 23 articles were examined for inclusion. Ultimately, 20 studies were included in the meta-analysis.
Characteristics of the included studies
Table 1 demonstrates the main characteristics of the included studies. A total of 2643 patients (60.88% males) were investigated for eight types of cancer, including cancers of the breast[10,11], cervix[12], colon[13], esophagus[14-16], head and neck[17-23], liver[24], lung[25],and stomach[26-29]. The sample sizes ranged between 20 and 307 patients. Excluding three studies in which the clinical stage was not reported[10,13,24], 1265 patients (54.98%)were at stage III or IV. Immunohistochemical staining was utilized in all studies to detect SOX2 expression. With respect to outcomes, only two studies did not report LNM[12,17], while DM was reported in 11 studies[12-14,17,19,20,22-24,26,27], OS in 11 studies[12,15-23,26], and DFS in 3 studies[17-19]. Two studies were performed in European countries[13,19], one in Africa[10], and the remaining studies in Asia. All of the included studies scored a NOS equal to or greater than 6, indicating the inclusion of highquality studies.
Prevalence of high SOX2 expression and its relationship to the clinicopathological parameters
The pooled prevalence of high SOX2 expression in different types of cancer was 46.22% (95%CI:39.07%-53.38%) with a significant heterogeneity among the included studies (I2= 94%). Subgroup analysis was performed to detect the sources of heterogeneity, yet it remained significant for most of the subgroups. As for the clinicopathological parameters, SOX2 expression was not significantly correlated to patient's age, sex and clinical stage as well as the size and differentiation of tumors(Table 2).
Relationship between SOX2 expression and metastasis
Although applying a random effects model yielded no remarkable association between high SOX2 expression and LNM (Figure 2A), the relationship was significant in the subgroup analyses for specific types of tumors, including cancers of the colon(OR = 4.15, 95%CI:1.43-12.09,P= 0.009), breast (OR = 2.78, 95%CI:1.50-5.50,P= 0.002,I2= 0%), and lung (OR = 1.74, 95%CI:1.02-2.89,P= 0.04) ( Table 3).
Interestingly, SOX2 was highly expressed in distant metastatic tumors (OR = 1.79,95%CI:1.20-3.25,P< 0.008; Figure 2B) despite the existence of a significant heterogeneity between studies (I2= 57%). Such relationship was also consistent for hepatic (OR = 4.80, 95%CI:1.56-14.76,P= 0.006), colon (OR = 3.04, 95%CI:1.15-8.04,P= 0.03), and head and neck tumors (OR = 2.46, 95%CI:1.63-3.72,P< 0.001) ( Table 3).
Relationship between SOX2 expression and patient prognosis
As compared to low SOX2 expression, high SOX2 expression was significantly associated with shorter OS and the studies showed no heterogeneity (HR = 1.65,95%CI:1.34-2.04,P< 0.001,I2= 44%; Figure 3A). Similarly, the included studies showed a significantly shorter DFS when SOX2 was highly expressed without heterogeneity (HR = 1.54, 95%CI:1.14-2.08,P= 0.005,I2= 15%; Figure 3B).
DISCUSSION
Cancer biology involves a number of hallmarks that enable cellular division and spread, including continued proliferative signaling, initiation and maintenance of metastasis, and evasion of cell death[30]. SOX2 has exhibited various roles in these hallmarksviapromoting cellular proliferation through the induction of cyclin D3 transcription and enabling S-phase entry[31]as well as evading apoptotic signals by precludingORAI1expression and subsequently reducing store-operated Ca2+entry[32].The present meta-analysis showed that SOX2 contributed to the development of a metastatic phenotype to distant organs and its high expression was associated with shorter OS and DFS in patients with cancer.
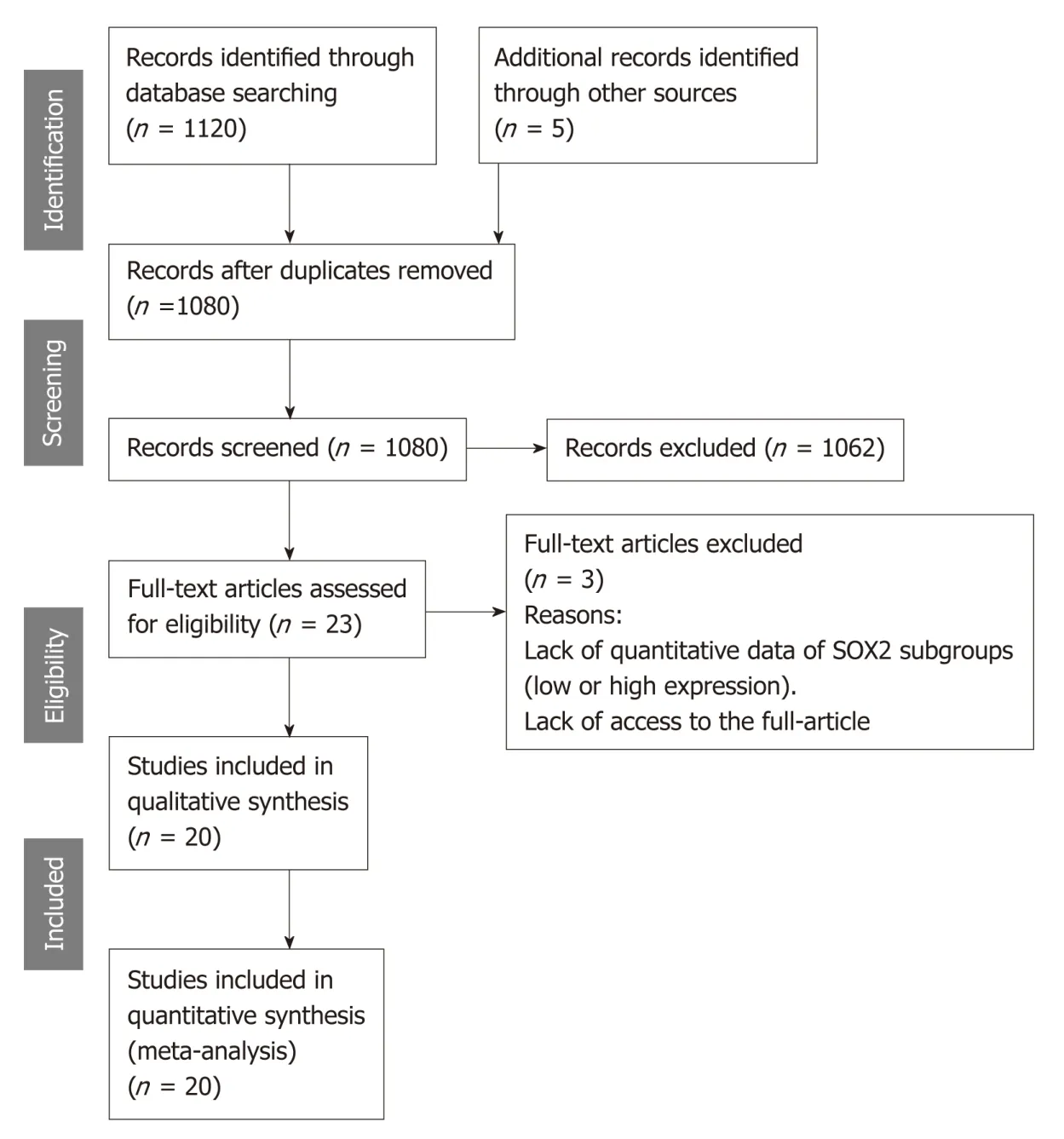
Figure 1 Flow diagram showing the search process used in the present study.
SOX2 is one of the embryonic cell fate determinants which have been linked to increasing tumor aggressiveness, metastasis, and poor prognosis. In addition to SOX2,these factors, namely the Yamanaka factors[3], includeOct4,Klf4, andMyc, among others. SOX2 determines distinct cell fate decisions during embryonic developmentviaantagonization of CDX2, NKX2-1, MITF, and other tissue-specific factors[33]. In cancer, evidence from experimental studies has revealed different mechanisms of SOX2-mediated metastasis. Girouardet al[34]showed that the transduction of G361 cells, which normally express low SOX2 levels, to enhance SOX2 overexpression led to a 3.8-fold increase in invasiveness (P= 0.0004) and, on the other hand, SOX2 knockdown in melanoma cells resulted in a significant reduction of invasiveness and diminished matrix metalloproteinase (MMP)-3 expression by 87.8%. Furthermore,invasion and migration of cancer cells in colorectal cancer (CRC), malignant glioma,and laryngeal squamous cell carcinoma were attributed to MMP-2-mediated effects and this was associated with SOX2 overexpression[35-37]. Therefore, in the current study, it was not surprising that SOX2 overexpression was associated with LNM and DM in colon cancer and DM in head and neck cancer. MMP-2 is a type of IV collagenase that has been implicated previously in carcinogenesis and metastasis[38]. In general, MMPs comprise a family of zinc-dependent endopeptidases that degrade extracellular matrix proteins and they are involved in metastasisviaseveral aspects,including tumor invasion, angiogenesis, and establishing metastatic foci[39].
In hepatocellular carcinoma, SOX2 induced cell invasion and hence was associated with DM. SOX2 expression was low in non-carcinogenic liver cells, moderate in noninvasive cells, and highest in the invasive cells[24]. Such an effect is mediated by enhancing cancer stemness properties through activation of the epithelial-tomesenchymal transition (EMT) process, which is characterized by remarkable activation of distinct transcriptional factors, including Snail, Twist, and Slug. The SOX2-Slug axis has been consistently reported not only in hepatocellular carcinoma but also in esophageal squamous cell carcinoma and muscle-invasive bladder cancer[24,40,41]. SOX2-mediated Slug expression was suppressed by inhibition of STAT3/HIF-1α signaling using siRNA, indicating a role of such pathway in the coexpression of SOX2 and Slug[40]. Nonetheless, the exact regulatory mechanisms between EMT and SOX2 and other stemness-related transcriptional factors,considering their contribution to cancer metastasis, have yet to be clearly revealed.Through its common activating effect on EMT, SOX2 exerts its metastatic potential in CRCviaa different mechanism. Activation of the WNT pathway, which is thought tobe critical for CRC tumorigenesis, was demonstrated in CRC and cisplatin-resistant lung adenocarcinoma cells[35,42].
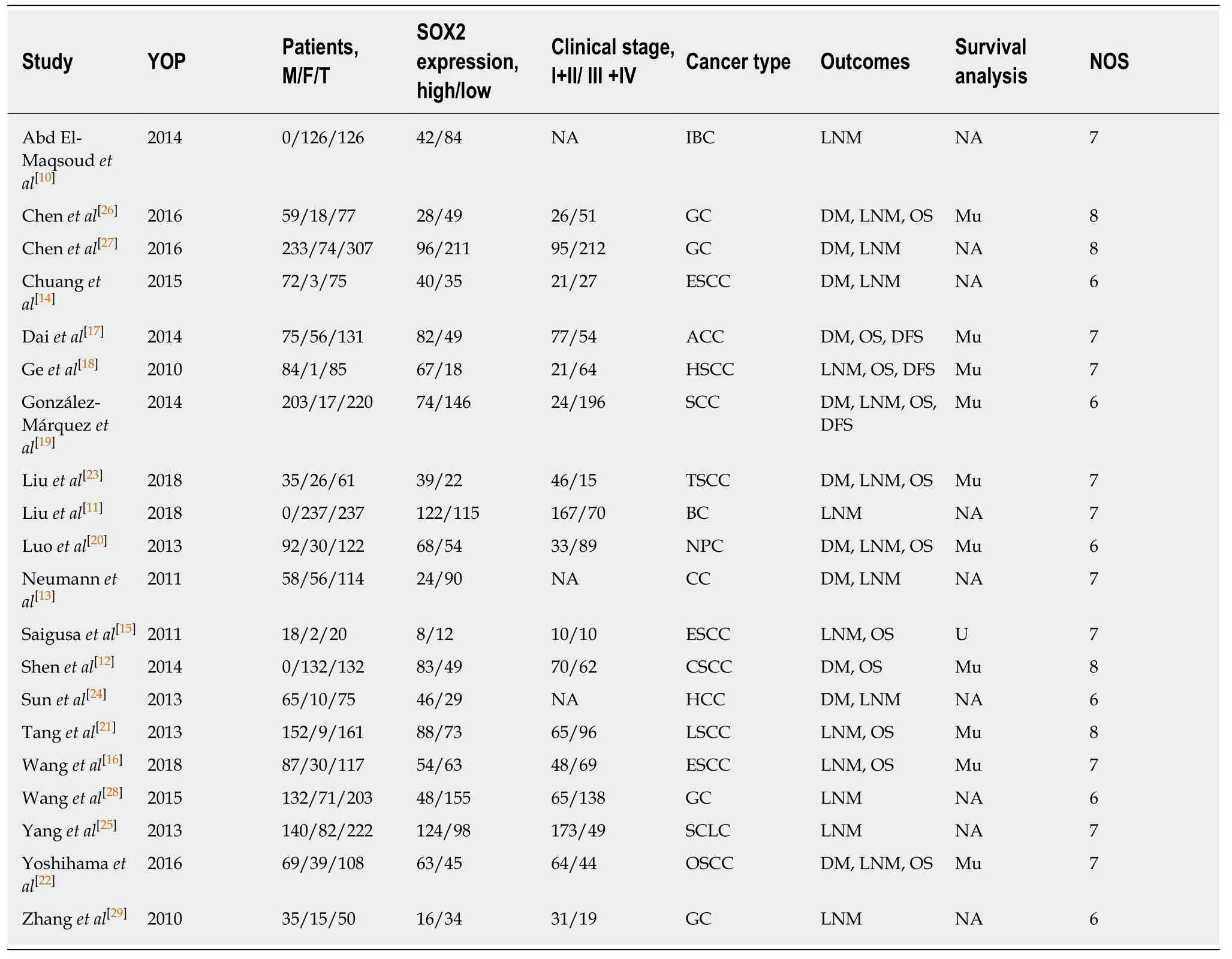
Table 1 Characteristics of the included studies
In contrast to our results, in ovarian cancer cells, SOX2 overexpression increased phosphorylation of Src and FAK proteins[43], both of which are well-established as prometastatic proteins through their abilities to mediate EMT[44]. Additionally, SOX2 functionality was linked to the Hedgehog signaling pathway in controlling different hallmarks of prostate cancer, such as reduction of apoptotic death, increasing cellular proliferation, and augmenting the metastatic ability[45]. This was also confirmed by the effects of SOX2 silencing, where it lowered the migration capabilities of different prostate cancer cell lines by 11%-31%[45]. Similarly,SOX2gene knockout or silencing led to loss of tumorigenic properties and reduced cellular proliferation in lung cancer[46], glioblastoma[47], and pancreatic cancer[31]. Paradoxically, SOX2 overexpression was found in gastric cancer and could contribute to cellular invasion[48],while it was found to be significantly downregulated in the same type of cancer in other studies[28,49,50].
In the present meta-analysis, SOX2 correlated with poor prognosis in different types of cancers. Other investigations have also indicated higher rates of recurrence and that its expression was associated with the parameters of worse outcome in primary cancers of the head and neck[51]. Furthermore, SOX2 was associated with advanced tumor stages in head and neck adenoid cystic carcinoma[52]. However, we failed to prove such a relationship, possibly because the included studies had to report at least one parameter of metastasis (LNM and/or DM) in addition to the prognostic data. Thus, several prognosis-relevant studies might have escaped inclusion.

Table 2 Pooled effect of the relationship between SOX2 expression (high vs low) and the clinicopathological parameters of cancer patients in the included studies
Given that SOX2 expression was detected not only in the nuclei of cancer cells but also in the their cytosols in hepatic cancer, pancreatic cancer and CRC[24,32,35], it is plausible that the intranuclear existence might explain its transcriptional role; yet, its functional role in the cytosol remains only fairly ununderstood. In light of the confirmed transcriptional roles, functional-depletion of SOX2,viaknockdown approaches using siRNA, is possible experimentally; although, its clinical application is challenging. Alternatively, researchers should strive to identify the possible regulatory mechanisms of SOX2 to tailor distinct targeted therapies accordingly.
In conclusion, SOX2 has exhibited a significant metastatic potential in various types of cancer, including colorectal, head and neck, liver and breast. It can also be regarded as a poor prognostic indicator, and recurrence may be expected in patients with SOX2 overexpression. Different transcriptional regulation mechanisms of SOX2 are involved, yet promotion of the EMT was apparently of paramount importance.Implementing targeted therapies aimed to counteract high SOX2 expression might be beneficial to treat this deadly disease.

Table 3 Subgroup analysis of the association between SOX2 expression (high vs low) and LMN and DM

Figure 2 Forest plot of the association between SOX2 expression and lymph node metastasis and distant metastasis.

Figure 3 Forest plot of the association between high SOX2 expression and lymph node metastasis and distant metastasis.
ARTICLE HIGHLIGHTS
Research background
SOX2 is a significant regulator of pluripotent cellular transcription and it helps in the reprogramming of somatic cells to the pluripotent properties. The involvement of SOX2 in cancer biology has been recently demonstrated.
Research motivation
Metastasis comprises the most important aspect of cancer-related mortality. Linking SOX2 expression to metastasis and subsequently to patient's survival may open novel horizons for the implementation of future therapeutic strategies that target SOX2 biological pathways.
Research objectives
To investigate the association between SOX2 overexpression and the development of a metastatic phenotype as well as the survival patterns of patients with increased SOX2 expression.
Research methods
A meta-analysis was conducted, including studies that recruited patients with different types of cancer and reporting SOX2 expression as either “low” or “high”, and evaluating patient survival using the relevant analytical methods [overall survival (OS) and/or disease-free survival (DFS)].A comprehensive search of articles published between 2010 and 2018 was performed in distinct scientific databases.
Research results
A total of 20 studies involving 2643 patients (60.88% males) were included. SOX2 overexpression was significantly associated with distant metastasis (odds ratio = 1.79, 95%CI:1.20-3.25,P<0.008), while it was associated with lymph node metastasis only in subgroup analyses of cancers of the colon, breast, and lung. Both OS and DFS were shorter in patients expressing high SOX2,as compared to those with low SOX2 expression (hazard ratio = 1.65, 95%CI:1.34-2.04,P< 0.001 and hazard ratio = 1.54, 95%CI:1.14-2.08,P= 0.005, respectively).
Research conclusions
The present study adds a comprehensive insight into the significant role of SOX2 in distant metastasis of different types of cancers and its correlation to poor prognosis rather than the outcomes obtained by individual studies. In line with the increased research interest in SOX2, we showed that it can be used as a prognostic marker in cancer patients, while, on the other hand,new therapeutic strategies are urgently needed to target the biological pathways implicated in SOX2 overexpression for more effective cancer treatment.
Research perspectives
Targeting SOX2 expression in cancer regimens is warranted. Future research studies should focus on developing novel drugs as well as the identification of the cut-off values of poor prognosis.

