The Natural Gas Composition is Key in Hydrate Formation
Jerome Rajnauth
[a]Society of Petroleum Engineer Member, Heritage Petroleum Company LTD., San Fernando, Trinidad and Tobago.
Abstract This analysis will evaluate the effects of the natural gas composition on the formation of hydrate for the purpose of storage and transporting natural gas.Results show that the composition of the natural gas can affect the temperature and pressure required for formation of the hydrate. Carbon dioxide, hydrogen sulfide and nitrogen impurities in natural gas affect the hydrate formation and may result in additional processing of the gas is required hydrate formation.
The composition of the sample also affects the water to gas mole ratio and hence the amount of water required for hydrate formation.
Key words: Gas; Composition; Hydrate formation
INTRODUCTION
Gas hydrate may be a viable means of capturing, storing and transporting stranded, flared and associated gas. The ability of natural gas to form hydrate in combination with water is a very interesting and useful concept which could be widely utilized in the industry.
The composition of the natural gas from the well is very important in the hydrate formation process as it affects the gas hydrate value chain. Gas from the well is first expanded to achieve the condition required for hydrate formation. The composition of the gas affects the expansion process since the resulting fluid may be in single phase or two phases. For hydrate formation the gas must remain in the single phase after expansion otherwise additional gas processing is required.
The composition of the gas captured in hydrate form would determine the heating value of the natural gas transported. This is important since natural gas is sold to meet a heating value standard. The particular natural gas composition of the sample must be evaluated for the hydrate formation process because it may not meet heating value requirement, remain in the single gas phase after expansion and require additional separation for samples with large amounts of impurities.
Technological advancement in utilizing gas hydrate as means of transporting natural gas could be a key component in capturing stranded, associated gas and in some cases unconventional gas. There is still about 40%of natural gas stranded reserves to be monetized (Fleisch,2006).
The composition of gas is very important and such proper evaluation must be done to ensure the gas sample is adequate for transportation via gas hydrate. The natural gas must remain in the gaseous phase after expansion prior to hydrate formation. Most of the previous studies focused on simple gases such as methane and ethane.Most natural gas has much more components than just methane and ethane and hence the composition can have significant impact on hydrate formation. In this analysis,twenty one natural gas compositions of typical wells are analyzed to convert and transport 5 MMscf/d of gas in hydrate state to neighboring islands (e.g.). The natural gas streams obtained from these wells were, generally, sweet gases (without H2S) and ranges in composition from C1to C10. These samples are shown in Table 1.
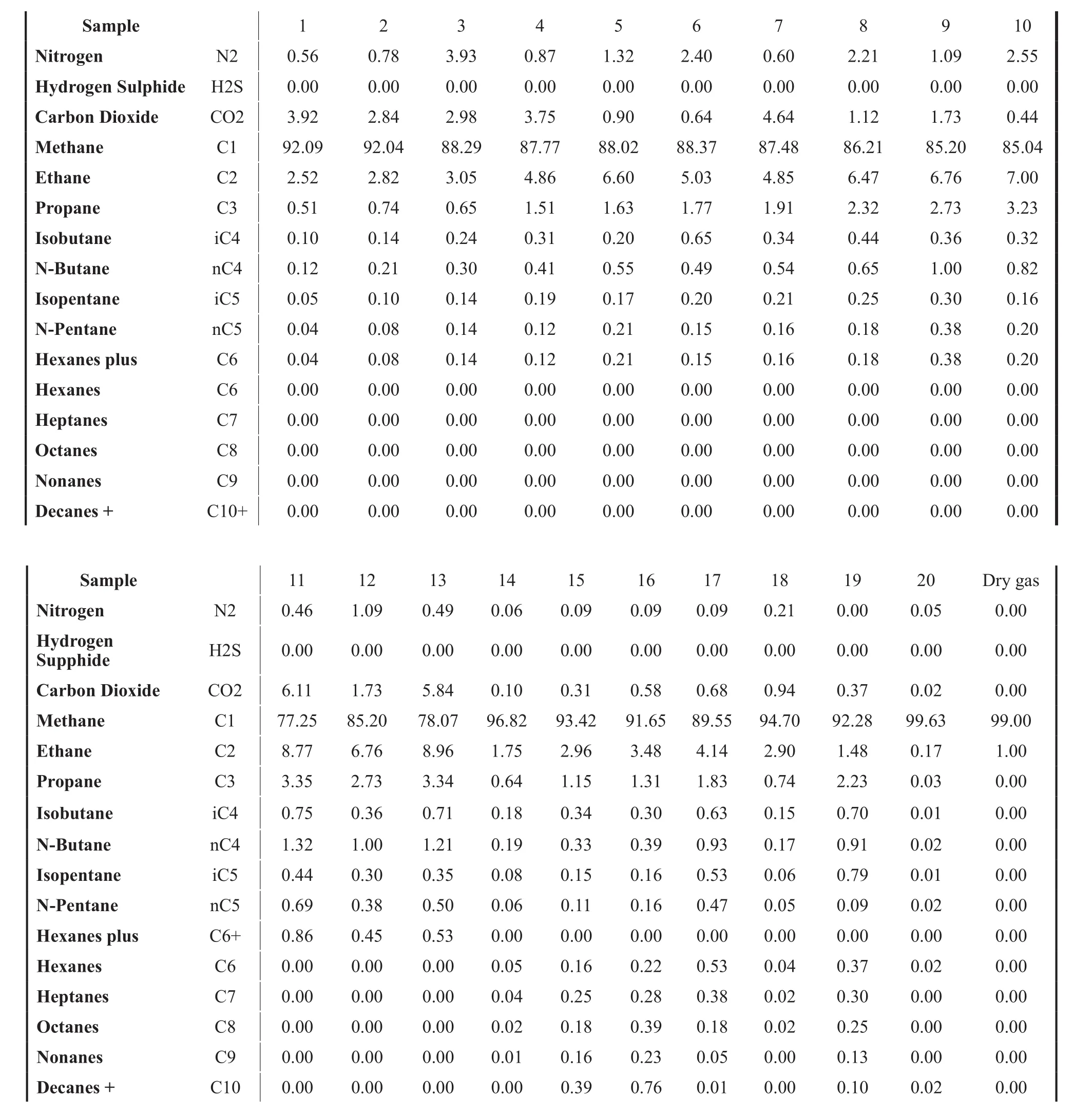
Table 1 Natural Gas Composition from Trinidad/Mole %
Effect on Natural Gas Hydrate Forming Conditions
The hydrate forming temperature/pressure for the twenty one natural gas streams given in Table 1 is plotted in Figure 1. This graph was developed on the basis of a large number of data points generated by computer using the PVTSim program (PVTSim, 18). Figure 1 shows that the hydrate forming temperature/pressure relationship for natural gases is greatly influenced by the composition of the gas mixtures.

Figure 1 Hydrate forming temperature/pressure
The uppermost curve corresponds to the natural gas obtained from sample Dry Gas while the lowermost curve corresponds to the natural gas obtained from the Sample 11. At a fixed pressure, i.e. 600 psi sample 11 will form hydrates if the temperature is lower than T2, while sample dry gas will form hydrate if T is lower than T1.
Effect of Composition on Hydrate Formation Pressure
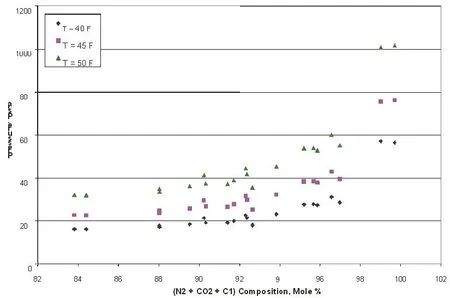
Figure 2 Hydrate formation pressure for different compositions (light components) from the 21 samples
Figure 2 shows the effect of the light components (N2 +CO2 + C1) on hydrate formation pressures at temperatures of 40 F, 45 F and 50 F. The points, in Fig. 2 correspond to the natural gas streams shown above. It is observed that as the (N2 + CO2 + C1) composition varied from 84 mol%to 99 mol%, the hydrate formation pressure increases from 161.5 psi to 572.1 psi at 40 °F. Similar trends can be seen at the temperatures 45 F and 50 F.
The influence of the heavier is opposite to that observed in Figure 2 i.e., varying (C2 + C3 + iC4 + nC4+ iC5 + nC5 +C6+) concentration from 0.3 mol% to 16.2 mol%, the hydrate formation pressure decreases from 565.2 psi to 161.4 psi approximately, at 40 F.
ESTIMATION OF MOLE RATIO MOLE REQUIRED FOR ALL SAMPLES
As indicated earlier, the formation conditions of 600 psia and 35 F were then chosen as it represents potential upscaling conditions deduced from experimental studies conducted by Okutani et al, 2007. The water to gas mole ratios for all samples was estimated for conditions of 600 psia and 35 F using the commercial simulator (PVTSim,18). This was done by changing the mole ratio at this specific temperature and pressure until 100 % volume of hydrate is formed. The results are shown in Table 22 below. If excess water is used in the formation of the hydrate (e.g 7:1 mole ratio), a hydrate phase and aqueous phase is obtained.
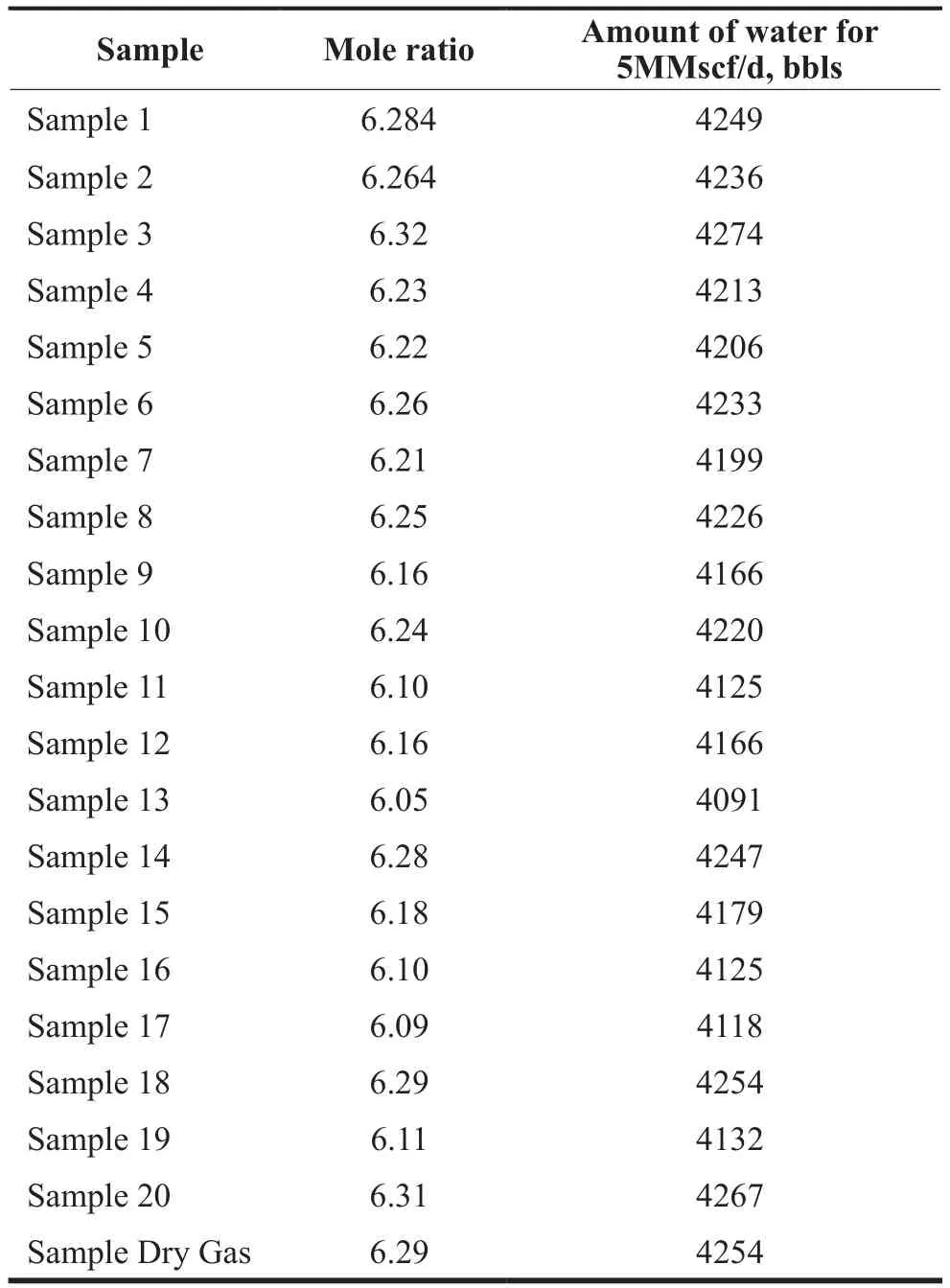
Table 2Actual Water to Gas Mole Ratio for all Samples (600 psia and 35 F)
The results show the range of mole ratio from 6.1:1 to 6.32:1 above the 6:1 mole. For 5 MMscf/d, this represent an additional 68–218 bbls of water required when considering all samples. This is an additional 1.6–5.1%change in water needed. Commercial flow meters are capable of handling such this % change. It is important to note that while the mole ratios do not change substantially,the actual water needs will change greatly with the amount of MMscf/d of gas processed (Rajnauth et al, 2012). The amount of water required for a gas rate of 5 MMscf/d for sample with a mole ratio of 6.29:1 is evaluated as follows.
This was calculated as follows:
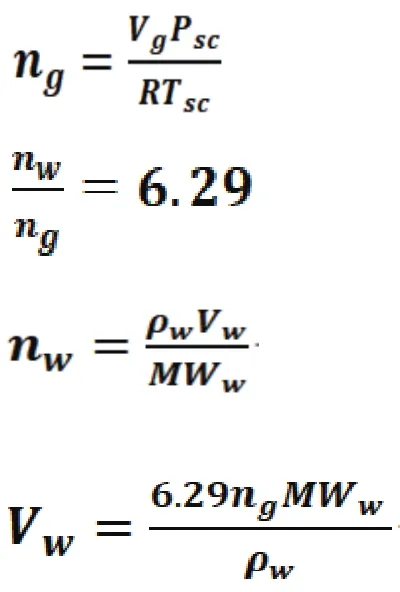
where
ngmoles of gas
Vgvolume of gas, 0.5 MMscf
Pscstandard pressure, 14.71 psi
Rgas constant, 10.73 ft3psiR–1lbmol–1
Tstandard temperature, 520 R
nwmoles of water
MWwmolecular weight water, 18
pdensity of water, 62.48 lbm/ft3
Vwvolume of water, bbls
For 5MMscf/d
For capturing 5MMscf of dry gas sample, 4250 bbls of water is required. The amount of water required for all samples are also shown in Table 2
ESTIMATION OF HEATING VALUES OF GAS SAMPLES
The heating values of all samples were estimated:
• To use in energy balance calculations for entire process since it gives an indication of the amount of energy transported in the gas.
• To determine gas acceptance at markets around the world or whether further gas processing required. This is important since gas is sold based on its heating value.
The heating values were estimated simply by multiplying the ideal heating values of the individual component by the mole fraction which gives a simply approximation.
The computed heating value of the gas Sample 2 was 1029 Btu/scf before the hydrate formation, and 1014 Btu/scf after. This is because the hydrate formation separates the heavier components (> C5) as useful natural gas liquid. 0.02 MMscf (3829 bbls) of natural gas liquids are obtained with a heating value of 4441 Btu/scf while 4.98 MMscf natural gas (C1to C4) is stored in hydrate form.The heating values of the samples are shown in Tables 3,4 and 5 below. These include Sample 2 before and after hydrate formation, and the natural gas liquids separated during hydrate formation.
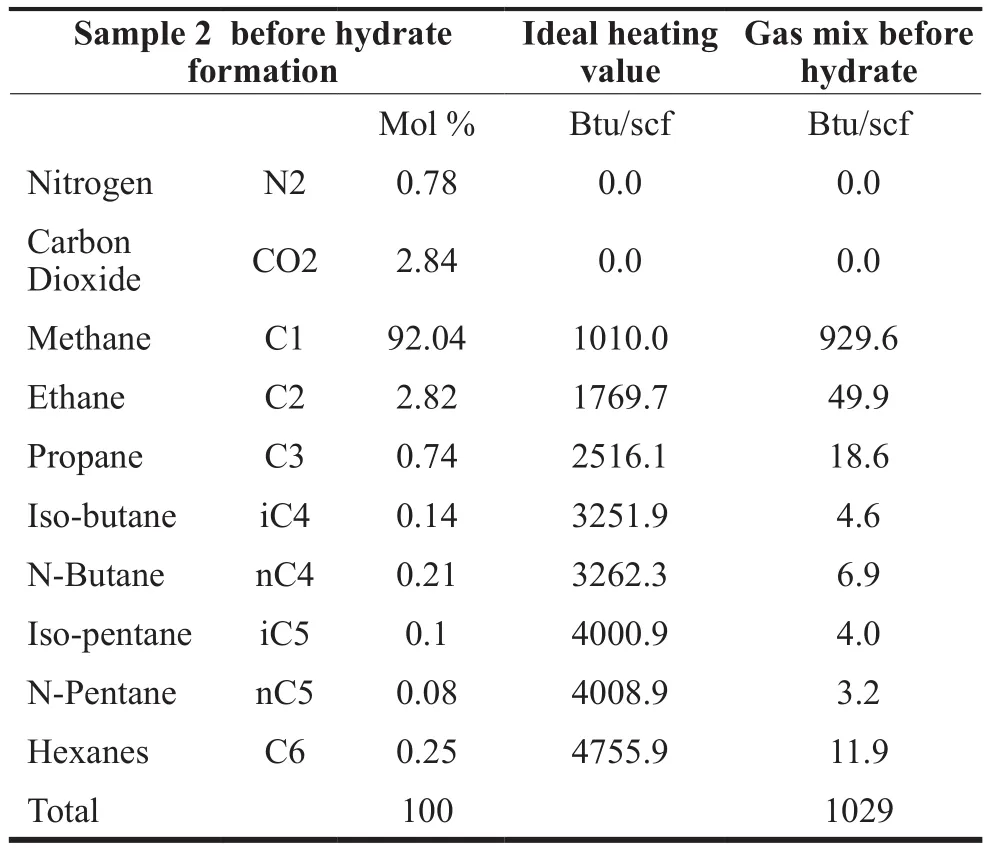
Table 3 Estimation of Heating Value of Sample 2 Before Hydrate Formation

Table 4 Estimation of Heating Value of Sample 2 After Hydrate Formation
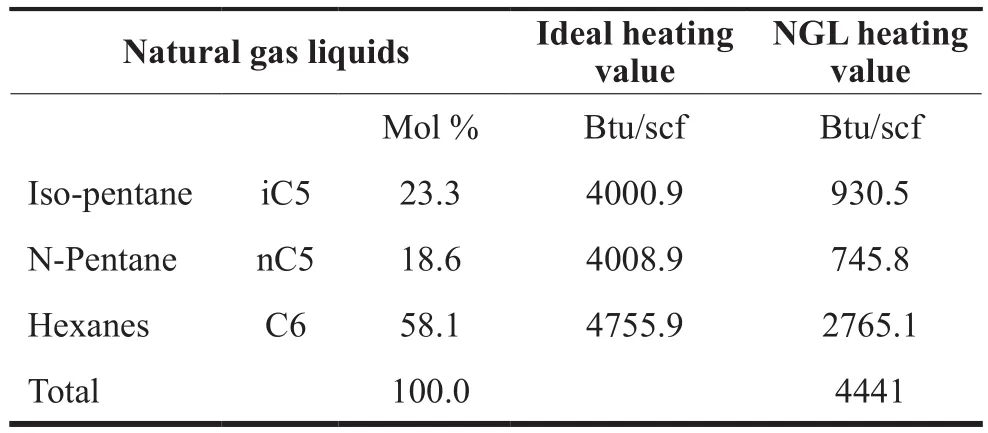
Table 5 Estimation of Heating Value of Natural Gas Liquids for Sample 2
The heating value estimates for all samples are shown in Table 6. The heating values before hydrate formation,the heating values of gas captured in hydrate form and the heating values of the natural gas liquids separate during the formation process are all highlighted in the table.
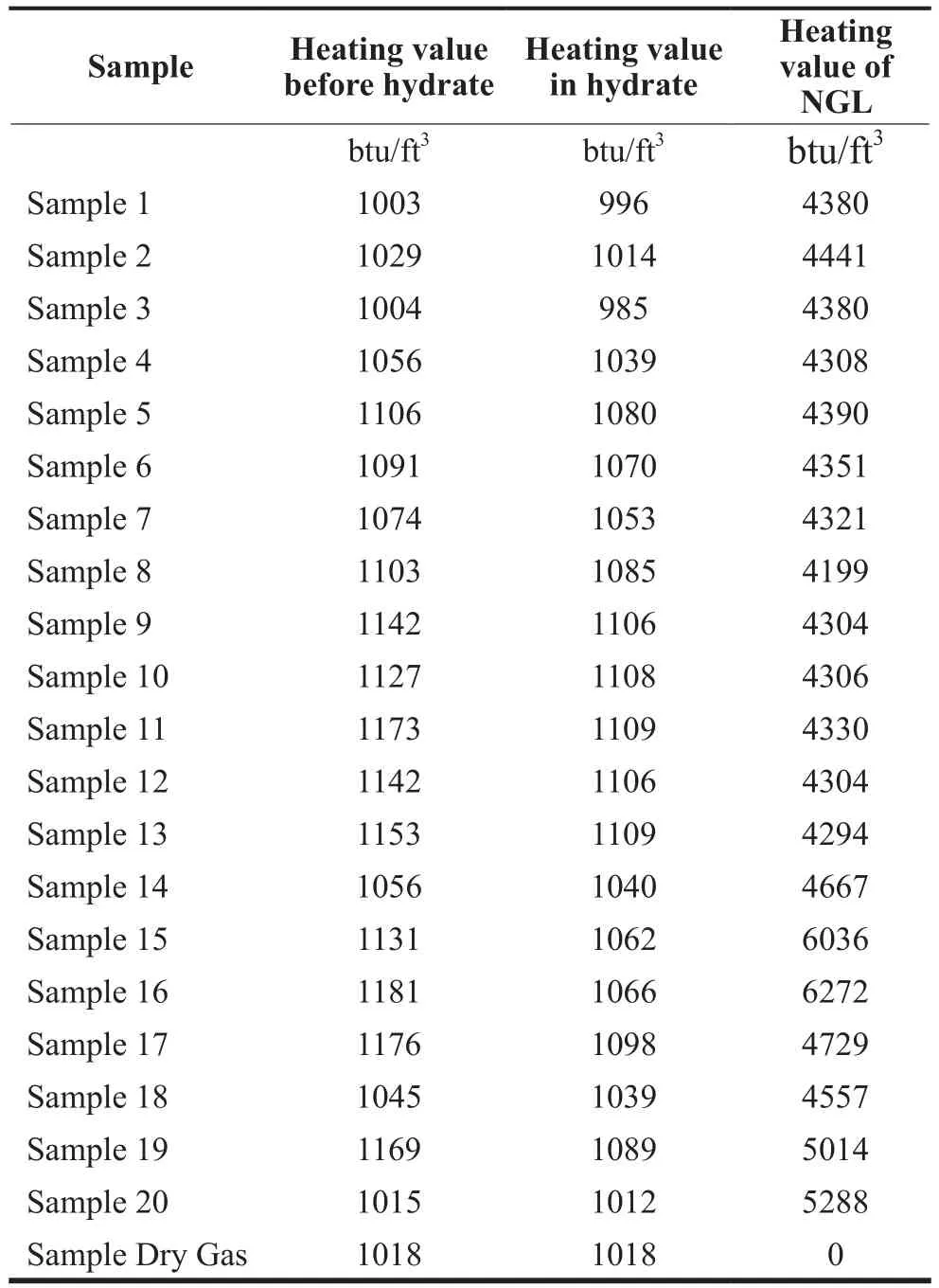
Table 6 Estimation of Heating Values Before and After Hydrate Formation and for Natural Gas Liquids for All Samples
Gas may be transported to different regions around the world and therefore would be required to meet that region’s heating value standard. The accepted heating value range accepted in the US is in the range 966–1120 Btu/scf. For Europe, the range is 940–1204 Btu/scf and for Japan 1065–1160 Btu/scf is required.
EXPANSION OF GAS SAMPLES TO HYDRATE FORMATION CONDITIONS
The expansion process must also ensure the gas sample remains in the single-phase region of the phase diagram,which is important in the design process (Rajnauth et al,2013). From the wellhead, the gas flows through a turboexpander, which causes the gas temperature to drop to 35 F, and the pressure to drop to 600 psia, assuming an efficiency of 85% or 90% depending on the sample.
Figure 1 shows the phase diagram for sample 2 with wellhead and outlet conditions, and with varying expansion efficiencies. Note that at 100% efficiency, the gas is very close to the two phase region.
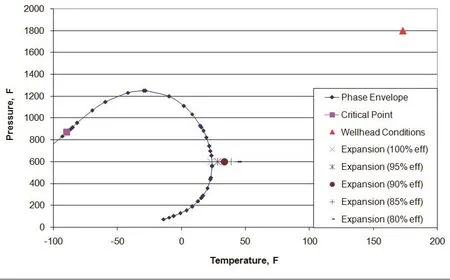
Figure 1 Phase diagram for sample 2
The expansion process for sample 2 remains in the single phase region of the phase diagram, which is important in the design process. However as seen in Figure 4 the expansion process for sample 9 goes into the two phase region of the phase diagram and therefore not suitable for natural gas hydrate formation unless further separation is carried out.

Figure 4 Phase diagram for sample 2
DISCUSSION
The composition of the sample must be evaluated for hydrate formation and transportation and should (a)Ensure heating value is within an acceptable range of 960-1120 Btu/scf. (b) Ascertain if the sample is appropriate for gas hydrate process since the sample must remain in the gaseous phase after expansion. If the sample goes into the two-phase region after expansion additional separation is required and this was not analyzed in this study.
The exact amount of water for a particular volume of gas can be determined. The composition of the sample affects the mole ratio and hence the amount of water needed.
Higher components of the natural gas such as pentane and higher are not captured in the hydrate and separated out as natural gas liquids (NGLs). This can provide additional fuel for many processes.
CONCLUSIONS
The composition of gas is very important and such proper evaluation must be done to ensure the gas sample is adequate for transportation via gas hydrate. The natural gas must remain in the gaseous phase after expansion prior to hydrate formation.
Impurities affect the hydrate PT equilibrium. Carbon dioxide increases the PT equilibrium line while nitrogen reduces it. At a fixed temperature, the higher the %methane in the sample the higher the hydrate formation pressure while the higher the propane levels the lower the formation pressure.
The mole ratio (hydrate number) is approximately 6; however, the actual hydrate number depends on the composition, temperature and pressure. It ranges from 6.1:1 to 6.32:1for the samples evaluated.
The expansion process ensured that sample 2 remain in the single-phase region of the phase diagram, while Sample 9 goes into the two-phase region and therefore require additional separation before expansion. This is important in the design process.
As shown in the analysis some natural gas sample:
• May not meet heating value requirement around the world
• May not remain in the single gas phase after expansion
• May require additional separation for samples with large amounts of impurities
 Advances in Petroleum Exploration and Development2019年1期
Advances in Petroleum Exploration and Development2019年1期
- Advances in Petroleum Exploration and Development的其它文章
- Performance of Combined Process of Air Flotation- Sedimentation- Biological Contact Oxidation - Membrane Biological Reactor Treating Heavy Oil Wastewater
- Analysis of Wastewater Membrane Pollutants in Joint Station and Research on Biological Control Technology
- Water Content of Sweet Natural Gas: A Simplified Formula-Based Approach
- Study and Application of Diagnosis Curves of Water Channeling Patterns for Horizontal Well in Bottom-Water Heavy Oil Reservoir
- Performance Evaluation of a Biomaterial in an Aqueous-Based Drilling Mud at High Pressure High Temperature
- Research on Sesmic Attribute Reservoir Prediction Method: Taking the Shang Block 2 of Sanzhao Sag as an Example
