Sofosbuvir/Ribavirin therapy for patients experiencing failure of ombitasvir/paritaprevir/ritonavir + ribavirin therapy: Two cases report and review of literature
Ken Sato, Yuichi Yamazaki, Takeshi Kobayashi, Satoshi Takakusagi, Norio Horiguchi, Satoru Kakizaki,Masayasu Andou, Yoshihiro Matsuda, Toshio Uraoka, Hiroshi Ohnishi, Hiroaki Okamoto
Abstract
Key words: Direct-acting antiviral agent failure; Hepatitis C; Genotype 2; Ribavirin;Sofosbuvir; Case report
INTRODUCTION
The combination therapies with sofosbuvir/ribavirin (SOF/RBV) and glecaprevir/pibrentasvir (GLE/PIB) are the 1st-choice therapeutic options for hepatitis C virus (HCV)-infected patients with genotype 2 in Japan according to the most recent version (ver. 6.2) of the Japan Society of Hepatology (JSH) guideline for the management of HCV infection. Ombitasvir/paritaprevir/ritonavir plus ribavirin(OBV/PTV/r+RBV) was the 1st-choice therapeutic option for HCV-infected patients with genotype 2 in Japan according to the previous version (ver. 5.4) of the JSH guideline, and this regimen was completely replaced by the combination therapy of GLE/PIB in the most recent version (ver. 6.2) of the JSH guideline.
The sustained virological responses at 12 wk after treatment (SVR12) for HCVinfected patients with genotype 2 are 97% and 91.5% for the 12-wk SOF/RBV regimen[1]and 16-wk OBV/PTV/r+RBV regimen[2], respectively. The combination therapy of GLE/PIB for HCV-infected patients with genotype 2 shows SVR12 rates of 97.8% and 100% for patients with chronic hepatitis and those with liver cirrhosis,respectively[3]. These direct-acting antiviral agent (DAA)-based therapies have a relatively favorable safety profile.
Although the efficacy of DAA-based therapy is quite high, we have recently experienced a certain percentage of patients with on-treatment virologic failure during treatment and virologic relapse during post-treatment for DAA-based therapy.Currently, three interferon (IFN)-free DAA-based therapies as mentioned above are available, and SOF/ledipasvir (LDV) combination therapy has just become available for HCV-infected patients with genotype 2 in Japan.
An SVR to the combination therapies of OBV/PTV/r+RBV and GLE/PIB in HCVinfected patients with genotype 2 who showed virologic failure with the SOF/RBV regimen was achieved[3,4]. However, it is unknown whether an SOF-based regimen can rescue patients with treatment failure by the combination therapy with OBV/PTV/r+RBV or GLE/PIB to date. We therefore examined the efficacy and safety of the combination therapy with SOF/RBV in two patients infected with HCV genotype 2a who could not achieve an SVR by the combination therapy with OBV/PTV/r+RBV.
CASES PRESENTATION
Baseline patient demographics and characteristics
The two patients were Japanese and comprised of a man and a woman. Both patients did not undergo liver biopsy. The severity of liver disease was judged as being chronic hepatitis in both patients by practical discriminant function to perform the distinction between chronic hepatitis and liver cirrhosis in patients with HCV infection[5]before the registration for the phase 3, randomized, open-label study with OBV/PTV/r+RBV[2]. Actually, the disease severity in both cases, however, was considered chronic hepatitis with advanced fibrosis that is close to liver cirrhosis based on the laboratory and imaging data. For example, although portosystemic shunt was not observed, FIB4 index and AST-to-platelet ratio index were relatively high in both cases (Table 1). HBsAg, HBcAb, and HBsAb were all negative in both patients. After starting the combination therapy with SOF/RBV, we principally checked the laboratory data and adverse events (AEs) at least once a fortnight. We obtained informed consent from both patients.
Diagnostic procedure
HCV genotypes were evaluated in commercial laboratories (BML, Inc., Tokyo, Japan).A TaqMan HCV test, version 2.0, real-time polymerase chain reaction (PCR) assay (F.Hoffmann, La Roche Ltd., Basel, Switzerland) was used to quantify HCV RNA.
Regarding resistance-associated variations (RAVs), we evaluated Y56 and D168 in the HCV/2a-NS3 for PTV[6], T24, F28, M31, and C92 in the HCV/2a-NS5A for OBV[6],and S282 in the HCV/2a-NS5B for SOF[7]. The genome sequences were analyzed using the serum samples before the combination therapy with OBV/PTV/r+RBV. PCR amplification and subsequent direct sequencing were performed to determine the RAVs. Table 2 shows the primers used for cDNA synthesis and amplifications.
The IL28B rs12979860 gene polymorphism of both patients was evaluated by the central laboratory of AbbVie Inc.
The HLA-DRB1 and DQB1 genotyping was determined by sequence based typing and HLA-DQA1 genotyping was determined by sequence-specific primers in commercial laboratories (SRL, Inc., Tokyo, Japan).
Study subjects
Patient 1:A 61-year-old female was diagnosed with chronic hepatitis C in 1995.Although the exact transmission source of the HCV could not be identified, the hemostatic agent used for a caesarotomy in 1989 might have been contaminated with HCV. She had received pegylated interferon/RBV therapy in 2013 after receiving partial splenic embolization. However, the treatment was discontinued due to erythema and itching at approximately 1 mo of the therapy. Resultantly, the viral response was a relapse. Then, she enrolled in a phase 3, randomized, open-label study with OBV/PTV/r+RBV. She received 25 mg OBV/150 mg PTV/100 mg r, which was administered orally once daily, and 600 mg RBV, which was administered orally twice daily. She was assigned to the 12w-treatment arm in the open-label study.Although the serum HCV RNA was disappeared at week 1 of the therapy and she achieved a rapid virological response (RVR), the HCV RNA reappeared at posttreatment week (PTW) 8 of the therapy.
Patient 2:A 50-year-old male was diagnosed with chronic hepatitis C in 2010. He has suffered from Vogt-Koyanagi-Harada (VKH) disease since 2011. He has a history of drug abuse and thus, the transmission source of the HCV may be from sharing needles. It has been reported that there is an association between VKH disease and IFN with RBV therapy, so he has not received IFN-based therapy. Although we had been concerned about the effect of RBV on VKH disease, we decided to apply DAAbased therapy including RBV due to the predicted advanced fibrosis based on laboratory data, including a relatively high level of alpha-fetoprotein and fluctuating serum alanine aminotransferase (ALT) level, although liver cirrhosis was denied due to practical discriminant function to perform the distinction between chronic hepatitis and liver cirrhosis in patients with HCV infection[5]. Then, he was enrolled in a phase 3, randomized, open-label study with OBV/PTV/r+RBV. He received the same dosage and administration of OBV/PTV/r and 800 mg RBV, which was administered orally twice daily. He was assigned to the 16w-treatment arm in the open-label study.He did not achieve an RVR. The HCV RNA disappeared at week 6 of the therapy and reappeared at the end of treatment. The serum ALT level of the patient increasedagain at PTW 4. Regarding the AEs, he became aware of being easily defocused near week 1 of the therapy. He was referred to an ophthalmologist. He was diagnosed with a recurrence of VKH disease at week 2 of the therapy due to choroidal thickening. He received a subtenon capsule injection of triamcinolone acetonide at week 2, week 12 and PTW 9. Then, he had a remission of the symptoms of VKH disease.
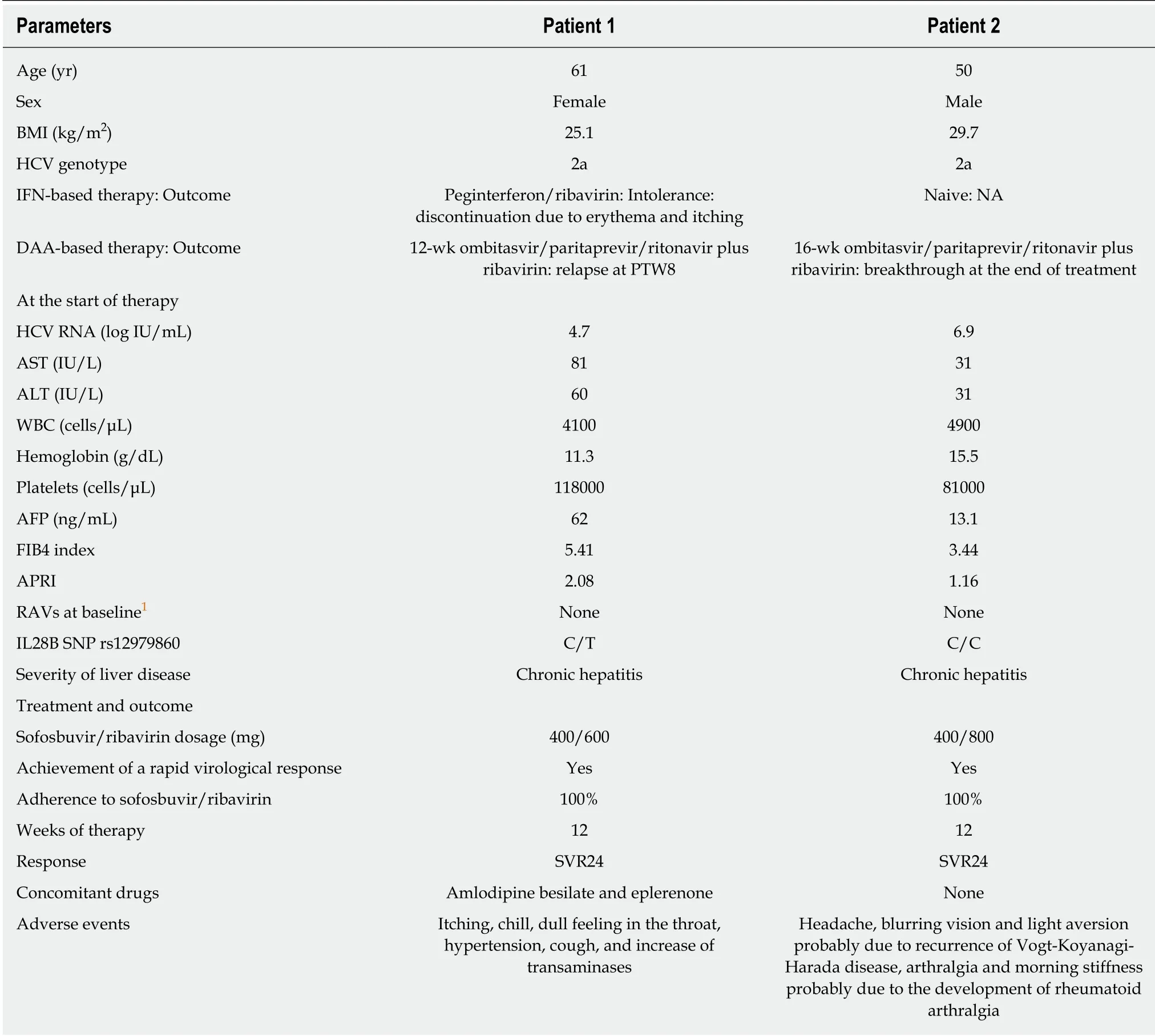
Table 1 Laboratory findings at baseline, and the treatments and outcomes of the patients
FINAL DIGNOSIS
Both patients were diagnosed with HCV genotype 2 with failure of OBV/PTV/r+RBV therapy based on laboratory findings and DAA treatment history.
TREATMENT
Patient 1
We tried to administer a different DAA-based regimen that is SOF/RBV. She did not have any RAVs regarding NS3, NS5A and NS5B before the treatment with SOF/RBV.Then, she received the combination therapy of SOF/RBV.
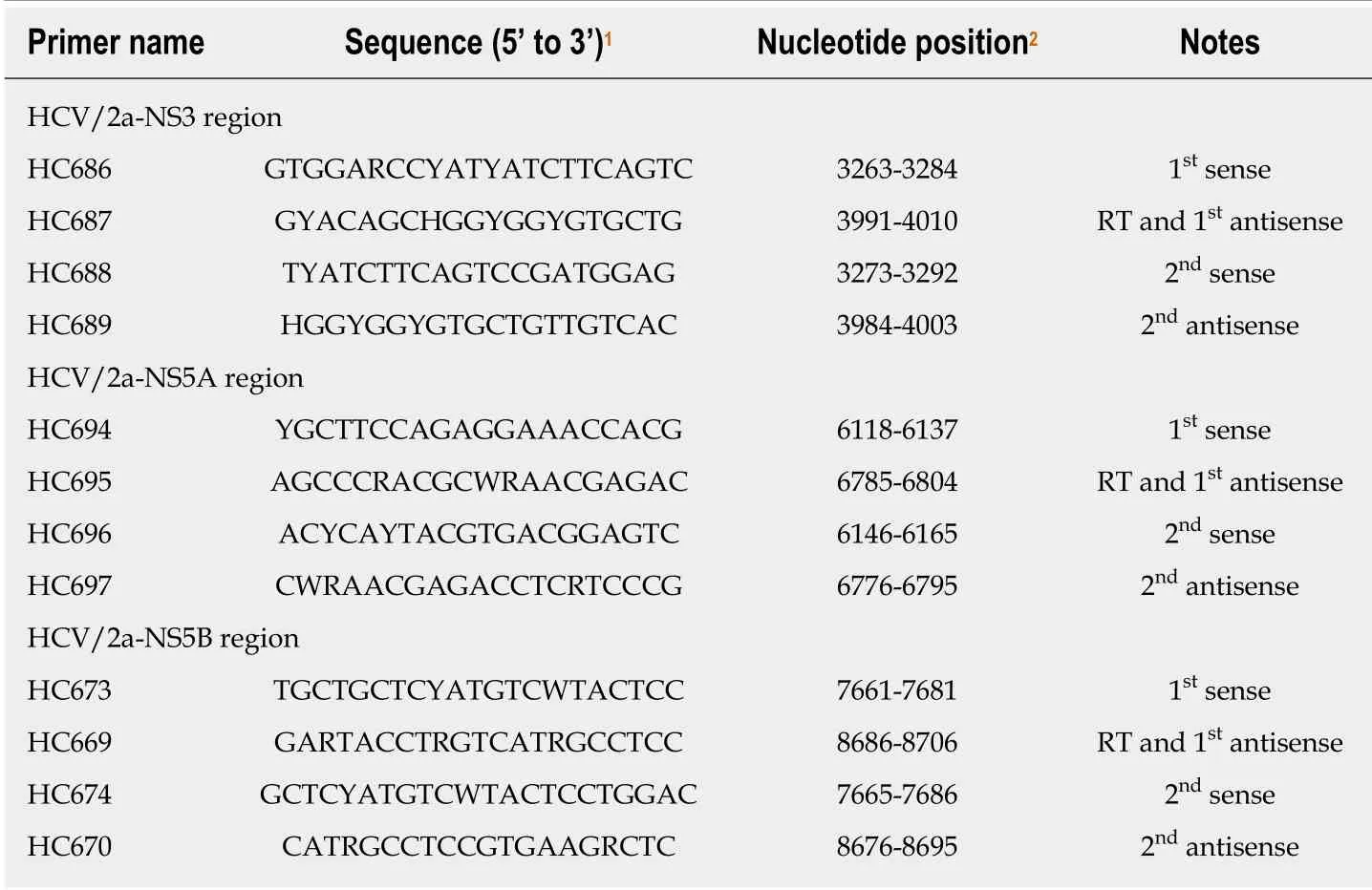
Table 2 Primers used for the amplification of the NS3, NS5A and NS5B regions of the genotype 2a hepatitis C virus genome
Patient 2
As we considered that the recurrence of VKH disease associated with DAA-based therapy can be controllable, we tried to administer a different DAA-based regimen that is SOF/RBV. He did not have any RAVs regarding NS3, NS5A and NS5B before the treatment with SOF/RBV. He received the combination therapy of SOF/RBV.
OUTCOME AND FOLLOW-UP
Patient 1
She achieved an RVR, SVR12 and SVR24. However, she developed itching, chill, a dull feeling in the throat, hypertension and cough. Her transaminases were temporarily increased. We considered elevation of transaminases as AE, but it may be due to discontinuation of ursodeoxycholic acid and Monoammonium glycyrrhizinate,Glycine, Aminoacetic acid, L-Cysteine hydrochloride hydrate (Stronger Neo Minophagen C®) just before the start of SOF/RBV. Although this is applied to Patinet 2 as well, the effect may vary in patients. After the treatment, these AEs disappeared completely.
Patient 2
He achieved an RVR, SVR12 and SVR24. Regarding the AEs, he developed a headache at week 1 and week 6 of the therapy. The recurrence of VKH disease was diagnosed at week 5 of the therapy on a regular clinical visit to his primary care ophthalmologist,and he was referred to the ophthalmologist in our hospital. Then, he received a subtenon capsule injection of triamcinolone acetonide, and resultantly, he had remission of light aversion. We retrospectively found that he had noticed a slightly blurring vision and light aversion one month before the combination therapy with SOF/RBV according to the detailed medical interview. As it remains a possibility that the symptom was deteriorated after the start of the combination therapy, this AE was however considered as being a treatment-emergent AE. Fortunately, the severity of the recurrence was mild because the single subtenon injection caused the remission of the disease. However, he developed arthralgia and morning stiffness approximately 7 wk after the end of treatment and turned out to have rheumatoid arthralgia (RA). The ultrasonography findings of the right ulnar intercarpal joints in patient 2 are shown in Figure 1. The RA was considered as being a treatment-emergent AE. The symptoms due to RA were improved using corticosteroid and disease modified anti-rheumatic drugs. HLA DNA typing showed that this patient had the DRB1*04:05:01,DQB1*04:01:01, and DQA1*03:03 genotypes. Serum HCV RNA levels remain negative even after treatment of RA.
The clinical course of both patients is shown in Figure 2. The laboratory findings,treatments and outcomes of both patients are shown in Table 1.
DISCUSSION
The major finding from this case series is that the combination therapy of SOF/RBV may have promise as an efficacious therapy in patients infected with HCV genotype 2 who could not achieve a SVR by the combination therapy with OBV/PTV/r+RBV.This is the first report showing the efficacy of the combination therapy with SOF/RBV for patients with failure of the combination therapy with OBV/PTV/r+RBV.OBV/PTV/r+RBV has been performed until very recently and was replaced by GLE/PIB combination therapy in Japan. However, there might be cases of treatment failure with OBV/PTV/r+RBV, and we believe that our study is useful for this current setting.
However, patient 1 received once-daily co-formulated OBV/PTV/r+RBV (25 mg/150 mg/100 mg) with twice-daily weight-based RBV for 12 wk but not the 16 wk that are covered by insurance at the present. The virological response for the combination therapy with OBV/PTV/r+RBV for patient 1 was a relapse. If she received this regimen for 16 weeks, she might have achieved SVR12 and SVR24. In fact, this regimen could achieve a numerically higher SVR12 rate in the 16-wk regimen compared to the 12-wk regimen[2]. In addition, the SVR12 rates based on this regimen were higher in patients with the HCV genotype 2a than in those with the HCV genotype 2b. However, both cases were HCV genotype 2a and were susceptible to this regimen, even the 12-wk regimen[2]. In this regard, patient 1, who received the 12-wk regimen, was considered relatively treatment-resistant. The reasons for the treatment failure due to the combination therapy with OBV/PTV/r+RBV were unclear. The patient characteristics that were statistically associated with virologic failure for the combination therapy with OBV/PTV/r+RBV were a prior treatment experience and male gender[2]. Patient 1 had a prior treatment experience and patient 2 is male. The RBV dose modification was a factor that was potentially but not statistically associated with virologic failure[2]. Neither of the patients had an RBV dose modification. RAVs before the combination therapy of SOF/RBV were not detected in NS3/4A, NS5A or NS5B in either patient. Patient 1 had no RAVs in NS3/4A or NS5A at the time of failure based on the clinical trial[2]. Regarding patient 2, the information about RAVs was not available at the time of failure. The genotypes of IL28B SNP rs12979860 were C/T and C/C in patient 1 and patient 2, respectively.Although, for the combination therapy with LDV plus sofosbuvir for patients with chronic HCV genotype 1, the multivariate analysis identified the FIB4 index (< 3.25),IL28B rs8099917 (TT type), and NS5A-L31 (wild-type) as significant determinants of SVR12[8]. The genotype C/T of IL 28B SNP in patient 1 might contribute to the failure of the combination therapy with OBV/PTV/r+RBV, although both of the cases were genotype 2a. The reason why SVR was achieved by SOF/RBV combination therapy but not OBV/PTV/r+RBV combination therapy was unclear but may have been due to the different functional mechanisms between each regimen. For example, the host innate and adaptive immune systems are closely associated with nearly every step of HCV infection[9]. In addition to viral factors, the host responses are critical for viral clearance[9]. The different regimens may cause the difference of host responses such as host cytokine cascade and resultantly affect viral clearance.
Regarding the safety of the combination therapy of SOF/RBV, the patient was generally well-tolerated in the phase 3 clinical trial[1]. Patient 2 might have had a recurrence of VKH disease caused by the combination therapy with OBV/PTV/r+RBV or SOF/RBV. Although the recurrence was not severe and was controllable by a subtenon capsule injection of triamcinolone acetonide, either therapy might have played a causal role in the deterioration from the VKH disease. IFN or IFN+RBV reportedly leads to the development of VKH disease or can cause a recurrence of VKH disease[10-17]. The assumed causes include a shift towards a predominant T-helper 1-type immune response, an alteration of the expression of histocompatibility class I and II antigens[12,18], and an increase in the production of endogeneous IFN-gamma that is induced by IFN-alpha[19,20], in addition to immunomodulation effects of IFN. In patient 2, however, IFN was not used. RBV may have an immunomodulatory effect by favoring the T-helper 1 cytokine response[21-23],as well as by enhancing the expression of IFN-stimulated genes[24], possibly leading to the development of VKH disease. In addition, RBV directly increases IL-8 expression in human hepatoma cell lines[25]. On the other hand, the high levels of IL-8 were observed in both the aqueous humor and plasma of the patient with VKH disease[26],and there is a significant correlation between the incidence of IL-8 detection in aqueous humor samples from patients with uveitis, including VKH disease and an increased disease activity[27]. The role of IL-8 levels on the pathogenesis of VKH disease or the action of RBV should be investigated in future studies.
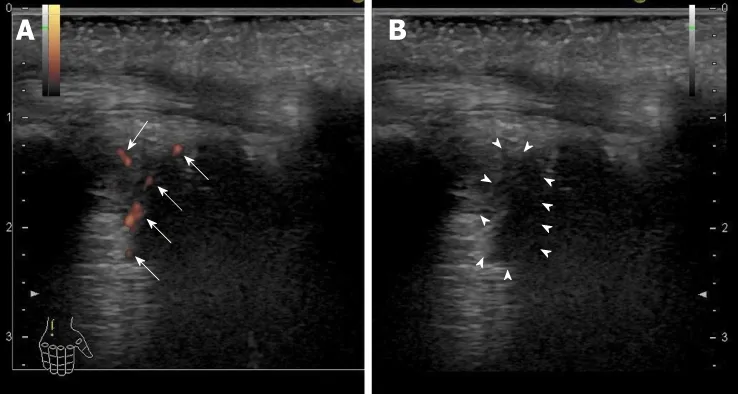
Figure 1 Ultrasonography findings of the right ulnar intercarpal joints in patient 2. A: Power Doppler imaging.There were clear synovial blood flow signals (arrows) with localization of the synovial thickening; B: B-mode imaging.Synovial thickening was observed as a low echoic lesion (surrounded by arrowheads).
Patient 2 developed arthralgia and morning stiffness approximately 7 wk after the end of the treatment. Rheumatoid factor, anti-nuclear antibody, and anti-cyclic citrullinated peptide antibody were negative. Serum matrix metalloproteinase-3, IgG,and C-reactive protein levels were within normal range. However, the ultrasound detected active synovitis in the right and left carpal joints and peritenon of the flexor muscle. RA was diagnosed according to the 2010 American College of Rheumatology/European League Against Rheumatism criteria[28]. Thus, our case was seronegative RA. As with the VKH disease, RA associated with IFN or PEG-IFN plus RBV treatment for chronic hepatitis C has been reported[29-33]. The possible reasons for the relationship between RA and IFN-based therapy were assumed to be similar to that with VKH disease and IFN-based therapy[29-33]. IFN-alpha reportedly induces the production of B-lymphocyte activation factor of which levels correlate with the autoantibody levels and synovitis in a subset of patients with early RA[34].Interestingly, there are reports showing an association between VKD disease and RA[35-37]. One case of this is myasthenia gravis complicated with RA and VKH disease[36]. The case had DR4, Bw54 and MT3 as subtypes of the HLA-DR antigen,which are frequently recognized in RA and VKH disease in common[36]. At the genomic level, our case had the DRB1*04:05:01, DQB1*04:01:01, and DQA1*03:03 genotypes. DRB1*04:05 is significantly increased compared to the healthy controls and is in a strong linkage disequilibrium with DQB1*04:01 in VKH patients[38]. DRB1*04:05 is also associated with a susceptibility to RA[39]. Thus, the development of RA and deterioration of VKH disease in our case were assumed to be associated with these genetic predispositions to autoimmune diseases.
The deterioration or development of autoimmune disease in our case may be affected by immune reconstitution/restoration due to HCV eradication with DAAbased therapy. DAA-based therapy reportedly improves innate immune function with restoration of type I IFN responses and normalization of NK cell function[40-43]and ameliorates various defective T cell functions[44,45]. This immune reconstitution/restoration may generate a harmful effect in patients with genetic predispositions to autoimmune diseases.
There is a case series study of DAA-based therapy for 12 patients with chronic hepatitis C complicated with autoimmune liver disease[46]. One cirrhotic patient with autoimmune hepatitis developed serum ALT elevation and was obliged to receive prednisolone during the therapy with SOF/LDV. Their report supports our study in which special caution regarding AEs should be taken in case of DAA-based therapy for patients with chronic hepatitis C complicated with autoimmune disease.
Several limitations associated with the present case series warrant mentioning. The sample size of our case series was very small, and the duration of combination therapy with OBV/PTV/r+RBV in one case was short compared to what is currently approved in Japan.
CONCLUSION
The combination therapy with SOF/RBV might have promise as an efficacious therapy, but caution regarding AEs should be practiced. Larger prospective studies of combination therapy with SOF/RBV for those with treatment failure for OBV/PTV/r+RBV are needed in the near future to confirm the findings in our case series.
 World Journal of Clinical Cases2019年9期
World Journal of Clinical Cases2019年9期
- World Journal of Clinical Cases的其它文章
- Coexistence of breakpoint cluster region-Abelson1 rearrangement and Janus kinase 2 V617F mutation in chronic myeloid leukemia: A case report
- Crizotinib-induced acute fatal liver failure in an Asian ALK-positive lung adenocarcinoma patient with liver metastasis: A case report
- Rare variant of pancreaticobiliary maljunction associated with pancreas divisum in a child diagnosed and treated by endoscopic retrograde cholangiopancreatography: A case report
- Adult-onset mitochondrial encephalopathy in association with the MT-ND3 T10158C mutation exhibits unique characteristics: A case report
- Nerve coblation for treatment of trigeminal neuralgia: A case report
- Management of the late effects of disconnected pancreatic duct syndrome: A case report
