Hydrate Process Analysis Focusing on Energy Requirement
Jerome Rajnauth
[a]Department of Reservoir Management, Heritage Petroleum Company Limited. Santa Flora. Trinidad and Tobago.
Abstract Transporting gas in the form of a gas hydrate can prove to be very useful in the supply chain of natural gas to meet future energy demand. There are major challenges that exist in effectively capturing, storing, transporting and utilizing form of energy. The details of the work flow in this paper evaluates the entire hydrate process with particular focus on energy balance using hydrate technology to capture and transport five (5) MMscf/d from Trinidad to Jamaica. The overall energy requirement of the process which involves heating, cooling and expansion is in the range 14-20% of the energy of the gas transported in hydrate form.
Key words: Energy requirement; Hydrate; Gas hydrate
INTRODUCTION
Gas hydrate may be a useful means of capturing,storing and transporting stranded and associated gas.This work proposes a workflow for capturing, storing and transporting gas in the hydrate form, particularly for Caribbean situations where there are infrastructural constraints such as lack of pipelines. The study analyzes the gas hydrate value chain for transportation of 5 MMscf/d of natural gas from Trinidad to Jamaica using a particular natural gas sample.
ENERGY BALANCE FOR THE HYDRATE PROCESS
The hydrate process energy requirements are shown Figure 1 for a particular natural gas sample producing 5MMscf/d. The heating value of the gas before and after hydrate formation is shown. This is particularly important since the gas is sold based on the heating value. Gas from the well is passed through an expander to lower the temperature and pressure suitable for hydrate formation.
The power developed by the expansion process is 4.8 x 105Btu/h. After the expansion the natural gas is pumped into the hydrate vessel with 4220 bbls of cold water. The cooling requirement for water is also shown in the figure. The formation of hydrate results in large amount of heat of formation that must be removed during the process.
The natural gas composition may have heavier components (C5or higher), as in the case of this gas sample. In the hydrate formation process, only C1to C4are captured, and the higher components (C5and higher)separate in the process as natural gas liquids (NGL). In this case 3830 bbls of NGL is separated out with a heating value of 4441 Btu/scf and is useful energy.
The same amount of energy removed during the formation of the hydrate is same energy requirement for dissociation.
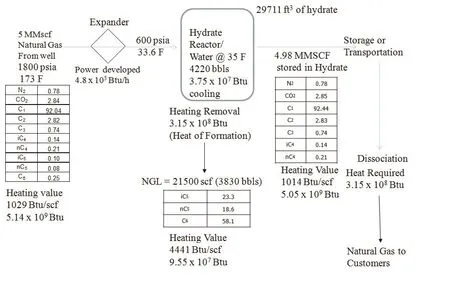
Figure 1 Hydrate process showing energy balance
Heating Value
The heating value of the gas sample is estimated to be 1029 Btu/scf and this is shown below in Table 1 For 5 MMscf of gas, the heating value translates to 5.14 x 109Btu.
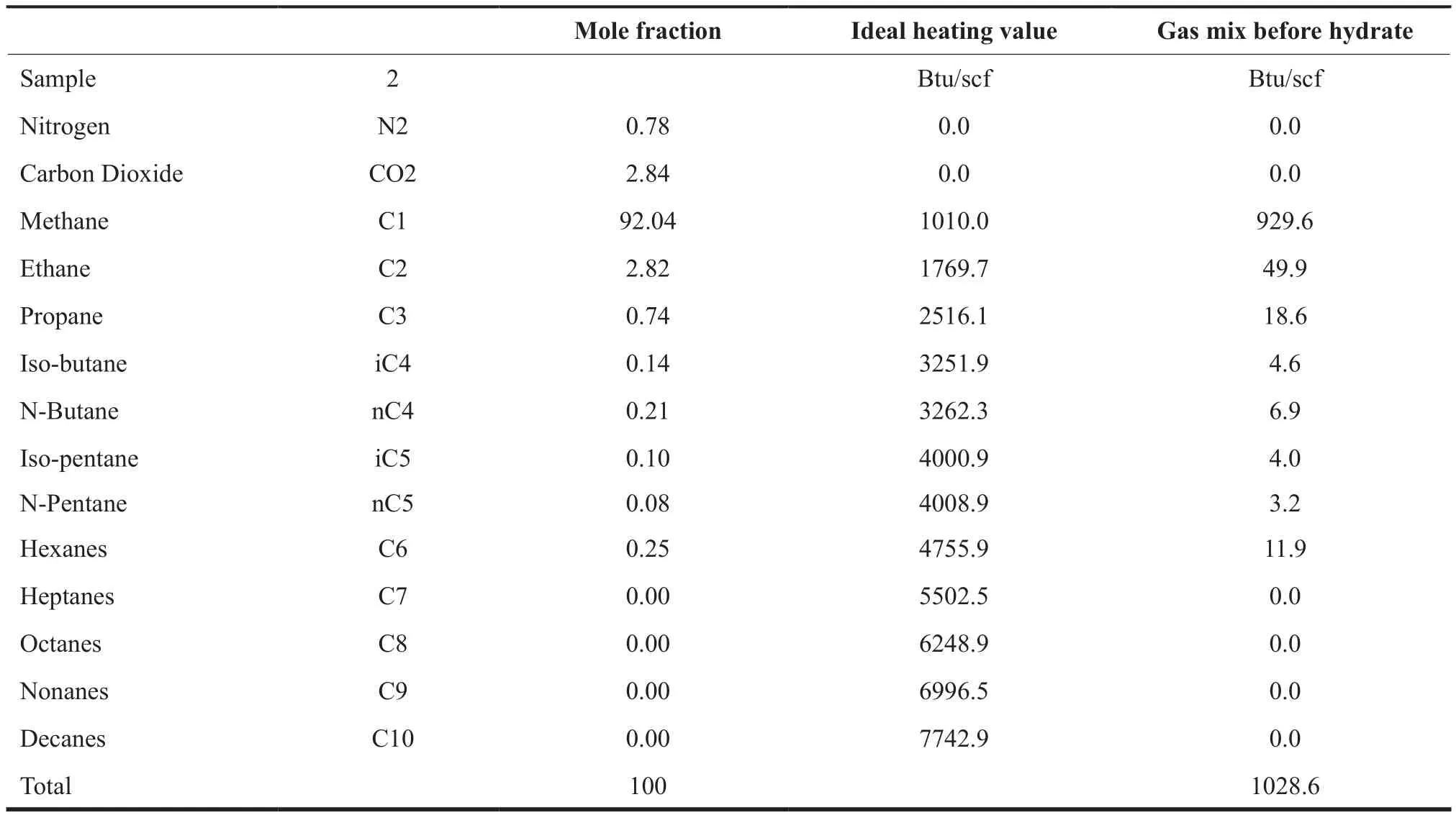
Table 1 Heating Value Estimation for a Gas Sample before Hydrate
Table 2 shows the heating value of the gas stored in the hydrate is estimated at 1014 Btu/scf with 29711 ft3of hydrate being formed.
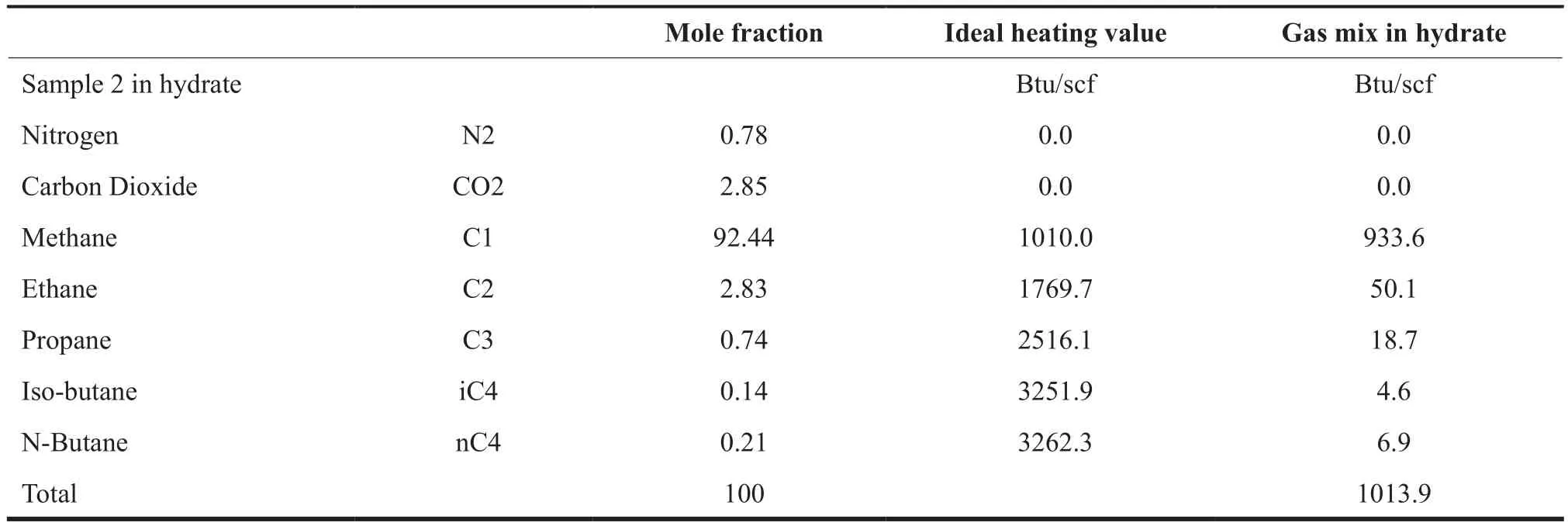
Table 2 Heating Value Estimation for a Gas Sample in Hydrate
The natural gas liquids may separate during the hydrate process for sample 2 and provides useful energy.The heating value of the NGL is estimated at 4441 Btu/scf. This is shown in Table 3.
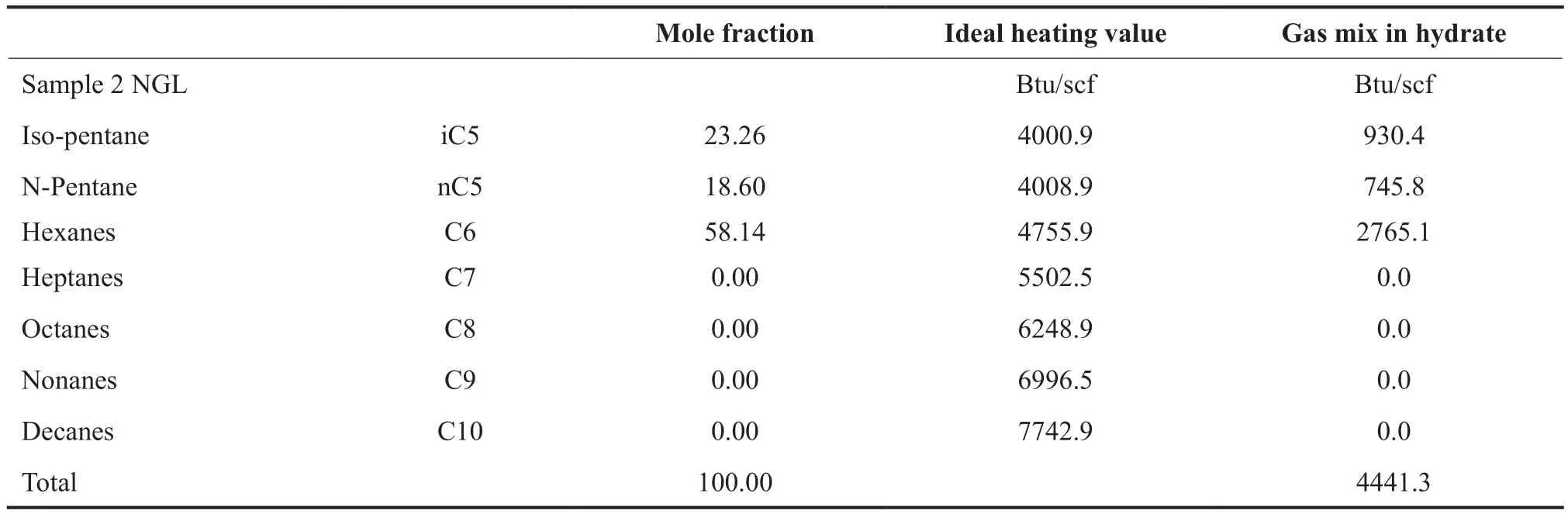
Table 3 Heating Value Estimation for Natural Gas Liquid (NGL)
Expansion
The expansion process must also ensure the gas sample remains in the single phase region of the phase diagram,which is important in the design process. From the wellhead, the gas flows through a turbo-expander, which causes the gas temperature to drop to 35 F, and the pressure to drop to 600 psia, assuming an efficiency of 85% or 90% depending on the sample.
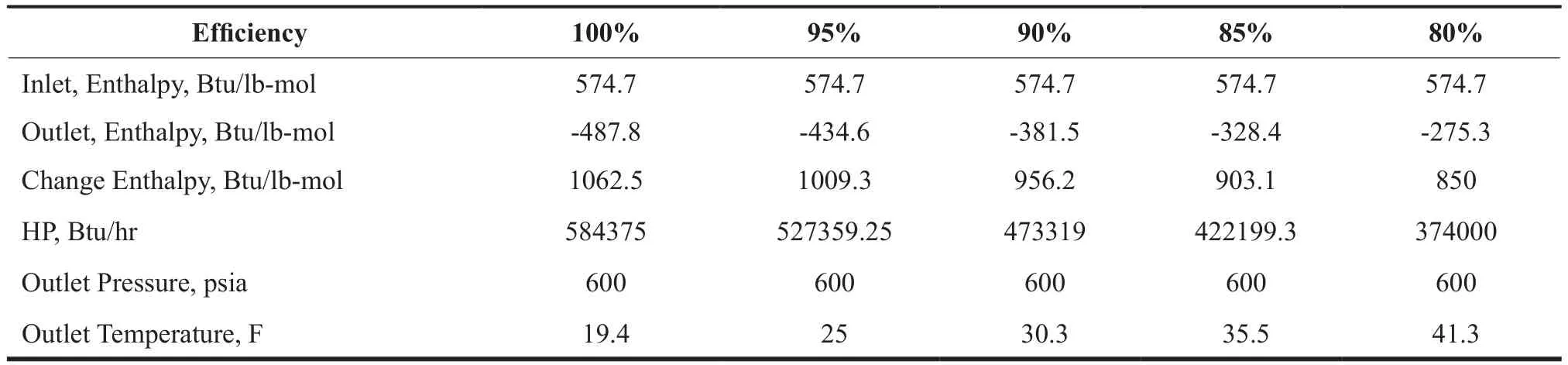
Table 4 Horse Power Generated and Outlet Temperature for Various Expansion Efficiencies
The power developed by the expander and the outlet temperatures are shown in Table 4. The data in the table were obtained from the commercial simulator. An efficiency of at least 85% is required to obtain an outlet temperature of 35 F, required for hydrate formation. This generates 1.01 x 107Btu of energy. Below is a sample calculation of power from the expander:
Power=∆h×w×ηe
where:
∆h= change in enthalpy, btu/lbmole,
w= flow rate, lbmole/hr
ηe= expander efficiency, %
For an 85% efficiency,h∆= 903.1 btu/lbmole and w= 550 lbmole/hr

Some commercial expanders can have up to 90%expansion efficiency (Turbo Expander, 2010).
Hydrate Formation
Water Cooling Requirements
The cooling requirement of water is estimated as follows.
The water enthalpies before and after the cooling are obtained NIST chemistry webBook (Themophysical,2008).
The sensible heat of cooling is estimated from
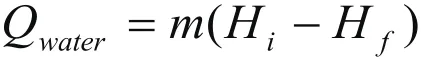
where
Qsensible heat of cooling
mno of moles of gas mix
Hiinitial enthalpy of water
Hffinal enthalpy of water
Sample calculation
For 5 MMscf/d gas mix, 79037.9 moles of water is required for hydrate formation
Water cooling from 60 F to 35 F is to be carried out.
m= 83046
Hi= 506.9 Btu/lbmole
Hf= 86.46 Btu/ lbmole
Q= 83046 (506.9 – 54.91)
Q= 37535961 Btu/d
Q= 3.75 x 107Btu/d
Latent Heat of Formation Estimation
During the formation of the hydrate large amount of heat is given out which must be removed to ensure the hydrate formed do not dissociate. The latent heat of formation is estimated based on the mole fraction of the gas as a weighting factor of the heat of formation of the gas components (Makogon, 1981) shown in Table 5.

Table 5 Estimation of Latent Heat of Formation
The latent heat of formation of the gas sample is 55.51 kJ/mol gas (63 Btu/scf).
For 5 MMscf/d gas of sample, the latent heat is calculated as follows.
Latent heat of formation of the hydrate
= Btu/scf gas x 5 MMscf/d
= 63 x 5,000,000
= 3.15 x 108Btu/d
Heat Gain through Insulation
NanoPore thermal insulation provides exceptional performance with a very low overall thermal conductivity of 0.004 btu.in/ft2.h.F (Nanopore, 2010). About 240 Btu/h of heat gained from the surroundings must be removed when using 1” thickness NanoPore material, compared to 4.35 x 107Btu/hr without any insulation.
Summary of Cooling Requirement (Hydrate Formation)
Table 6 summarizes the cooling requirement for formation of hydrate for 5 MMscf/d. The largest energy requirement is removal of the latent heat of formation which is approximately 3.15 x 108Btu/d. Heat gain through insulation is at about 2880 Btu/d. Overall the total cooling requirement for hydrate formation for 5 MMscf/d is 3.53 x 108Btu.
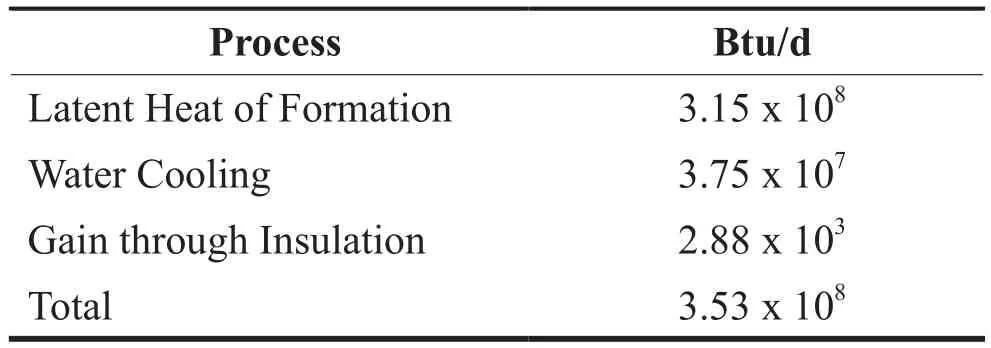
Table 6 Summary of Energy Requirement for Hydrate Formation
Hydrate Dissociation
For hydrate dissociation, it estimated that the same amount of latent heat removed during the formation process is required. In this case, 3.15 x 108Btu/d is required for dissociation of gas hydrate storing 5 Mmscf/D.
SUMMARY
The total energy requirement including hydrate formation and dissociation is 6.68 x 108Btu/d. The energy content of 5 MMscf/d gas is 50.7 x 108Btu/d. The overall energy requirement for the process is about 14% of the energy value of the gas transported in hydrate form depending on the composition of the gas. The value ranges from 14–20% depending on the natural gas sample.
CONCLUSIONS
Commercial expander can have an efficiency of up to 90%. Therefore the selected expander efficiency must allow the expansion of the gas to the required hydrate formation conditions. The power developed by the expander is of the magnitude 4.2 x 105Btu/h.
The cooling requirement for the hydrate formation(5MMscf/d) was estimated to be 3.5 x 108Btu/d. About 89% of this requirement is from the latent heat of formation.
For dissociation of the hydrate, 3.15 x 108Btu/d is required; this represents the same amount of latent heat removed during hydrate formation.
The overall energy requirement for the process using a selected natural gas sample is about 14% of the energy value of the gas transported in hydrate form.
 Advances in Petroleum Exploration and Development2019年1期
Advances in Petroleum Exploration and Development2019年1期
- Advances in Petroleum Exploration and Development的其它文章
- The Natural Gas Composition is Key in Hydrate Formation
- Performance of Combined Process of Air Flotation- Sedimentation- Biological Contact Oxidation - Membrane Biological Reactor Treating Heavy Oil Wastewater
- Analysis of Wastewater Membrane Pollutants in Joint Station and Research on Biological Control Technology
- Water Content of Sweet Natural Gas: A Simplified Formula-Based Approach
- Study and Application of Diagnosis Curves of Water Channeling Patterns for Horizontal Well in Bottom-Water Heavy Oil Reservoir
- Performance Evaluation of a Biomaterial in an Aqueous-Based Drilling Mud at High Pressure High Temperature
