Can microcaIcifications’ characteristics predict the risk of breast cancer metastasis to bone?
Rita BonfigIio, Manuel Scimeca, Alessandro Polidori, Clara Nazzaro, Giselda De Silva, Elena Bonanno,5
1Department of Experimental Medicine, University of Rome “Tor Vergata”, Via Montpellier 1, Rome 00133, Italy.
2Department of Biomedicine and Prevention, University of Rome “Tor Vergata”, Via Montpellier 1, Rome 00133, Italy.
3San Raffaele University, Via di Val Cannuta 247, Rome 00166, Italy.
4Assing S.p.a, Via Edoardo Amaldi 14, Monterotondo 00015, Italy.
5Diagnostica Medica’ & “Villa dei Platani”, Neuromed Group, Avellino 83100, Italy.
Abstract Aim: To correlate the microcalcifications’ characteristics, such as morphology and elemental compositions, with the occurrence of bone metastatic lesions at 5 years from diagnosis.Methods: In this retrospective study, we enrolled 70 patients from which we collected one breast biopsy each. From each biopsy, paraffin serial sections were obtained to perform histological classifications, immunohistochemical analyses and Energy Dispersive X-ray evaluation.Results: Microcalcifications analysis showed a significant association between the presence of calcium crystals made of magnesium substituted hydroxyapatite and the development of bone metastasis from 5 years from diagnosis.No significant association was observed by evaluation the morphological appearance of microcalcifications.Immunohistochemical analysis displayed a significant association between the expression of bone morphogenetic proteins 2 and pentraxin-3, two osteoblast induction factors, and the formation of bone metastatic lesions.Conclusion: Results here reported highlighted the possible use of breast microcalcifications as a negative prognostic marker of bone metastatic diseases. In particular, the association between elemental composition of breast microcalcifications and the formation of bone lesions can lay the foundation for the development of new in vivo diagnostic tools based on the analysis of microcalcifications and capable to predict the formation of bone metastasis.
Keywords: Microcalcifications, breast cancer, bone metastasis, breast osteoblast-like cells, bone morphogenetic proteins 2, pentraxin-3
INTRODUCTION
Bone metastasis from breast cancer represent the main disability associated to breast cancer[1,2]. Indeed, the occurrence of bone metastatic lesions affected the patients’ quality of life by inducing pain, hypercalcemia,bone fracture and spinal compression[2]. Also, the progression of metastatic lesions is often the cause of the patient’s death[2].
They wandered forth11 to seek another country, but nowhere did they find a shelter, or a human being to give them a mouthful of bread, and their need was so great that they were forced to appease12 their hunger with nettles13
In this context, the early identification of breast cancer lesions with high propensity to form bone metastasis could improve the patient’s survival allowing to clinicians to choose the more appropriate therapeutical protocol.
In the last years, several studies investigated the cellular and molecular mechanisms involved in the breast cancer osteotropism[3-6]. In particular, our group described, for the first time, a new breast cancer cell type showing an osteoblast-like phenotype, the breast osteoblast-like cells (BOLCs)[7]. As the real osteoblasts, it is demonstrated that these cells are capable to product calcium crystals made of hydroxyapatite (HA) of magnesium substituted hydroxyapatite (MgHAp) in a process similar to the physiological mineralization[7].Several molecules have been associated to the process of the formation of microcalcifications in breast cancer. Among them, deserve particular mention the bone morphogenetic proteins 2 (BMP-2) and pentraxin-3 (PTX3). BMP-2 is currently considered the most powerful osteoblast induction factor[8], whereas it is recently demonstrated the ability of PTX3 to induce both osteoblast proliferation and activity[9,10]. In particular, studies of Scimecaet al.[7]highlighted the essential role of PTX3 in bone metabolisms founding a correlation between the impairment of PTX3 expression and the inhibition of osteoblast activity. In these reports authors suggested a direct role of PTX3 in the assembly of HA crystals. Surprisingly, the presence of BOLCs into breast cancer lesions is also associated to the development of bone metastatic lesions at five years from diagnosis. Therefore, the presence of microcalcifications made of HA or MgHAp in breast lesions could be considered as a negative prognostic factor for bone metastatic diseases as well as the BOLCs.
Starting from these considerations, the aim of this study was to correlate the microcalcifications’characteristics, such as morphology and elemental compositions, with the occurrence of bone metastatic lesions at 5 years from diagnosis.
METHODS
In this retrospective study, we enrolled 70 patients from which we collected one breast biopsy each. Our study protocol was approved by independent ethical committee. From each biopsy, paraffin serial sections were obtained to perform histological classifications, immunohistochemical analyses and energy dispersive X-ray (EDX) evaluation. Exclusion criteria were history of previously or concomitant other neoplastic diseases, autoimmune diseases, viral chronic infections (HBV, HCV, and HIV), and any antitumoral treatment received before biopsy.
Histology
The data used to support the findings of this study are included within the article. Further details can be made available upon request.
EDX microanalysis
The EDX microanalysis is a technology that performs the elemental and chemical analysis of a sample in a transmission electron microscope. When the electron beam in an electron microscope hits a thin sample,some atoms of the sample will be excited or ionized. When they return into their ground state, they will emit characteristic X-rays. The X-ray emission at different wavelengths may then be measured by a photonenergy-sensitive detector[12,13].
While the poor girl was falling she happily caught one of thebranches of the willow tree, by the help of which she held herselfover the water, and as soon as the baron with his company and the dogs had disappeared through the gate, the girl endeavoured to scramble9 up, but the branch broke off, and she would have fallen backward among the rushes, had not a strong hand from above seized her at this moment. It was the hand of a pedlar; he had witnessed what had happened from a short distance, and now hastened to assist her. Everything in the right place, he said, imitating the noblebaron, and pulling the little maid up to the dry ground. He wishedto put the branch back in the place it had been broken off, but itis not possible to put everything in the right place; therefore hestuck the branch into the soft ground.
All breast samples underwent ultrastructural microanalysis. Following to the identification of microcalcifications, six-micrometer-thick paraffin sections were embedded in Epon resin as previously described[12]. Briefly, sections were de-paraffinized, hydrated, osmium tetroxide-fixed, dehydrated in ethanol and propylene oxide and infiltrated in Epon. The embedding capsules were positioned over areas containing microcalcifications identified by Toluidine Blue staining previously. Unstained ultra-thin sections of approximately 100-nm-thick were mounted on copper grids for microanalysis. EDX spectra of microcalcifications were acquired with a Hitachi 7100FA transmission electron microscope (Hitachi,Schaumburg, IL, USA) and an EDX detector (Thermo Scientific, Waltham, MA, USA) at an acceleration voltage of 75 KeV and magnification of 12,000. Spectra were semi-quantitatively analyzed by the Noram System Six software (Thermo Scientific, Waltham, MA, USA) using the standardless Cliff-Lorimer k-factor method[12,13]. The EDX microanalysis apparatus was calibrated using an X-ray microanalysis standard(Micro-Analysis Consultants Ltd., Cambridgeshire, UK).
Immunohistochemistry
In conclusion, results here reported highlighted the possible use of breast microcalcifications as a negative prognostic marker of bone metastatic diseases. In particular, the association between elemental composition of breast microcalcifications and the formation of bone lesions can lay the foundation for the development of newin vivodiagnostic tools based on the analysis of microcalcifications and capable to predict the formation of bone metastasis. In this scenario, thein vivoanalysis of elemental composition of microcalcifications by RAMAN spectroscopy could improve the clinical armamentarium available for the diagnosis and stadiation of breast cancer.
Every trade was located in the basement of the houses or inthe side thoroughfares; and the sun shone with such heat, and theair was so close, that one seemed to be in an oven full of beetles,cockchafers, bees and flies, all humming and buzzing together
Statistical analysis
Separateχ2tests were used to assess the associations between morphological appearance and experimental groups and between elemental composition and experimental groups. Difference between the expression of biomarkers evaluated by immunohistochemistry were evaluated by Mann Whitney test.Immunohistochemical values were reported as mean value ± standard error.
RESULTS
Histology
In the last years, numerous studies highlighted the role of breast microcalcifications in the pathophysiogenesis of both breast cancer occurrence and progression[3-6,15,16]. In particular, we recently demonstrated the presence of breast cancer cells with an osteoblast phenotype (breast osteoblast-like cells-BOLCs) able to product microcalcifications made of HA or MgHAp[7]. Noteworthy, the breast cancer lesions characterized by the presence of BOLCs showed high propensity to form bone metastasis[7]. Thus, the presence of microcalcifications in breast lesions could represent a negative prognostic marker for metastatic diseases. Starting from these considerations, the aim of this study was to correlate the microcalcifications’characteristics, such as morphology and elemental compositions, with the occurrence of bone metastatic lesions at 5 years from diagnosis. To this end, we retrospectively collected breast cancer lesions of patients with (BM+) or without (BM-) clinical evidence of bone metastatic lesions. Our results clearly indicate that elemental composition, but not morphological appearance, of breast microcalcifications can predict the presence or development of bone metastatic lesions. In particular, according with the data reported by Bonfiglioet al.[15], in this study the breast cancer lesions of BM+ patients were frequently characterized by the presence calcifications made of MgHAp. Thus, the presence of MgHAp could play an active role in breast cancer progression. Indeed, as hypothesized in our previously study, the ability of HA to bind Mg can support the neoplastic progression by inhibiting the activity of DNA repair enzymes that require Mg as co-factor. These data further support the hypothesis that the bone metastatic process of breast cancer can be driven by BOLCs. Indeed, we also noted that breast cancer cells of BM+ patients acquired the capability to express two of the most important osteoblast induction factors such as BMP-2 and PTX3. BMP-2 is a member of TGF-β superfamily that which regulate numerous process in bone metabolisms[8]. Specifically,BMP-2 is involved in both mesenchymal stem cells recruitment and differentiation into mature osteoblasts[8].In a recent paper, Scimecaet al.[7]demonstrated the expression of BMP-2 by BOLCs. PTX3, also known as TNF-inducible gene 14 protein, is a molecule involved in several process of innate immunity[17,18]. It is also demonstrated that PTX3 have a role in the extracellular matrix formation as well as bone formation[9]. Inthis context, the expression of BMP-2 and PTX3 in BM+ lesions can provide a scientific rationale for BOLCs development and microcalcifications formation. Also, it is known that both BMP-2 and PTX3 are involved in the EMT phenomenon. Thus, it is possible to speculate that these molecules have role both in BOLCs generation that in the production of HA or MgHAp crystals by the BOLCs themselves.
NEAR the grass-covered rampart which encircles Copenhagen lies agreat red house. Balsams and other flowers greet us from the long rows of windows in the house, whose interior is sufficientlypoverty-stricken; and poor and old are the people who inhabit it.The building is the Warton Almshouse.
The EDX detector system performs a simultaneous display of all mid-energy (1-20 keV) X-rays collected during any individual analysis period. Therefore, it is possible to detect those elements with N.A. > 10.The minimal detectable elemental concentration, which requires some signal averaging, is approximately 0.1 mmol/kg of dry specimen (i.e., 10 ppm), whereas spatial resolution ranges from about 10 nm to a few micrometers[12,13].
EDX microanalysis
Analysis of the elemental composition of microcalcifications revealed the presence of two form of calcium crystals in our samples: HA, and MgHAp [Figure 1D]. Statistical analysis shows a significant distribution of these types of calcifications in BM+ and BM- [Figure 1D]. In particular, calcifications of BM+ group were 70% MgHAp and 30% HA, whereas in BM- group we noted 37.5% of MgHAp and 62.5% of HA [Figure 1D].
Immunohistochemistry
Made substantial contributions to conception and design of the study and performed data analysis and interpretation: Bonfiglio R, Scimeca M, Bonanno E
DISCUSSION
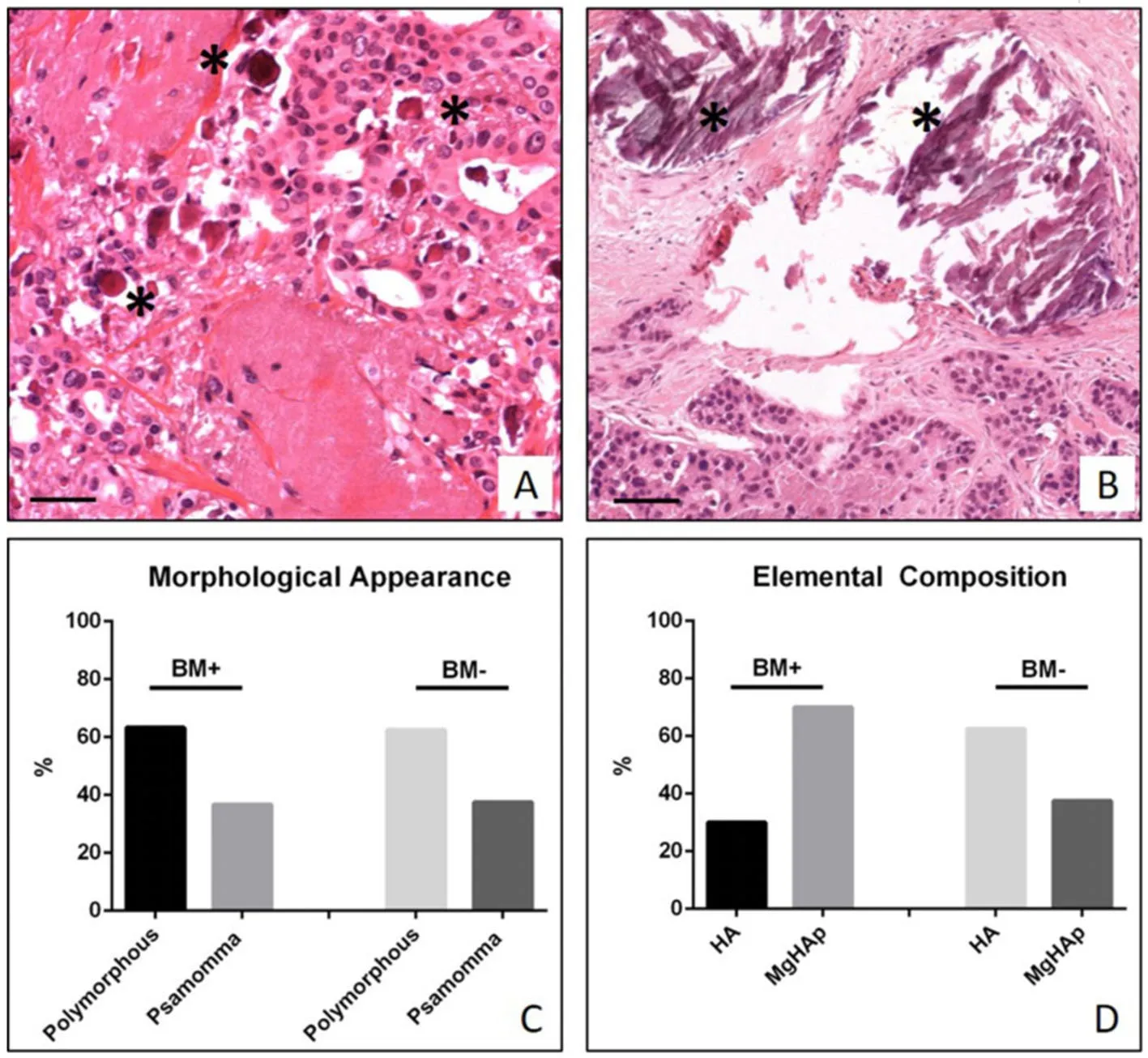
Figure 1. Study of breast microcalcifications. A: Representative image of ductal infiltrating breast carcinoma with several psammoma bodies (asterisks); B: image shows a ductal infiltrating breast carcinoma with polymorphous calcifications (asterisks). Scala bars represent 100 μm for all images; C: graph displays the percentage of psammoma bodies and polymorphous calcifications in BM+ and BM- patients; D: graph displays the percentage of hydroxyapatite (HA) and Magnesium substituted hydroxyapatite (MgHAp) in BM+and BM- patients
The study of H&E sections allowed us to classify breast biopsies in ductal infiltrating carcinomas according to Nottingham Histological system[14]. Specifically, we observed 15/70 G1 infiltrating carcinomas, 38/70 G2 infiltrating carcinomas and 17/70 G3 infiltrating carcinomas. Also, based on the presence of metastatic lesions at 5 years from diagnosis, biopsies collected in the study were classified as follow: 30 infiltrating carcinomas of patients with clinical evidence of bone metastasis (BM+) (59.65 ± 1.23 years) and 40 infiltrating carcinomas of patients without clinical evidence of bone metastasis (BM-) (57.91 ± 0.96 years).Microcalcifications were detectable in 63.3% of BM+ and in 62.5% of BM-. From morphological point of view, we observed 63.3% of psammomabodies and 36.7% of polymorphous calcifications in BM+ and 62.5%of psammomabodies and 37.5% of polymorphous calcifications in BM- [Figure 1A-C].
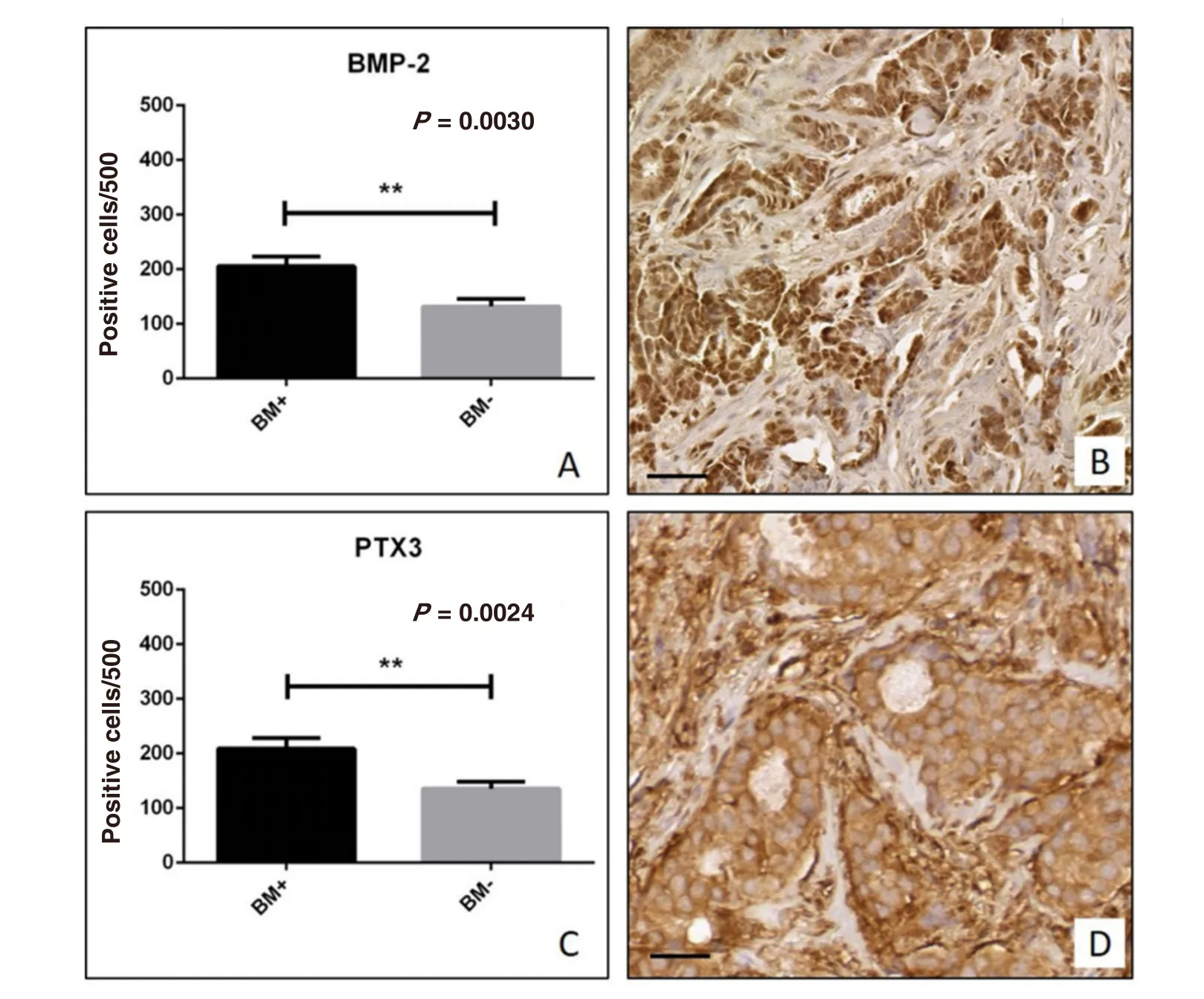
Figure 2. Immunohistochemical analysis of bone morphogenetic proteins 2 (BMP-2) and pentraxin-3 (PTX3). A: Graph shows the number of BMP-2 positive breast cancer cells in BM+ and BM- patients; B: representative image of BMP-2 expression in a ductal infiltrating breast carcinoma; C: graph shows the number of PTX3 positive breast cancer cells in BM+ and BM- patients; D: image displays the PTX3 expression in a case of ductal infiltrating breast carcinoma. Scala bars represent 100 μm for all images
We employed immunohistochemical techniques to study the expression of molecules associated to microcalcifications production, PTX3 and BMP-2. Briefy, antigen retrieval was performed on 3-μm-thick paraffin sections using EDTA citrate pH 7.8 buffers for 30 min at 95 °C. Sections were then incubated for 1 h at room temperature with the following primary antibodies diluted 1:100: BMP-2 (clone N/A; Novus Biologicals, USA) and PTX3 (clone MNB1; Abcam, UK). Reactions were revealed by HRP-DAB Detection Kit (UCS Diagnostic, Italy). To assess the background of immuno-staining we included a negative control for each reaction by incubating the sections with secondary antibodies (HRP) and detection system (DAB).Reactions have been set-up by using specific control tissues as indicated in the data sheets.
DECLARATIONS
Authors’ contributions
We employed immunohistochemical techniques to study the expression of two osteoblast induction factors,BMP-2 and PTX3. Immunohistochemical positivity was evaluated on digital images (Iscan Coreo, Ventana,Tucson, AZ, USA) by a semi-quantitative approach. Specifically, immunoreactions for BMP-2 and PTX3 were evaluated by counting the number of positive breast infiltrating cells (out of a total of 500 in randomly selected regions). Our results showed a significant increase of BMP-2 expression in BM+ breast lesions as compared to BM- group (BM+ 205.6 ± 17.57; BM- 131.8 ± 14.17;P= 0.030) [Figure 2A and B]. In agreement with this, we also observed a significant increase of PTX3 expression in patients of BM+ group as compared to those of BM- (BM+ 209.0 ± 19.32; BM- 135.6 ± 13.10;P= 0.0024) [Figure 2C and D].
Performed data acquisition and performed administrative, technical, and material supports: Polidori A,Nazzaro C, De Silva G
Availability of data and materials
After fixation in 10% buffered formalin for 24 h, breast tissues were paraffin embedded. Four μm thick sections were stained with haematoxylin-eosin (H&E)[11].
The young man fetched the pillows, and shook out all the feathers, and oh! what quantities of them there were! He was thinking to himself, as he spread them out carefully, how lucky it was that the sun was so bright and that there was no wind, when suddenly a breeze sprang up, and in a moment the feathers were dancing high in the air
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conficts of interest.
The following day she was dressed from head to foot in silk and velvet16, and they invited her to stay at the palace for a few days, and enjoy herself, but she only begged for a pair of boots, and a little carriage, and a horse to draw it, so that she might go into the wide world to seek for Kay
Ethical approval and consent to participate
Our study protocol was approved by independent ethical committee. Experimental procedures were carried out according toThe Code of Ethics of the World Medical Association (Declaration of Helsinki). Specimens were handled and carried out in accordance with the approved guidelines.
Consent for publication
Not applicable.
But on the evening of the day in which she had driven poor Rapunzel away, the Witch fastened the plaits on to a hook in the window, and when the Prince came and called out:
Copyright
©The Author(s) 2019.
 Journal of Cancer Metastasis and Treatment2019年4期
Journal of Cancer Metastasis and Treatment2019年4期
- Journal of Cancer Metastasis and Treatment的其它文章
- Metabolic alterations and the potential for targeting metabolic pathways in the treatment of multiple myeloma
- Contralateral axillary metastasis: is surgical treatment the best option?
- Breast cancer metastasis to the stomach
- Targeting autophagy with small molecules for cancer therapy
- Peripheral biomarkers for pediatric brain tumors:current advancements and future challenges
- Sensitive and specific detection of circuIating tumor ceIIs promotes precision medicine for cancer
