Evaluation and management of acute pancreatitis
Ahmed T Chatila, Mohammad Bilal, Praveen Guturu
Abstract
Key words: Acute pancreatitis; Necrotizing pancreatitis; Resuscitation; Gallstone pancreatitis
INTRODUCTION
Acute pancreatitis (AP) is one of the most common gastrointestinal causes for hospitalization in the United States. In 2015, AP accounted for 390940 hospitalizations making it one of the most frequent causes of gastrointestinal hospitalizations in the nation with the annual incidence only expected to increase over time[1-3]. Despite recent advances in gastroenterology, AP continues to be associated with substantial mortality, morbidity and healthcare resource utilization[2,3].
In this report, we provide a comprehensive review of the epidemiology, pathophysiology, evaluation, and management of AP.
EPIDEMIOLOGY
The annual incidence of AP ranges from 15.9 to 36.4 per 100000 persons. The burden of the disease on the healthcare resource utilization is expected to increase in the near future[2-5]. Despite the improvement we have seen in access to healthcare, imaging modalities and interventions, AP continues to have significant morbidity and mortality that has largely remained unchanged over time. The overall mortality rate being 5% to 17% in severe AP, and 1.5% in mild AP[2,4,6].
The three most common causes of AP are gallstone/biliary related, alcohol related,and idiopathic. These three causes account for the majority of cases of AP[2,7-10]. Biliary pathology was estimated to be 28%-38% of the cases while alcohol accounted for 19%-41% of the cases[8,9,11].
Prior reports have shown a significant relation of gender and race in regards to etiology of AP. Overall, a markedly higher frequency of AP was seen among blacks than whites, followed closely by Hispanics, Asians, and then American Indians.Patients with AP due to alcohol use were significantly younger and were more likely to be male and/or black, with blacks having the highest frequency of alcohol related pancreatic disease[5,9,12]. Females are more likely to have biliary related pancreatitis[5,12].The increase in incidence of AP has been mostly seen in woman ages < 35 and men between the ages of 35 and 54[2].
ETIOLOGY
AP is the inflammation of the pancreas that is often associated with systemic inflammatory response syndrome (SIRS) that may impair the function of other organs. The etiology of AP can be readily identified in 75% to 85% of cases[8]. The American Gastroenterological Association (AGA) provides a comprehensive guide to determine the etiology of pancreatitis.
The evaluation should begin with a detailed history focusing on symptoms and presentation. The investigation should focus on evaluation of any previous documented gallstones, alcohol use, history of hypertriglyceridemia or hypercalcemia, family history of pancreatic diseases, prescription/non-prescription drug history, history of trauma, and presence of autoimmune disease. On presentation to the hospital, patients should have a serum amylase or lipase level checked along with liver chemistries (bilirubin, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase), and abdominal ultrasound assessing for cholelithiasis or choledocholithiasis. Extensive or invasive evaluation should be avoided on presentation[10,13,14]. The most common etiologies of AP have been summarized in Table 1.
Biliary Tract disease
Gallstone pancreatitis is the most common cause of AP and is estimated to be 28%-38% of all cases of AP[8,9,11]. Gallstone induced pancreatitis is caused by duct obstruction by gallstone migration leading to temporary impaction of migrating stones at the duodenal ampulla, increased duct pressure, and unregulated stimulation of the digestive enzymes secreted by the pancreas[15,16]. This obstruction can be due to calculi lodged in the duodenal ampulla, spasms, and fibrosis of the sphincter of Oddi[15,17-19].
Alcohol
Alcoholic pancreatitis is the second most frequent cause of AP and is estimated to be 19%-41% of all AP cases[9-11]. The association between alcohol abuse and pancreatitis is poorly understood, but it is known that the majority of patients who abuse alcohol do not develop pancreatitis[10,19]. In addition, two thirds of patient's who present with acute alcoholic pancreatitis already have developed an underlying chronic pancreatitis[20]. In about 8% of cases of AP related to alcohol, mutations in the pancreatic secretory trypsin inhibitor gene (SPINK1) have been seen[21].
Hypertriglyceridemia
Hypertriglyceridemia induced pancreatitis is a rare cause of AP and is estimated to make up 1%-4% of cases[22,23]. Hypertriglyceridemia induced pancreatitis is thought to be due to the hydrolysis of excessive triglyceride rich lipoproteins releasing high concentration of free fatty acids which injure the vascular endothelium and acinar cells of the pancreas. This injury causes a self-perpetuating ischemic and acidic environment with resultant toxicity[23-26]. Specific genes associated with cystic fibrosis transmembrane conductance regular mutation and a tumor necrosis factor were found to be risk factors for AP secondary to hypertriglyceridemia[22,27]. We recommend checking a triglyceride level in all patients with AP in which history is not suggestive of alcohol use and imaging does not indicate a biliary pathology.
Genetic
Several genetic mutations have been associated with the development of AP. Specific cystic fibrosis gene (CFTR) genotypes have been shown to be significantly associated with AP, with the highest risk seen in mild phenotypic genotypes[28]. Hereditary pancreatitis is an autosomal dominant disease caused by cationic trypsinogen (PRSS1)gene mutation, but is usually associated with chronic pancreatitis[29,30]. In younger patients, with no identifiable cause of AP, genetic etiologies should be considered.
Drug
Drug induced AP is a rare entity. There should be a high level of suspicion after common causes of AP have been ruled out. An estimated 2%-4.8% of reported cases of AP have been related to some medications[31,32]. A wide variety of drugs have been reported as possible causes of AP including 6-mercaptopurine, sulfonamides,diuretics, didanosine, pentamidine, tetracycline, azathioprine, estrogen, and steroids[33]. The proposed mechanisms of drug induced AP include immunologic reactions, direct toxic effect, toxic metabolite, ischemia, and thrombosis[33].
Infectious
Various infections have been associated with AP including viral, bacterial, fungal, and parasitic. Infections which have been reported to cause AP include mumps, coxsackie virus, hepatitis B virus, cytomegalovirus, varicella-zoster virus, herpes simplex virus,Mycoplasma, Legionella, Leptospira, Salmonella, Aspergillus, Toxoplasma, and Cryptosporidium[34-36]. From infectious causes, viruses are the leading etiology of AP[35].
Trauma
Any blunt trauma to the pancreas can cause AP, but this diagnosis should be made when there is a high suspicion. The incidence of pancreatic injury comprises 0.2% to 12% of all abdominal traumas[37,38]. The majority of pancreatic trauma is related to direct trauma with only a minority associated with blunt trauma[39].
Post-endoscopic retrograde cholangiopancreatography (ERCP)
Serum amylase elevations up to three times the upper limit of normal have been reported after 24 h of an ERCP[40]. It has been reported in 1.3%-4.3% of ERCP procedures. The most common risk factors for post-ERCP related AP are younger age,female gender, history of sphincter of Oddi dysfunction, pancreatic duct opacification,cholangitis, and duodenal perforation[41-44].
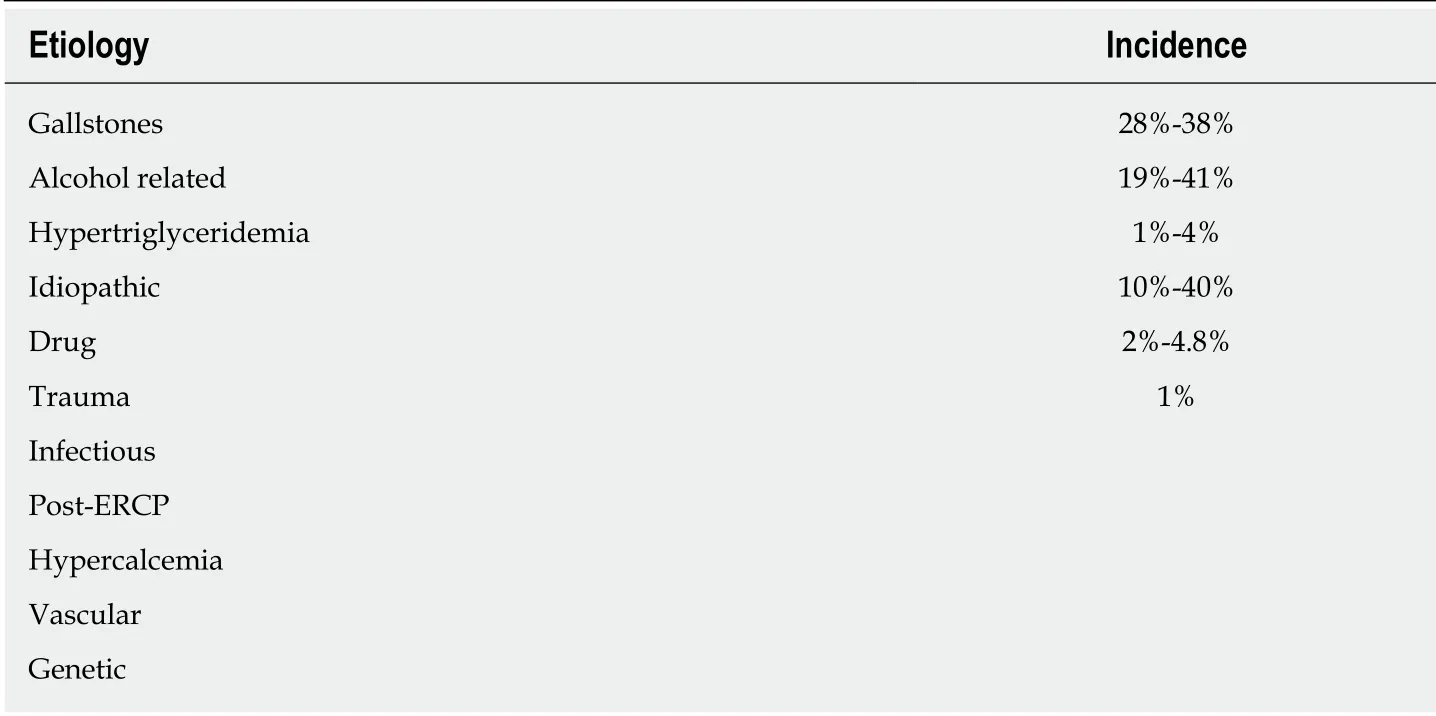
Table 1 Etiologies of acute pancreatitis
Hypercalcemia
Elevated calcium levels have also been linked to AP. The mechanism behind It stems from an exposure to high concentrations of calcium leading to toxicity, disruption of intracellular signaling, and cell damage[45]. In addition, AP has been reported in 1.5%of patients with hyperparathyroidism which is thought to be due to hypercalcemia[46].
Pancreatic anatomical abnormalities
Anatomical abnormalities of the pancreas includin g annular pancreas and pancreatic ductal stricture are accepted rare causes of AP as well as recurrent AP. However the role of pancreas divisum remains as an etiology of AP is controversial[9,47]. Pancreas divisum is a common variant seen in up to 14% of patients[48-50]. The clinical implications remain controversial and currently there is no consensus if pancreatic divisum alone can cause AP[9,47,51].
Vascular
Pancreatic ischemia secondary to rheumatological disease, ischemia secondary to shock, and atheromatous embolization have also been reported as rare causes of AP[52-54]. AP has been reported in numerous rheumatic diseases including systemic lupus erythematosus, Sögren's syndrome, scleroderma, and rheumatoid arthritis[52].AP has also been reported as a rare but potential event within 48 h of transabdominal angiographic procedures secondary to atheromatous embolization[53].
Pregnancy
AP has been rarely reported in pregnancy. In these cases five of them were found to be due to gallstones while the other three were reported as idiopathic causes[55].
Malignancies
AP can present as a manifestation of underlying malignancy. Specifically in intraductal papillary mucinous neoplasms (IPMNs), AP has been reported to be a presenting symptom with AP occurring in up to 21% of patients diagnosed with IPMNs[56].
Autoimmune pancreatitis (AIP)
AIP is a rarely identified disorder that is presumed to be autoimmune in etiology associated with IgG4 cholangitis, salivary gland disorders, mediastinal fibrosis, and inflammatory bowel disease. AIP should be considered in patients presenting with AP, particularly those with previously diagnosed autoimmune disorders[57,58]. AIP is classified into two types - Type 1 and 2. Type 1 AIP is part of a systemic IgG4 positive disease meeting the HISORt criteria proposed by the Mayo Clinic[59]. The HISORt criteria includes the presence of one or more of the following: diagnostic histology,characteristic imagining on computed tomography (CT) scan, elevated serum IgG-4 levels, other organ involvement, and/or response of symptoms to glucocorticoid therapy[59]. Type 2 AIP or idiopathic duct-centric pancreatitis is defined by granulocytic lesions in the absence of IgG-4-positive cells and systemic involvement[58].
Idiopathic
Idiopathic or unidentifiable causes of pancreatitis have been reported in about 10%-40% of all AP cases[9,60]. Idiopathic pancreatitis is often due to microlithiasis which is not picked up on routine abdominal imaging.
DIAGNOSIS
The revised Atlanta classification for AP helps to standardize the diagnosis of AP. The classification system defined pancreatitis as the presence of any two of the following three criteria being present in the patient: Abdominal pain consistent with that of AP,serum amylase and/or lipase levels greater than three times the upper limit of normal, and characteristics findings of AP seen in cross-sectional abdominal imaging[10,13,14,61-64].
Presentation
The diagnosis of AP begins early on in a patient's course and should be suspected in patients presenting with clinical symptoms and features consistent with AP -epigastric abdominal pain, nausea, vomiting, abdominal pain radiating to the back(seen in 40%-70% of patients)[10,13,14,61,64]. This pain can last several hours to several.Nausea has also been seen in about 90% of patients with AP which can last for several days as well[64].
Physical exam
Pancreatitis is an inflammatory condition of the pancreas extending to local and distant extra-pancreatic tissues[65]. Exam findings associated with AP vary greatly based on the severity of AP. Patients with mild disease may present with little tenderness to palpation throughout the abdomen, while patients with severe disease may present with severe abdominal pain to palpation and absence of bowel sounds[10,13,14].
Cullen's and Turner signs are seen in about 3% of patient's and are associated with a mortality of about 37%. These signs are many times associated with hemorrhagic pancreatitis, however neither sign is not specific to hemorrhage[66].
Laboratory tests
The evaluation of pancreatic enzymes (Lipase and Amylase) released from inflamed tissue is the cornerstone of biochemical diagnosis of AP[10,13,14,62,63,65,67]. The Atlanta criteria identified a serum amylase and/or serum lipase greater than three times the upper limit of normal as a contributory factor to the diagnosis of pancreatitis[10,13,14,62,63,67]. Although there is no optimal diagnostic test for pancreatitis, lipase is preferred over amylase in routine clinical practice[10].
Tyrpsinogen activation peptide is cleaved from trypsinogen to produce active trypsin and can also be seen in AP[68]. This can be measured in both the urine and serum. These tests are not readily available and hence not routinely used in clinical practice.
All patients with AP should get a complete blood cell count, basic metabolic panel,liver function tests (LFTs), coagulation profile, C-reactive protein (CRP), and total albumin as part of their initial laboratory work-up. An arterial blood gas should be performed in patients with hypoxia[10,13,14].
Imaging
Contrast enhanced CT scan of the abdomen and magnetic resonance imaging (MRI) of the abdomen is the best imaging modalities for visualization of pancreatic pathology.Although these tests are not routinely indicated in patients with mild AP. The classic feature seen in AP is the presence of focal or diffuse enhancement of the pancreas[10,13,14,69].
CT scan of the abdomen is used to both diagnose pancreatitis and many times helps establish scales of severity[69]. A non-contrast CT scan helps to establish the extent of pancreatic and extra-pancreatic inflammation[10,13,14]. A contrast enhanced CT scan of the abdomen is the gold standard for the establishment of severity of pancreatitis[10,13,14]. The CT severity index is based on a combination of peri-pancreatic inflammation, phlegmon, and degree of pancreatic necrosis seen on initial CT scan study and was developed to grade the severity of pancreatitis and establish the correlated mortality[10,13,14,70]. A high CT severity index correlated with a 92% morbidity and 17% mortality rate, while a low CT severity index correlated with a 2% morbidity and 0% mortality rate[70]. An early CT scan of the abdomen on admission has not been shown to affect the disease course as the CT scoring system for severity of AP is similar to that of clinical scoring system. Thus, a CT scan of the abdomen on admission solely to assess severity is not recommended[10,13,14,71]. It is recommended that an ultrasound of the abdomen be obtained on all patients with AP to assess the presence of biliary tract obstruction from gallstones[10,13,14].
An MRI of the abdomen is indicated in patients who have elevated LFTs with suspected common bile duct disease which cannot be visualized on ultrasound.Otherwise, an MRI is not indicated for the diagnosis of pancreatitis[10,13,14,71].
ASSESSMENT OF SEVERITY
About 15%-20% of patient with AP will develop severe disease and will have an elongated hospital stay with likely complications including possible death[10,13,14]. Thus,the determination of the severity of AP is one of the most important first steps in the management of AP. It helps in selecting appropriate treatments, ensuring proper patient triage, initiation of applicable therapies, and stratifying patient risk for complications. This is important because of the possibly of death related to severe AP.Mortality rates with AP in tertiary care centers alone is reported to be between 4.8%-9%, and when considering severe forms of the disease the mortality rates increased to 13.5%[72-74]. Several tools and scoring systems have been developed to assess the severity of AP. These scoring systems have been summarized in Table 2. We have reviewed the most commonly used scoring systems below:
Acute Physiology and Chronic Health Examination (APACHE) II Score
The APACHE II score was originally developed for patients in the intensive care unit(ICU) and utilizes 12 variables in order to help calculate a score that can be used upon admission, 24 h, and 48 h. This allows the advantage of the score being recalculated throughout the patient's stay allowing for appropriate adjustments and interventions.Each of the 12 variables are translated into weights using the original APACHE score and help to stratify a patient's risks[75].
In comparative studies the APACHE II score was the most accurate score in predicating the severity of the disease and the outcome of the disease. After 48 h, the APACHE II score predicted the outcome in 88% of cases and outperformed both the Ranson's and Imrie scores[10,76]. The APACHE II score has many limitations that make its use cumbrous. The complexity and difficulty to use, inability to distinguish between interstitial and necrotizing pancreatitis, and poor predictive value at 24 h are just a few of the limitations of the APACHE II score[64].
Bedside Index of Severity in Acute Pancreatitis (BISAP) Score
This score was developed in 2008 to be a mortality based prognostic tool for physicians to use within the first 24 h of admission[77]. The scoring system takes into account 5 variables: Blood urea nitrogen (BUN) > 25 mg/dL, impaired mental status, SIRS, age greater than 60, or the presence of a pleural effusion. Mortality was shown to be greater than 20% in the highest risk group or a score of 5 and less than 1% in the lowest group or score of 0[77]. The prognostic value of the BISAP score was found to be similar to those of other scoring systems such as the Ranson's, APACHE II, and computed tomography severity index (CTSI) in determining pancreatic necrosis and mortality[78]. The BISAP score is easy to use and authors recommend that all patients with AP should have BISAP score calculated to assess the severity of the disease.
Glasgow criteria
This scale is also known as the Imrie score and includes 8 of the variables used in the Ranson's Criteria. This scale has been used in gallstone induced AP. This scale must be determined after 48 h and uses SI units making its use difficult in the United States[10,13,14,79].
Ranson's criteria
The Ranson's criteria were one of the earliest criteria developed for assessing the severity of AP. The score takes into account 11 variables: 5 of which are measured at admission while 6 of these are measured 48 h after admission[10,79]. The limitation to this scoring system is that the criteria must be taken promptly at the correct time, and results cannot be determined until 48 h after admission. Many times the criteria are not completely measured during the hospital stay[10]. The mortality rises with increasing scores. Mortality was reported to be 0%-3% in patients with a score less than 3, 11%-15% in a score greater than or equal to 3, and 40% when the score was greater than or equal to 6[64]. The Ranson's criteria can be cumbersome to use in routine clinical practice at times.
Computed Tomography Severity Index
The CT severity index is based on a combination of peri-pancreatic inflammation,phlegmon, and degree of pancreatic necrosis seen on initial CT scan of the abdomenwithin one week of AP. This study was developed to grade the severity of pancreatitis(Balthazar score) and establish a correlated mortality rate[10,13,14,70]. The presence of necrosis on CT scan of the abdomen was a predictor of worse outcome[77,79]. In prior studies, a high CT severity index correlated with a 92% morbidity and 17% mortality while a low CT severity index correlated with a 2% morbidity and 0% mortality[70].

Table 2 Scoring systems for assessing severity of acute pancreatitis
Atlanta Criteria for Severity and Revised Atlanta Classification
The Atlanta criteria was developed in 1992 and helped identify severity of AP based on organ failure, local complications, and unfavorable prognostic signs[10,63]. The criteria helped define specific definitions of organ failure such as shock, pulmonary insufficiency, renal failure, and gastrointestinal bleeding. The Atlanta criteria also helped define pancreatic complications which were the development of pseudocyst,abscess, or parenchymal necrosis[10,63]. The Atlanta Criteria was then revised in 2012 in an attempt to further classify the severity of AP. The revised Atlanta Classification divides AP into interstitial edematous or necrotizing pancreatitis, distinguish early and late phase pancreatitis, and emphasizes the importance of SIRS and multiorgan failure[62]. Furthermore, severe AP is defined by persistent organ failure lasting greater than 48 h[62].
Laboratory findings
There are a wide range laboratory markers that have been identified as being elevated in AP including interleukin-B, interleukin-6, CRP, procalcitonin, antithrombin III,substance P. However, only two of these markers have been used regularly including C-reactive protein and hematocrit[10]. Seventh day CRP concentration was shown to have similar accuracy to both Ranson's and Glasgow criteria. This is a quick and simple test that can be performed to help aid in assessing severity[80]. AP results in third spacing increasing the hematocrit, which has been shown as an early marker for organ failure and necrotizing pancreatitis[81].
In conclusion, no one system or criteria exists to precisely predict prognosis of AP on admission. However, the use of clinical judgment, appropriate laboratory values,BISAP and the APACHE II scoring systems serve as an important guide for the triage,management and prognostication in patients with AP. CT scan of the abdomen after 72 h can help provide additional information about the severity of disease[10,13,14,71].Both the American College of Gastroenterology and International Association of Pancreatology/American Pancreatic Associations suggest the following as predictors of disease severity: advanced age, any comorbid disease, body mass Index greater than 30, presence of pleural effusion, rising hematocrit, hematocrit > 44, BUN > 20,elevated creatinine, SIRS score > 1, and persistent organ failure[13,82].
MANAGEMENT
The cornerstones in the management of AP include aggressive early intravenous hydration, appropriate nutrition, necessary interventions, and pain management.Below we review the current and most up to date treatment options in AP.
Initial assessment and triage
A crucial early step in the management of patients with AP is the initial assessment and triage to the appropriate hospital setting. This should be addressed early within the hospitalization to allow for appropriate management. Recent guidelines from the AGA recommend that patients with organ failure and/or severe inflammatory response syndrome (SIRS) should be admitted directly to the ICU[13]. The BISAP scoring system in particular is very useful with initial assessment of AP patients with a score of greater than or equal to three being an appropriate score to identify a patient with a high risk of mortality and hence should undergo evaluation for ICU admission[83]. However, we recommend that patients should be assessed on a case by case basis.
Fluid resuscitation
The current guidelines regarding fluid resuscitation in the management of AP are evolving. Despite these changes, there continues to be consensus in the importance and need for aggressive early fluid resuscitation[10,13,14,84]. Early goal directed fluid resuscitation has been shown to reduce mortality in patients with severe sepsis[85].However, the administration of excessive fluid has been shown to have worse outcomes after 24 h[86]. We suggest that the need of aggressive fluid resuscitation should be evaluated after 6 and 24 h of admission and rate of fluids should be adjusted based on changes in mean arterial pressure, urine output, changes in BUN and respiratory status. Two recent articles showed the importance of aggressive intravenous fluids in hastening clinical improvement in patients with AP, as well large volume fluid resuscitation in severe AP within the first 24 h is associated with decreased mortality[87,88].
The choice of fluids for patients with AP has been a topic of great debate in recent years. Prior guidelines suggested that Ringer's Lactate was superior to Normal Saline and should be used as the initial fluid therapy in patients with AP, with one randomized trial showing Ringer's Lactate reduced the incidence of systemic inflammation in comparison to Normal Saline[10,89]. However, the more recent guidelines from the AGA suggest that Normal Saline and Ringer's Lactate are equally efficacious in the management of AP. This is based on poor quality of evidence behind prior studies and not focusing on important clinical outcome such as organ failure,pancreatic necrosis or mortality[84,90]. The guidelines do state against the use of hydroxyethyl starch (HES) fluids, since the literature has showed no differences in mortality when comparing fluids with and without HES[84,91,92].
Nutrition
The paradigm of nutrition in AP has shifted to early initiation of nutritional supplementation as compared to the conventional nil per oral strategies used in the past.The AGA now recommends initiating early oral feedings (within 24 h) in patients with mild AP. There was no type of diet that was specified in these recommendations,but it is thought that beginning early feedings helps to protect the gut-mucosal barrier and reduce bacterial translocation, which in return will reduce the risk of worse outcomes associated with AP. These findings were based on the results of 11 randomized control trials which examined earlyvsdelayed feeding. Although enteral tube feeding was started within 24 h in some of these studies, no study reported the initiation of oral feeding within 24 h. Thus, whether the 24-h time-frame is appropriate for the oral intake of food is still unclear and will need to be studied further[10,13,14,84,93,94].
In patients who are unable to eat, enteral feeding should be considered early through nasogastric/nasojejunal routes as opposed to total parenteral nutrition[10,13,64,82,84]. There has been no difference in outcomes when compared with nasogastricvsnasojejunual feeds.
Pain management
Pain management remains essential in the management of AP. Uncontrolled pain can lead to hemodynamic instability leading to worse outcomes. Opioids remain the first line choice of pain medication in AP. Recent studies showed no differences in the risk of complications related to pancreatitis or adverse events when comparing different opioids and routes of administration[95].
Role of antibiotics
There is no role for prophylactic antibiotics in patients with AP. Recent studies have shown no association between the initiation antibiotic therapy in AP and severe outcomes such as organ failure, necrosis or mortality[13,84,96].
Antibiotics do however play a large role in patients with infected pancreatitis.Infected necrosis should considered in patient's failing to improve after one week.This should be assessed promptly with acquisition of a CT scan guidance fine-needle aspiration for gram stain or presence of gas on CT scan[13,82,97]. In these patients,empiric treatment should be effective against common pathogens including:Escherichia coli, Bacteroides species, Enterobacter species, Klebsiella species,Streptococcus faecalis, Staphylococcus epidermidis and Staphylococcus aureus[14,97].Appropriate antibiotic choices include carbapenems, quinolones, and metronidazole which are all known to penetrate pancreatic necrosis and target these bacteria. The routine use of antifungals is not recommended in these patients[13]. Antibiotics should be initiated early in patients who have infected pancreatitis and may help prevent the need for surgical necrosectomy. Delaying intervention may result in poor outcomes for these patients[98-100].
Endoscopy
Endoscopic intervention is indicated in patients with AP who have concurrent cholangitis or biliary obstruction. In a small subgroup of patients, persistent choledocholithiasis can become obstructive and can lead to pancreatic/biliary tree obstruction. This will eventually lead to severe AP that can be complicated with cholangitis[13]. Guidelines recommend patients who have cholangitis should undergo ERCP within twenty four hours of admission[10,13,14]. Prior reports have shown that patients undergoing ERCP within 24 hvspatients with conservative management had fewer complications[101]. In addition, patients with AP complicated with cholangitis or biliary sepsis who receive early ERCP have been shown to have lower morbidity and mortality rates[102].
However, timing of ERCP in patients with biliary pancreatitis continued to be controversial. Recent studies have shown that urgent ERCP in patient's having acute biliary pancreatitis without cholangitis had no impact on clinical outcomes such as mortality, pancreatitic infections, and organ failure[84,96].
Surgery
Indications for surgical intervention include the presence of gallstones in the gallbladder or biliary tree, infected necrosis preferably for more than 4 wk after antibiotics if stable, and necrosectomy in symptomatic patients[13,84].
All patients with mild AP related to gallstones should undergo cholecystectomy during the same admission prior to discharge. Early surgical intervention in biliary pancreatitis drastically reduces mortality and gallstones related complications[103]. In addition, patients with moderately severe and severe AP should undergo an interval cholecystectomy after discharge[104]. Overall, cholecystectomy in patients with gallstone related pancreatitis have been shown to drastically reduce the incidence of recurrent AP[9].
Patients who are asymptomatic with findings of pseudocysts and/or necrosis of the pancreas or extrapancreatic tissue do not require surgical intervention[105]. While historically the treatment for pancreatic necrosis was surgical intervention, most recent guidelines point away from immediate surgical intervention[10,13,14]. Current guidelines recommend postponing necrosectomy for four weeks in patients who are stable[10,13,14]. This delay in surgery was shown to be associated with a decreased mortality from 39% to 12% in patients with severe AP[106]. However, in symptomatic patients with infected necrosis, necrosectomy is still recommended with minimally invasive methods such as endoscopic necrosectomy as compared to surgery[10,13,14].
Alcohol cessation
All patients admitted with AP should undergo counselling for alcohol cessation[84]. A single randomized controlled trial showed that alcohol cessation counseling at the time of AP leads to decreased incidence of recurrent AP over a 2-year period[107]. We suggest that all patients admitted with AP should be provided with resources to assist with cessation of alcohol use on discharge from the hospital.
COMPLICATIONS
Local complications
The most common complications following AP include acute peri-pancreatic fluid collection, pancreatic pseudocyst, acute necrotic collections, and walled off necrosis[10,13,14,62]. The complications of AP have been outlined in Table 3.
Interstitial edematous pancreatitis:Interstitial edematous pancreatitis is an acute inflammation of the pancreatic parenchyma and peri-pancreatic tissues. However,this does not have any signs of recognizable tissue necrosis. On contrast enhanced CT scan, enhancement of the pancreatic parenchyma with no signs of necrosis isseen[10,13,14,62].
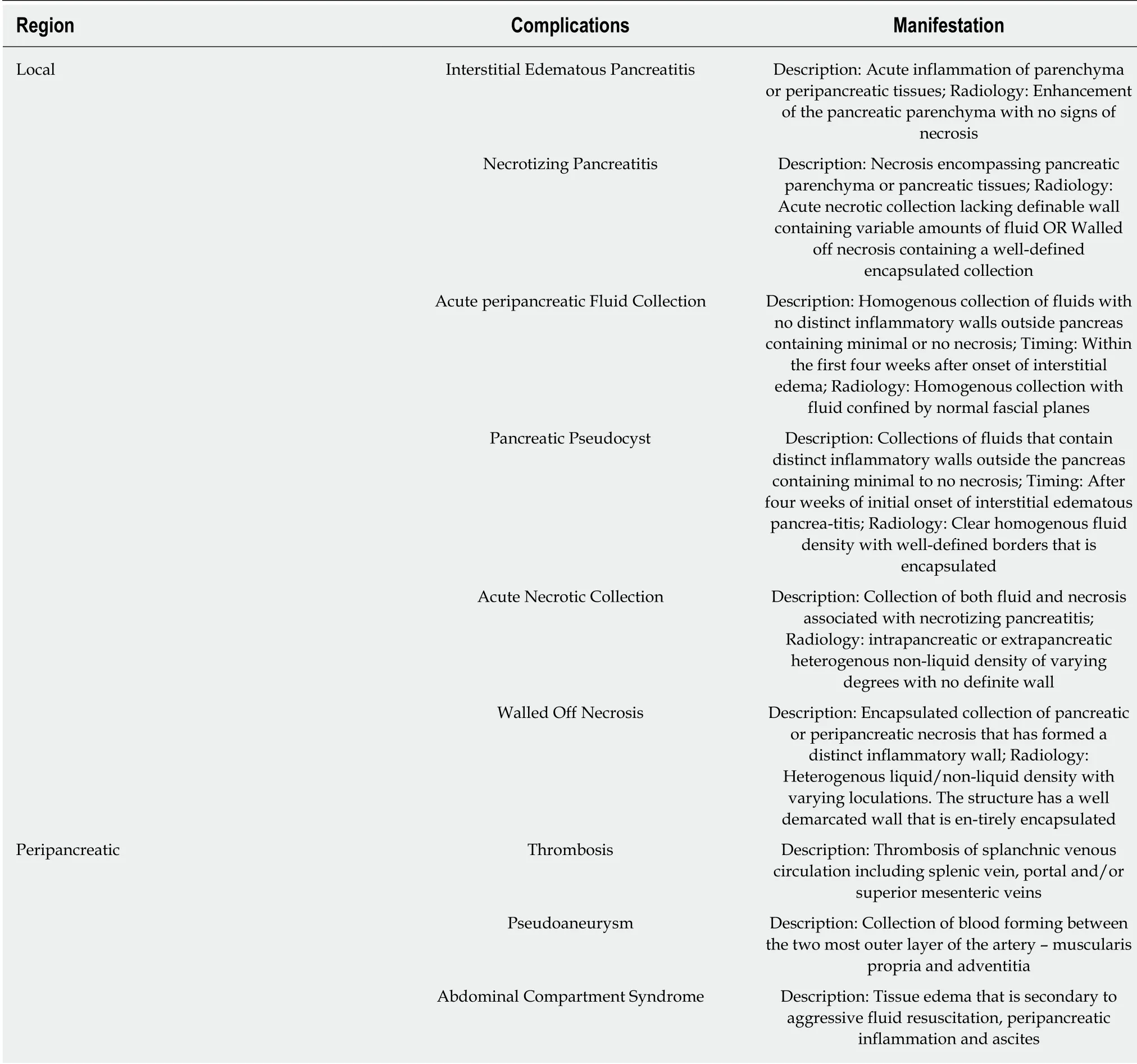
Table 3 Complications of acute pancreatitis
Necrotizing pancreatitis:Necrotizing pancreatitis commonly manifests as necrosis encompassing pancreatic parenchymal and/or peripancreatic tissues. On imaging,these findings manifest either as an acute necrotic collection lacking a definable wall containing variable amounts of fluid, or walled off necrosis containing a well-defined encapsulated collection of pancreatic parenchymal and/or peripancreatic tissues.These findings are initially sterile and may eventually become infected[10,13,14,62].
Acute peripancreatic fluid collection (APFC):APFC are homogenous collections of fluids with no distinct inflammatory walls outside the pancreas containing minimal to no necrosis. APFC often occurs within the first four weeks after the initial onset of interstitial edematous pancrea-titis. On contrast enhanced CT scan, APFC is visualized as a homogenous collection with fluid that is confined by normal fascial planes adjacent to the pancreas[10,13,14,62].
Pancreatic pseudocyst:Pancreatic pseudocysts are collections of fluids that contain distinct inflammatory walls outside the pancreas containing minimal to no necrosis.This often occurs four weeks after the initial onset of interstitial edematous pancreatitis. Contrast enhanced CT scan criteria include a clear homogenous fluid density with well-defined borders that is encapsulated[10,13,14,62].
Acute necrotic collection:An acute necrotic collection is a collection of both fluid and necrosis associated with necrotizing pancreatitis. This involves either the pancreatic and/or peripancreatic tissue. Contrast enhanced CT scan shows an intra-pancreatic or extra-pancreatic heterogenous non-liquid density of varying degrees with no definite wall[10,13,14,62].
Walled-off necrosis:Walled off necrosis is defined as an encapsulated collection of pancreatic or peri-pancreatic necrosis that has formed a distinct inflammatory wall.This occurs greater than four weeks after the initial onset of necrotizing pancreatitis.Contrast enhanced CT scan of the abdomen shows a heterogenous liquid/non-liquid density with varying loculations. The structure has a well demarcated wall that is entirely encapsulated[10,13,14,62].
Hemorrhagic pancreatitis:Although rare, hemorrhagic complications can be seen and are considered late sequelae of AP. Hemorrhage may develop secondary to ruptured or leaking pseudoaneurysms, bleeding associated in pancreatic necrosis, and hemorrhagic pseudocysts. Early detection of this complication is important and surgical embolization or intervention has been shown to decrease mortality[108].
Peripancreatic complications
Peripancreatic complications encompass a number of complications. An uncommon complication of AP includes thrombosis of the splanchnic venous circulation. This predominantly occurs in the splenic vein but can occur in the portal and/or superior mesenteric veins. This manifestation is seen in up to 24% of patients with AP[109]. This can also lead to development of gastric varices leading to gastrointestinal bleeding.
Another rare but serious complication that may occur in AP includes a pseudoaneurysm. This should be suspected when patients develop sudden gastrointestinal bleeding, drop in hemoglobin and worsening abdominal pain[62,82]. CT scan can often show signs of hemorrhagic pancreatitis. These patients benefit from angioembolization which is often performed by interventional radiology and surgical intervention is reserved as the last resort.
Patients with AP are also at increased risk for abdominal compartment syndrome secondary to tissue edema from aggressive fluid resuscitation, peripancreatic inflammation, and ascites[110].
Systemic complications
Any patient with AP is at an increased risk for exacerbation of underlying conditions including cardiac, lung, hepatic, and nephrogenic disease. These complications should be treated as they arise[10,14,62,82]. We suggest that patients with AP who develop serious systemic complications should be managed in the ICU with the assistance of other colleagues including pulmonologists, cardiologists and nephrologist.
CONCLUSION
AP continues to be a common reason for hospitalization. The most common etiologies include gallstones, followed by alcohol. It has significant impact on healthcare resource utilization, morbidity and mortality. Disease can vary from mild disease to severe disease with systemic complications. The cornerstones to management include aggressive early fluid resuscitation, appropriate nutritional supplementation and management of complications. Patients with mild acute gallstone related pancreatitis should undergo cholecystectomy prior to discharge to prevent recurrent episodes.Severe AP and pancreatitis with local and systemic complications should be managed in a multidisciplinary approach with involvement of internists, gastroenterologists,hepatobiliary surgeons and interventional radiologists.
 World Journal of Clinical Cases2019年9期
World Journal of Clinical Cases2019年9期
- World Journal of Clinical Cases的其它文章
- Coexistence of breakpoint cluster region-Abelson1 rearrangement and Janus kinase 2 V617F mutation in chronic myeloid leukemia: A case report
- Crizotinib-induced acute fatal liver failure in an Asian ALK-positive lung adenocarcinoma patient with liver metastasis: A case report
- Rare variant of pancreaticobiliary maljunction associated with pancreas divisum in a child diagnosed and treated by endoscopic retrograde cholangiopancreatography: A case report
- Adult-onset mitochondrial encephalopathy in association with the MT-ND3 T10158C mutation exhibits unique characteristics: A case report
- Nerve coblation for treatment of trigeminal neuralgia: A case report
- Management of the late effects of disconnected pancreatic duct syndrome: A case report
