Isolation and characterization of biosurfactant producing bacteria from groundnut oil cake dumping site for the control of foodborne pathogens
Obula Reddy Chittepu
Department of Biotechnology,Chaitanya Bharathi institute of Technology,Hyderabad 500075,India
Keywords:
Bacillus
Biosurfactants
Lipopeptide
Control
Foodborne
Pathogens
ABSTRACT
Infection and intoxication are two common types of foodborne illnesses throughout the world.The aim of the present work was to isolate and characterize biosurfactant producing bacteria from groundnut oil cake dumping sites and evaluate their biocontrol effects against foodborne pathogens.Bacteria were isolated by enrichment culture technique and preliminary screening method.Biosurfactant activity evaluation was carried out by oil displacement,drop collapse test,lipase activity,hemolytic activity,emulsification index and emulsification assay methods.16s rRNA sequence analysis was used for the isolate identification.Crude biosurfactant was extracted by acid precipitation method and characterized using FTIR(Fourier Transform Infrared Spectroscopy).Antibacterial activity was investigated using disc diffusion method.CMC and surface reduction were analyzed by DuNouy tensiometer.Top 10 strains were selected for biosurfactant activity assessment from the total 30 isolates.In addition,16s rRNA sequence identified that the potential isolate was Bacillus pseudomycoides OR 1.Then,FTIR result of the extracted biosurfactant established the extract as a lipopeptide based on the absorption peaks at 3500 to 3200 cm-1 and 2963 to 2854.68 cm-1,respectively.50 μg/mL of lipopeptide showed the highest antibacterial activity. Critical Micelle Concentration (CMC) of the lipopeptide was 60 mg/L and it reduced the surface tension of water from 71.6 to 31.6 mN/m.Hence,this study widens the scope to employ the bacterial lipopeptide surfactant as a promising biocontrol agent against foodborne pathogens.
1.Introduction
According to the World Health Organization,foodborne diseases are severe diseases owing to their toxicity[1].The poisonous substrates(toxins)can be present even without pathogens and cause diseases in animals and humans [2]. Studies on foodborne diseases revealed that the primary sources of food and water are contaminated with pathogens along with toxins and some chemicals[3].Foodborne diseases are detected when the patients suffer severe neurological and gastrointestinal problems within 72 h of consumption of contaminated food items[4].Majorly possibility of food contamination was explained that the food is mixed with the pathogen and mixed with toxin substances[5].Majority of the microbiologists explained the role of microorganisms to cause foodborne diseases. The pathogens causing food borne illnesses are multiplied immediately before food consumption and cause infections wherein the food acts as a vector for microbial contamination.The other mode of foodborne diseases is the intoxication of food with chemicals and microbial toxin substances[6].The primary foodborne disease causing pathogens are Escherichia coli,Klebsiella pneumoniae,and Staphylococcus,which result in illness via food poisoning[7].For controlling these pathogens,novel bioactive molecules need to be developed taking their drug resistance properties into consideration[8].
In the current decade,microbial surfactant molecules are being used in various applications,i.e.,food,pharma and agriculture based on their origin and ecofriendly nature[9,10].Biosurfactants have fulfilled almost all the regulations and demands of the society regarding the replacement of toxic chemicals [11]. The recent trend of using more natural additives than the synthetic ones due to their positive effects on health and environment has also created a demand for“green”additives in foods[12].Microbes produce surfactants with surface active, antimicrobial and antibiofilm properties[13].Based on their activities,these are used as natural additives for the control of foodborne pathogens in the food processing sector[14].These are amphiphilic in nature with hydrophilic and hydrophobic moieties, showing the ability to reduce the surface tension between immiscible interfaces[15].The concentration of biosurfactant required for reducing surface tension by forming a micelle is called Critical Micelle Concentration(CMC).Based on their chemical structures and properties,biosurfactants are classified as glycolipids,lipopeptide,and phospholipids.Due to their lower toxicity,ecofriendly and biodegradable properties,microbial surfactants are used as an alternative for chemical substances[16].
2.Materials and methods
2.1.Isolation of biosurfactant producing bacteria
Soil samples were collected(October 30th,2018)from groundnut oil cake dumping sites at different areas of Hyderabad,Telangana,India.The collected soil samples were kept in sterile bags and transported to experimental laboratory within 2 h before sampling.Enrichment culture method of Brzozowski et al.[17]was adopted with a slight modification:10 g of soil sample was mixed into 100 mL of mineral salts medium, supplemented with 5%(V/V)sterile crude oil and incubated in a rotary shaker at 37°C and 200 r/min for 7 days.After incubation,the samples were serially diluted and spread on NA(nutrient agar plates),and incubated at 37°C for 24–48 h.Isolates were subcultured and used for further studies.
2.2.Screening methods for biosurfactant producers
2.2.1.Penetration method
ELISA 96 well microplates were used in this method;200µL hydrophobic paste containing oil and silica gel were added into the wells.10µL of crude oil, 90 µL of culture supernatant and 10 µL of safranin solutions were added subsequently and the biosurfactant activity was observed[18].
2.2.2.Oil displacement method
In this method,50 mL of distilled water was added into the petridish and 2 mL of the crude oil was added such that it was spread uniformly on the water surface.Then,500µL of the culture supernatant was added and clear zones on the oil surface were observed[19].
2.2.3.Drop collapse method
The method described by Morais et al.[20]was performed with slight modifications. A drop of crude oil and culture supernatant were added onto the glass slide and the drop collapse activity was visualized.
2.2.4.Lipase assay
In this assay,10 μL of overnight culture broth of the selected isolates were plated onto tributyrin agar medium and the plates were incubated at 37°C for 48 h.Then,the zones of lysis around the colonies were observed[18].
2.2.5.Hemolytic activity
In this method,10 μL of overnight culture broth of the selected isolates were inoculated onto 5%sheep blood agar and the plates were incubated at 37 °C for 48 h. The presences of zone of lysis around the colonies were checked[21].
2.2.6.Emulsification index(EI%)
48 h grown culture supernatants were collected by centrifugation at 10,000 r/min for 10 min and EI percentages were estimated by adding 2 mL of each oil(6 different oils were used in this study)and 2 mL supernatant,separately.The above prepared mixture was vortexed for 15 min at room temperature and left intact for 24 h and the emulsification index percentages were calculated[22].
2.2.7.Emulsification assay
This assay was performed by following the method described by Campos et al.[23]with slight modifications.3 mL of culture supernatant and 0.5 mL of oil were mixed,vortexed for 5 min and allowed to stand for 1 h at room temperature.The aqueous phase of the mixture was then collected and the absorbance at 400 nm was measured.
2.3.Molecular identification
The selected isolate Bacillus sp.OR1 was identified through 16S rRNA sequencing.The protocol was followed according to the manufacturer's(MACROGEN (Seoul, Korea)) instructions. Standard primers (785F 5′-GCATTAGATACCCTGATA-3′and 907R 5′-CCGTCAATTCMTTTRAGTTT-3′)were used.The following conditions were applied to the polymerase chain reaction(PCR),i.e.,95°C,5 min,95°C for 1 min,55°C for 50 s,72°C for 55 s,and 72°C for 5 min(Bio-Rad,USA).Sanger sequencing method was used for determining the nucleotide sequence of the amplicon in Applied Biosystems 3730 XL sequencer,USA.BLAST and phylogenetic analysis were performed by using Molecular Evolutionary Genetics Analysis(MEGA) version .7 [18]. Finally, the sequence was submitted to the GenBank database.
2.4.Extraction of biosurfactant
Bacillus sp.OR1 culture supernatant was collected by centrifugation at 10,000 r/min for 15 min and the pH of the supernatant was adjusted to 2.0 using 6 mol/L HCl.The supernatant was then allowed to the precipitate for 24 h at 4°C and the biosurfactant was collected by centrifugation at 10,000 r/min for 10 min with methanol[24].
2.5.Characterization of the extracted biosurfactant by FTIR
To determine the chemical structure and components of the extracted biosurfactant, FTIR spectroscopic analysis (Shimadzu, Japan) was performed. Samples were prepared by homogeneous dispersal of 1 mg of biosurfactant sample in pellets of potassium bromide.Infra-red(IR)absorption spectrum was obtained using a built-in plotter.The IR spectrum was obtained in the range of 450–4500 cm-1with a resolution of 4 cm-1[25].
2.6.Antibacterial activity
In this method,the extracted lipopeptide was diluted and the minimum inhibitory concentration (MIC) was determined [26]. The following foodborne pathogens enlisted in Microbial Type Culture Collection(MTCC)database namely E.coli(MTCC 43),Klebsiella pneumoniae(MTCC 530),and Staphylococcus aureus(MTCC 96)were provided by the Department of Biotechnology,Mahatma Gandhi University,Nalgonda and were used for antibacterial activity studies.
2.7.Critical micelle concentration(CMC)
In this method,surface tension was measured by the ring method using a DuNouy tensiometer at room temperature. The concentration of biosurfactant required for micelle formation in the solution is known as CMC.Various concentrations(0–500 mg/L)of the extracted biosurfactant were prepared using distilled water and the surface tensions were measured,respectively[27].
2.8.Statistical analysis
All the experiments were performed in triplicates.The mean value of the three independent trials and the standard errors were calculated using Microsoft Office Excel 2007.
3.Results
3.1.Biosurfactant producers
Soil samples were collected from groundnut oil cake dumping sites at Hyderabad,Telangana,India.Soil samples were then analyzed by enrichment culture technique for the selection of potential biosurfactant producers, and henceforth, thirty of them were selected. All the thirty bacterial isolates were evaluated by penetration assay and based on the results of the penetration assay(color change),top ten strains were selected(data not shown). Oil displacement and drop collapse assay are rapid methods for the selection of microbial biosurfactant producers;the results revealed that all the ten isolates were good producers.In lipase and hemolytic activity assays,all the ten strains showed zones of lysis around thecolonies.In the present investigation,EI%and emulsification capacity were estimated using six different oils. The isolate namely, Bacillus sp. OR1 showed the highest EI%(Table 1).Compared to other isolates,the selected isolate Bacillus sp. OR1 showed the highest emulsification capacity(Table 2).
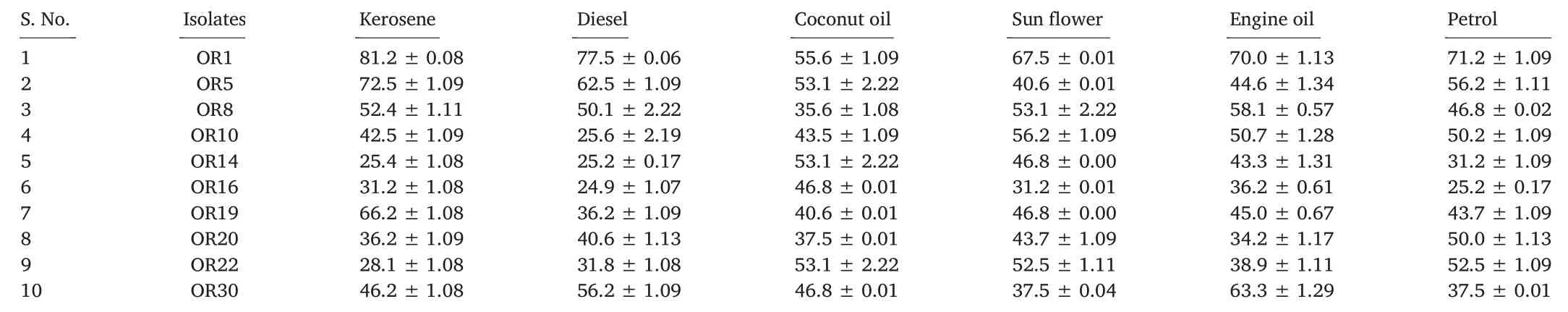
Table 1 Emulsification index(EI%)of selected bacterial isolates using different hydrocarbons.
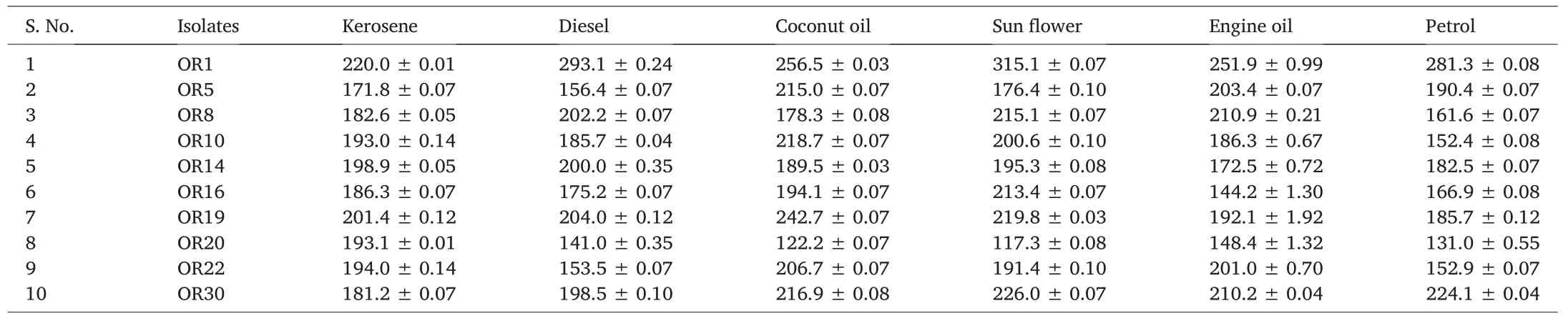
Table 2 Emulsification capacity assay.
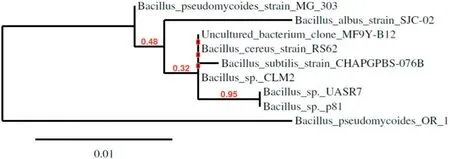
Fig.1.Phylogenetic tree analysis of Bacillus sp.OR1.
3.2.16S rRNA sequencing
The morphological studies of the selected isolate,Bacillus sp.OR1,revealed it to be Gram-positive, rod-shaped, motile and a sporulating bacterium in nature. BLAST results demonstrated the isolate as Bacillus pseudomycoides OR 1 with the accession number, MK490967(Fig. 1).
3.3.FTIR analysis
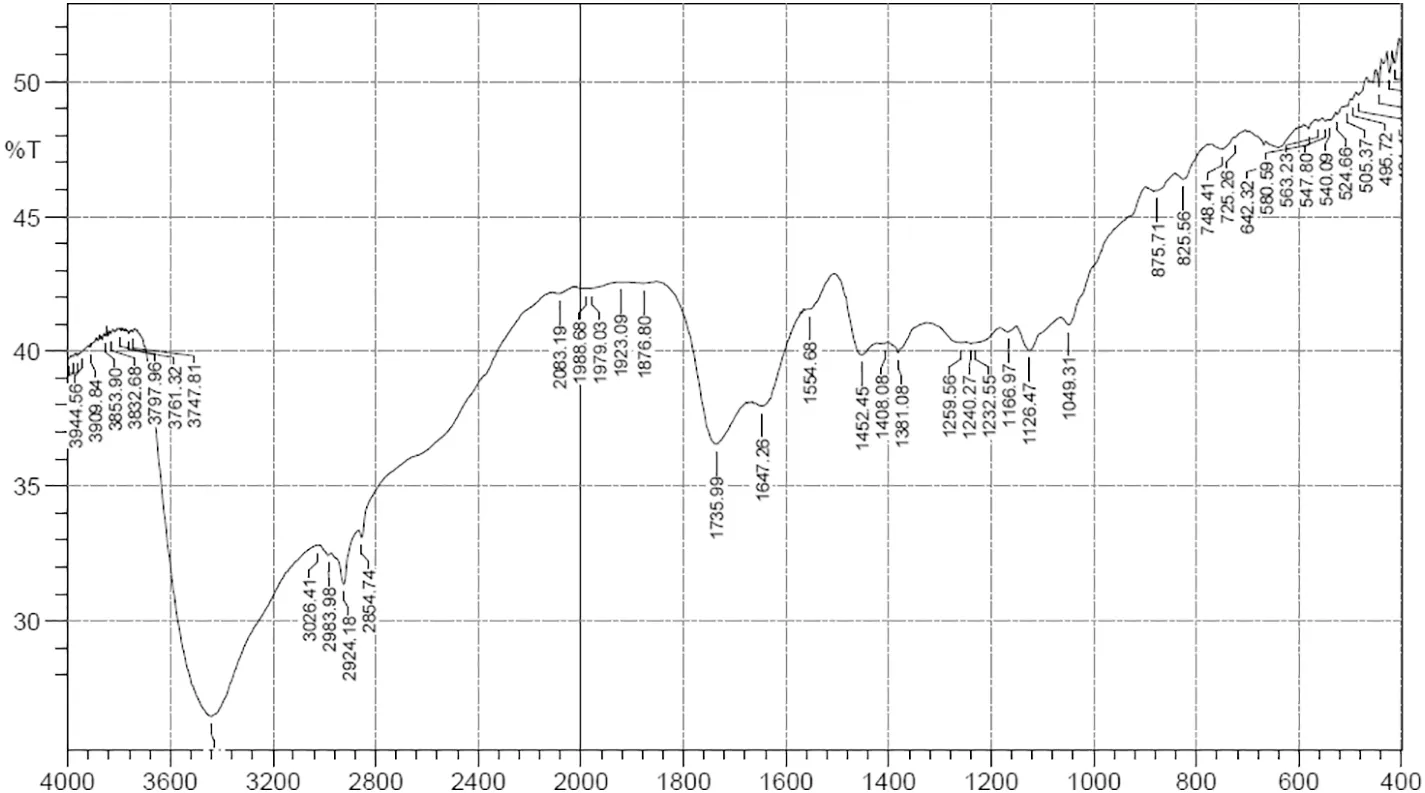
Fig.2.FTIR analysis of extracted biosurfactant.
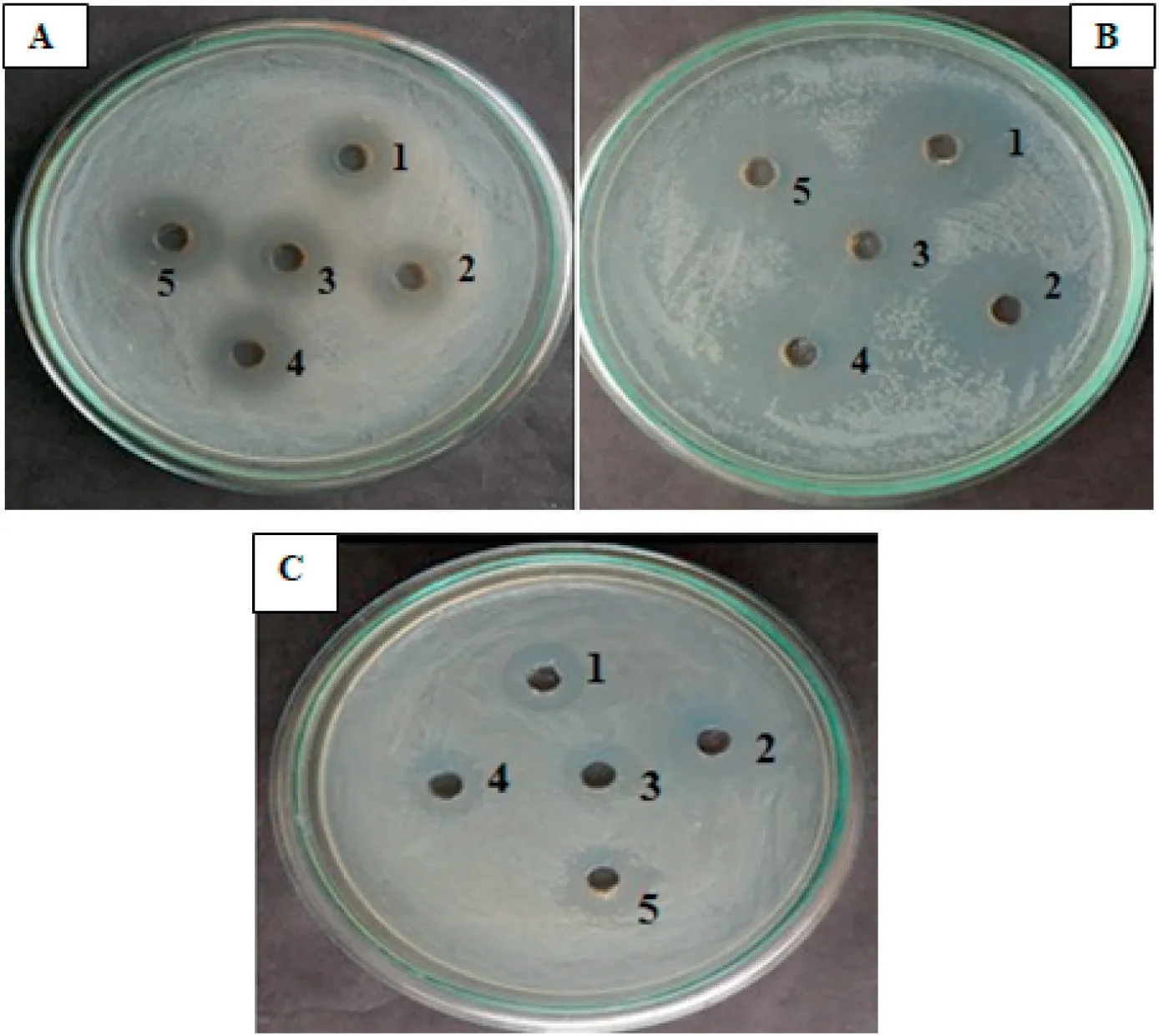
Fig.3.Antibacterial activity of extracted lipopeptide.A:Escherichia coli;B:Klebsiella pneumoniae;C:Staphylococcus aureus.Here 1 to 5 is different concentration of extracted lipopeptide from 10 to 50 μg/mL.
The biosurfactant extracted from B.pseudomycoides OR 1 was characterized by using FTIR.In their spectrum of the biosurfactant,the peak at 3500 to 3200 cm-1was due to N—H and O—H stretching,indicating amine and hydroxyl groups (Fig. 2). The peaks between 2963 and 2854.68 cm-1disclosed the presence of the aliphatic chain as suggested by the C—H modes. The strong absorption peak at 1647.26 cm-1revealed the stretching of the CO—N bond,and an absorption peak at 1554.68 cm-1showed the stretching mode of the C—N bond.The absorption peaks between 1452.71 cm-1and 1408.08 cm-1indicated the presence of CO and it was confirmed from the band at 1240.27 cm-1,which corresponded to CO deformation vibrations.Based on the above results,the biosurfactant produced by B.pseudomycoides OR 1 could be identified as a lipopeptide type of biosurfactant(Fig.2).
3.4.Antibacterial activity
Different concentrations of the extracted lipopeptide were used for in vitro antibacterial activity studies against foodborne pathogens(Fig.3).Lipopeptide at 50 μg/mL concentration showed maximum inhibitions against E. coli (7 mm), K. pneumoniae (10 mm) and S. aureus(6 mm),respectively.The zones of inhibition(mm)exhibited by different concentrations of lipopeptide are listed in Table 3.
3.5.CMC of extracted lipopeptide
The surface active properties of the biosurfactant mainly depend on its lower surface tension and CMC concentrations.The CMC values confirm the minimum concentration of biosurfactant required for surface tension reduction. Thus, CMC is an essential method to detect the efficiency of a biosurfactant producer. In the present work, the extracted lipopeptide showed the lower CMC value of 60 mg/L while causing a significant surface tension reduction of water up to 31.6 mN/m(Fig.4).
4.Discussion
The aim to include additives is to boost and maintain the biological process, feel, safety, taste and worth, and bringing out food modifications within the trend of customers towards natural additives from artificial ones and also the increasing health and environmental issues have created demand for brand spanking new“green”additives in food[28,29].Bacteria produce biosurfactants extracellularly,and these compounds show amphiphilic moieties[30].Based on the chemical composition and nature,different types of biosurfactants are reported[31].The biosurfactant producers isolated from various sources such as rhizosphere,endophytes,metal,hydrocarbon polluted soils,petrol,and diesel contaminated soils,oil industries and effluent samples, marine sources, mangrove sediments and tannery effluents,etc.,have been documented well in literature[32].In the present study, biosurfactant producing bacteria were isolated from groundnut oil cake dumping sites in Hyderabad.
Various techniques have been reported in literature to screen biosurfactant producers since 1970.Nevertheless,in recent years,due to automation and miniaturization, high throughput screening methods,which can lead to the desired upsurge of novel commercial biosurfactant producers, are being developed. According to Jing and Bingbing [33],several processes are needed to detect biosurfactant producers including hemolysis,drop collapse,oil spread on surfaces and emulsification activity assay.In the present study,thirty bacteria were isolated from crude oil contamination sites based on penetration method and ten isolates among those were selected for further studies.According to Kumar et al.[18],qualitative and quantitative methods are generally needed to screen biosurfactant producing bacteria.According to the previous report of Garrett et al.[34],oil spreading,the results of the lipase and hemolysis activity assays would reveal the concentration of biosurfactant involved in producing lysis zones.In the present study,all the ten isolates showed positive results in all the screening methods.The previous report of Kumar et al.[18], suggested that the EI and EA methods would be inevitable to detect potential biosurfactant producers;Bacillus subtilis(MTCC 2422),showed the highest EI%of 68%with kerosene and the highest emulsification potential on petrol(291.4 EU/mL),respectively.Bacillus sp.OR1 isolate showed the highest EI% of 81.2 (Table 1) with kerosene and an emulsification capacity of 315.1 EU/mL with sunflower,respectively(Table 2).Morphological and growth characteristics of the bacterial isolate Bacillus sp.OR1,revealed it as Gram-positive and rod-shaped. Identification of the isolated was achieved by 16S rRNA gene sequence analysis at Macrogen, Korea and the sequence was searched in different bioinformatics servers. The sequence was then submitted to NCBI and the phylogenetic tree was constructed by using bioinformatics tools (Fig. 3). Previously, Gandhi and Skebba[35]and Araujo et al.[36]revealed the absorbance bands of similar lipopeptide FTIR peaks as observed in the present study.
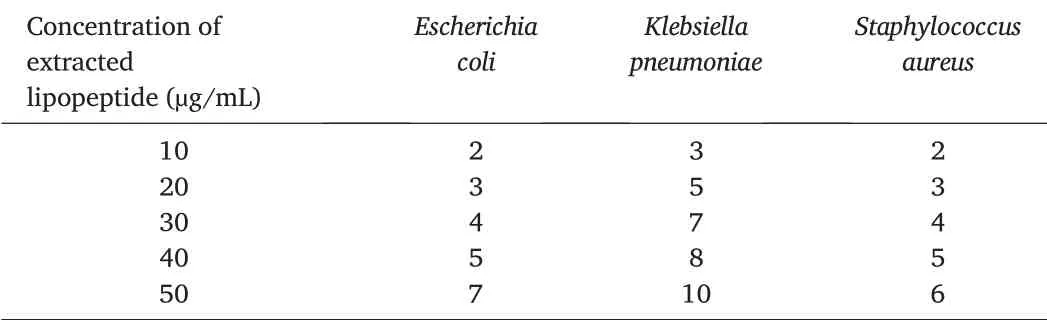
Table 3 Antibacterial activity of extracted lipopeptide.Unit:mm.
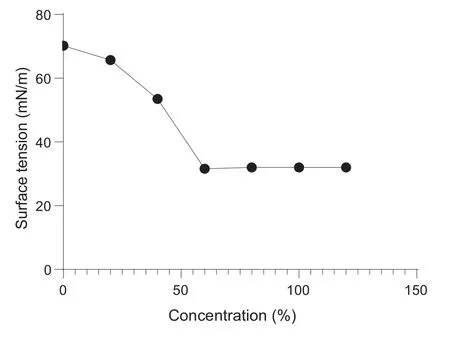
Fig.4.Critical micelle concentration of extracted lipopeptide.
Food processing business will utilize biosurfactants in two different ways: (a) indirect use for sterilization or cleaning of contact surfaces;(b) direct use as a food supplement [37]. Several food companies are using the waste source as a substrate for the production of biosurfactants[38].In recent trends of past years,the number of research works and reports were published on control of foodborne pathogens by using microbial surfactants[39].Based on the important of biosurfactants in the food industry have gained the interest for exploitation[40].In the present study,the extracted lipopeptide was used for antibacterial activity studies and the results revealed that at 5 μg/mL concentration showed better antibacterial activity against E.coli(7 mm),K.pneumoniae(10 mm)and S.aureus(6 mm),respectively. Members of the lipopeptide family include iturin A,bacillomycin D, bacillomycin F, bacillomycin L, and bacillomycin Lc,which contain a β-hydroxy fatty acid that is known to inhibit different species of fungi and bacteria[41–43].The mode of action of a lipopeptide is binding to the bacterial surface bilayer and altering the local lipid organizational linking on negatively charged fatty acids, resulting in the restructuring of the lipid bilayer and preventing cellular processes[44,45].According to Mulligan et al.[46],a wide range of CMC concentrations(5 to 386 mg/L),has been reported.In the present study,CMC value of the crude extracted lipopeptide from Bacillus sp. OR1 was recorded as 60 mg/L.
5.Conclusion
The extracted lipopeptide (50 μg/mL) from B. pseudomycoides OR 1 showed significant inhibition against foodborne pathogens and seemed to be a promising biocontrol agent use in the food industry.
Declaration of Competing Interest
All the authors confidently declare that there is no conflict of interest,and paper is approved by all authors for publication.
Acknowledgment
The author wishes to express his special thanks to the Principal,Chaitanya Bharathi Institute of Technology,Hyderabad,and Telangana,India.
 Grain & Oil Science and Technology2019年1期
Grain & Oil Science and Technology2019年1期
- Grain & Oil Science and Technology的其它文章
- Study on the effect of wheat bran dietary fiber on the rheological properties of dough
- Antidepressant activity and HPTLC fingerprinting of stearic acid in different days of wheat seedlings
- Consolidated bioprocess of corn stover to polysaccharide using Chaetomium globosum CGMCC 6882
- Synergism of essential oils with lipid based nanocarriers:emerging trends in preservation of grains and related food products
