沉积物电缆细菌研究进展
张海涵,王 跃,黄廷林,赫慧艳,王 玥,张梦瑶
沉积物电缆细菌研究进展
张海涵*,王 跃,黄廷林,赫慧艳,王 玥,张梦瑶
(西安建筑科技大学,陕西省环境工程重点实验室,西北水资源与环境生态教育部重点实验室,陕西 西安 710055)
目前,在沉积物中发现一种丝状多细胞微生物—电缆细菌,该菌属于变形菌门的脱硫球茎菌科(Desulfobulbaceae).电缆细菌通过远距离电子传输将沉积物表面的氧还原与深处缺氧层的硫化物氧化电耦合进行完整的氧化还原反应,调节氧分子和硫化物迁移转化.电缆细菌有3种连接方式,表层存在脊段结构进行电子运输,并在富含硫酸盐和季节性周期变化的自然环境中广泛存在.这种新型的多细胞合作方式对沉积物的生物地球化学循环产生显著影响,促进沉积物硫化区铁锰的溶解,并抑制硫和磷等元素的释放.此外,电缆细菌参与硫化物和烃类污染物的降解,为污染沉积物生物修复领域提供新的研究方向.本文对电缆细菌的发现、生理代谢、栖息环境以及生物地球化学循环的影响等研究进行了综述.
电缆细菌;远距离电子传输;氧化还原反应;生物地球化学循环
多细胞生物是地球生物圈重要组成部分[1-3].其一个基本特征是能有效的利用能量并维持细胞功能[4].他的胞外电子传输方式包括:细胞的直接接触[6-7];利用可溶性氧化还原电子穿梭体[8-11];“纳米导线”(铁氧化菌的菌毛)直接将细胞外电子转移到固体基质并有效进行电子长距离的运输[12-17].这3种方式广泛存在于土壤、沉积物和活性污泥等环境中,并应用于微生物修复和重金属还原等方面[18].然而,最近在海洋沉积物中,发现一种新型多细胞合作方式,即细胞间通过电流的方式合作以提供能量、发挥生态功能[19].
生物地球化学循环的主要能量由生物体的氧化还原反应直接或间接提供[20].氧化还原作用会导致沉积物表层出现高度氧化的环境,该环境中富含的有机质、硫化物和甲烷等还原产物可作为电子供体,而微生物体内C、N、Mn、Fe、S和P等元素的氧化形式作为电子受体[21-28].传统的氧化还原反应在同一局部环境中需要同时提供电子供体和电子受体[29].然而,多细胞合作的长丝状电缆细菌可在厘米级的距离产生并传递电子,在不同空间分别进行氧化半反应和还原半反应[19].电缆细菌参与沉积物中元素的生物地球化学循环,促进沉积物底部铁锰的溶解,在水-沉积物界面积累铁锰氧化物,抑制硫和磷的释放.并对污染物(硫化物和烃类化合物)的迁移降解也发挥重要作用.此外,电缆细菌栖息环境广泛,在海洋、湖泊和红树林等自然环境中均发现其的存在.电缆细菌不仅为微生物生态学的研究提供了新方向,并在生物修复、生物电等应用领域有着巨大潜力.因此,本文对沉积物中电缆细菌的生长繁殖、能量传输及栖息环境等研究进行综述.
1 电缆细菌的长距离电子传输
1.1 长距离电子传输(LDET)
Nielsen等[19]将奥尔胡斯海岸沉积物与上覆水培养.揭示沉积物表面存在一个较窄的有氧区.随后是1.5cm的次氧区,最后是黑色硫化物沉淀形成低硫化区.此外,实验中观察到pH值垂向变化显著,其在沉积物表面的有氧区达到峰值,在次氧区和硫化区急剧下降.按照常规的好氧沉积过程,与氧分子发生的反应是在中性或者pH值下降的环境中,所以对于实验中pH值异常变化,只能由电化学氧化还原解释(图1).阴极位于表层有氧区,氧气的还原半反应消耗H+;阳极位于硫化区,游离硫化氢(H2S)通过氧化半反应被消耗,为了使这种空间分离的半反应发生,电缆细菌将阳极硫化物氧化产生的电子运输到阴极进行还原反应,相隔很远的细胞能协同进行氧化还原反应,这是由LDET驱动的好氧硫化物参与的电氧化反应(e-SO)[30].
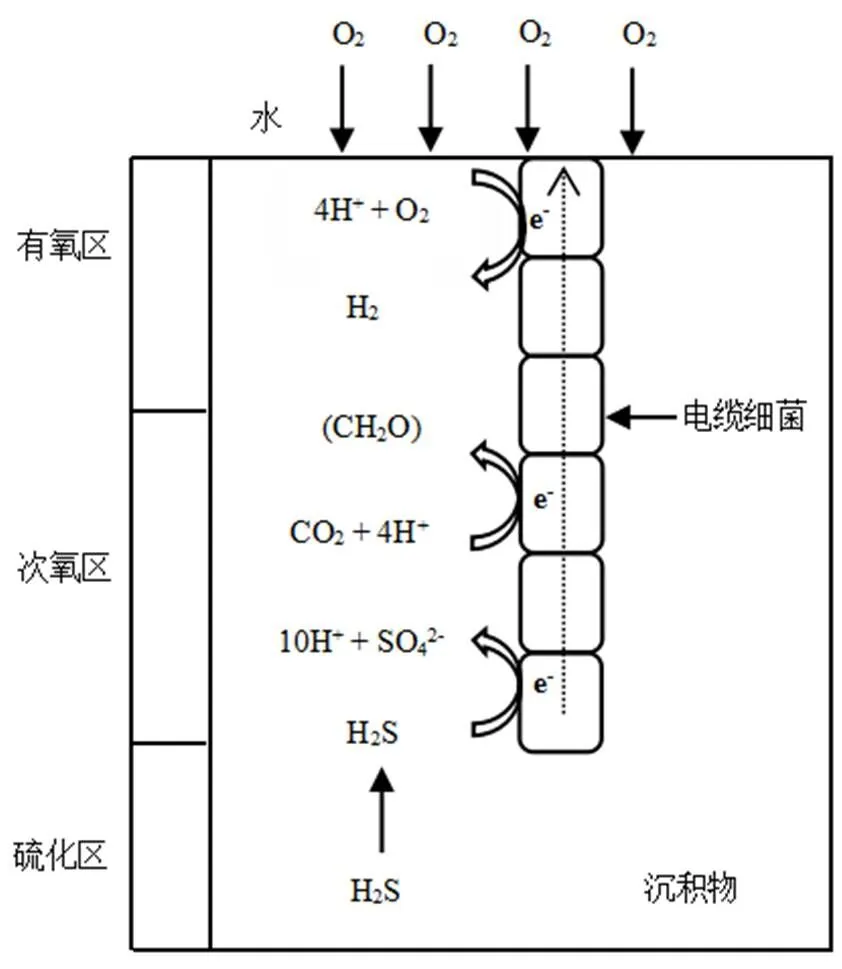
图1 电缆细菌的LDET
1.2 电缆细菌的发现
LDET被发现时,一些学者推测其是通过细菌纳米导电线、腐殖质颗粒和半导电矿物传递电子[8,11,14].然而,在2012年,Pfeffer等[30]证明电流是由生物介导的:一种丝状多细胞的e-SO与LDET密切相关,间隙水中O2、H2S和pH值垂向偏移现象表明氧分子还原反应和硫化物氧化反应在空间上是分离的.e-SO在沉积物中传递电子时,会产生相应的电场,从而驱动间隙水中的离子移动以维持电荷平衡,这揭示了沉积物可以提供电活性环境[31-32].
实验进一步表明,电子传递是在电缆细菌内部发生的,而不是通过分子扩散或者可溶性电子穿梭体的偶然接触.实验中在有氧-缺氧界面下1~2mm处水平穿过一根钨丝(直径50mm),有氧区pH值峰值在1h后变得不明显,同时氧气和硫化物的消耗量显著下降[30].Nielsen等[19]也通过改变上覆水中氧分子浓度发现沉积物中硫化氢浓度在有氧区以下迅速变化(1h内),这远快于分子扩散的速度.将孔径2µm的过滤器插入沉积物中心,沉积物中出现明显的LDET特征,而0.8µm孔径中的沉积物则未发现该现象,表明电子传递需要细菌大小的物质介导.最后当非导电玻璃微球层代替5mm厚的沉积物层时也发生了LDET,表明沉积物颗粒不是电子运输的重要介质.这些实验均间接的为LDET是由电缆细菌内部介导的观点提供了科学支撑.
然而,电缆细菌通过菌丝传输电子的现象仍然缺乏直接证据证明.直到Bjerg等[33]检测单个丝状体细胞色素氧化还原电位梯度.当在缺氧或激光切割丝状菌体时该梯度立即消失,电子受体失效,细胞色素明显减少,所以细胞色素氧化还原状态的迅速变化必须依赖于从硫化物区到有氧区的电子传输.这为电缆细菌远距离电子传递提供了直接证据.
1.3 电缆细菌电子传递结构
确定电缆细菌的导电结构和机理不仅加深对电缆细菌的认识,还可以进一步扩展生物电的应用.在扫描电镜下观察,电缆细胞外表面结构独特,沿着电缆细菌的轴存在均匀的脊状结构[30].丝状体相邻的细胞被200nm宽的间隙隔开,间隙由脊状物填充桥接而成,并被外膜紧紧包裹,一般有15~58个标记脊(图2),外观上类似于电缆,脊段结构表面存在弦(strings)充当“导电线”,电子在其内部的周质空间传递,并具有显著的极化性或电荷存储能力,并且是连接多个细胞的关键结构.这些弦引导电子穿过细胞间交界处,加强了节点的连接.弦与弦之间相互缠绕呈二聚体结构,提高电缆细菌稳定性,也是电缆细菌抵抗外界压力和保持厘米长度的必要结构[34].
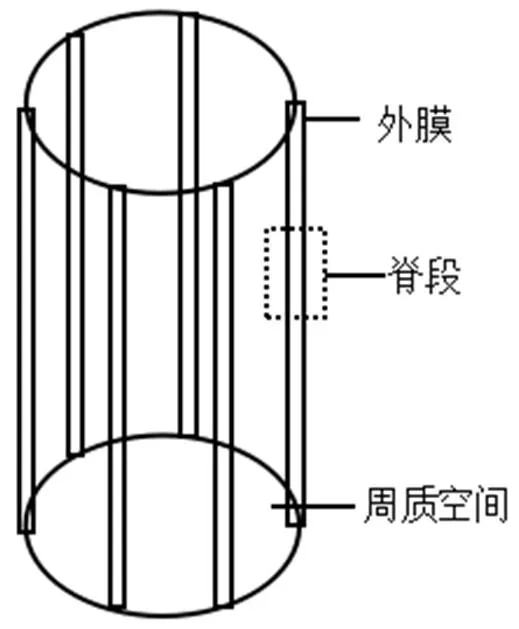
图2 电缆细菌形态示意
2 电缆细菌的生理特性
2.1 电缆细菌的代谢
Schauer等[35]基于荧光原位杂交(FISH)监测电缆细菌密度以探究电缆细菌的代谢途径和增殖速率,结果表明丝状菌生长过程中的菌直径在垂向无明显变化,在所有时间点和不同的沉积物层中均可以观察到沿整个细丝的细胞分裂的不同阶段,细菌是连续分裂的.在21d丝状菌密度达到最大(2km/cm2),间隙水的化学特征完全显现.随着时间的变化(21~53d),电缆细菌的密度和活性大幅度下降,可能是由于次氧区的FeS耗尽[36].Jiang等[34]提出了一个模型来表示细胞分裂的不同阶段(图3)也是丝状菌不同类型的连接方式(J1、J2和J3).J2出现在细胞复制时期,分裂的细胞从中间开始膨胀,形成一个新的连接J2,随着细胞分裂,当两个细胞之间形成细胞膜时,过渡型的连接J3出现,在分裂将要结束时,两个新的细胞形成,菌丝伸长,J2收缩形成J1连接,J1和J2交替出现.根据细胞分裂模型,所有细胞的分裂是均匀连续的.
电缆细菌能够在几天内大量繁殖,这意味着它有高效的碳固定方式.Vasquez-Cardena等[37]通过纳米级次级离子质谱法(NanoSIMS)和磷脂衍生脂肪酸稳定同位素探针法(PLFA-SIP)用13C标记丙酸和碳酸氢盐结合细菌脂肪酸来研究电缆细菌的代谢方式,表明电缆细菌与化能无机自养生物密切相关,e-SO是由化能有机型电缆细菌、化能自养型ε-变形菌和丙型变形菌共同完成.然而,对这两组代谢类型之间的联系还有待进一步研究.
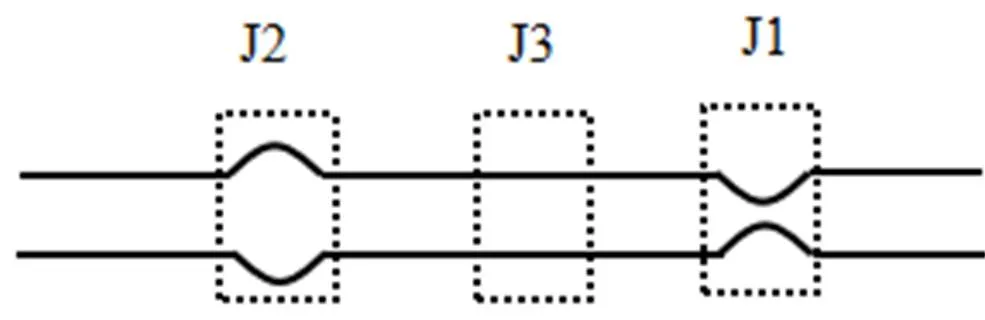
图3 电缆细菌的3种连接方式
2.2 电缆细菌的能动性
电缆细菌的演替通常是快速增长到种群密度峰值,根据电缆细菌生长及增殖表明,细胞分裂并具有能动性才能使菌丝向下延伸.Malkin等[38]研究电缆细菌与光合藻类生物膜的关系时,发现在明暗交替的状态下培育沉积物,深部硫化物浓度随光/暗周期的变化呈规律性循环.所以Malkin假设电缆细菌是运动的,并且随氧化还原梯度的变化进行移动.Bjerg[39]等利用时差显微成像技术观察到电缆细菌通常是沿着菌丝的轴线滑行运动,但是部分电缆细菌有时会反向运动.当电缆细菌的末端滑动遇到障碍停留,菌丝继续移动从而成环,或者不同的菌丝反向运动,形成环状凸起.电缆细菌群体中有3种运动状态:不运动(22%),末端直线滑动(35%)和环状滑动(43%).通过了解电缆细菌的运动不仅揭示了其在环境中的作用,还加深了对电缆细菌生态作用的理解.
2.3 电缆细菌的系统发育分析
目前发现的所有电缆细菌均属于脱硫球茎菌(Desulfobulbaceae),它们确切的系统发育分类尚不明确,因为大多数研究只通过16S rRNA序列测序,依靠丝状形态鉴定或利用Desulfobulbaceae探针荧光原位杂交进行鉴定[32].Trojan等[40]从淡水和海洋沉积物中获取电缆细菌,对16S rRNA和AB基因序列进行全基因组扩增、测序,并构建电缆细菌16S rRNA基因序列的环境分布.电缆细菌16S rRNA基因序列可按相似性分为2个属水平,6个不同的种水平.2个新增的属分别为(来自海洋)和Candidatus(来自淡水). Candidatus附属4个种,分别为:,,,和; Candidatus附属2个种,分别为:,和.对含有分离亚硫酸盐还原酶的全部或部分AB基因的系统发育分析也得到了相同分组.目前,对于LDET是否局限于电缆细菌,会不会存在于不同种类的微生物中,如光合菌,化能自养菌等还有待进一步深入探究.
3 电缆细菌栖息环境
电缆细菌在各种自然环境的沉积物中被发现,其中以富含硫酸盐、季节性周期变化和缺少生物强烈扰动等特征的海岸地区为主[41,49].目前发现的栖息地包括盐沼[41-43]、红树林[44]、海岸潮间带[45]、海岸淤泥堆积地[46]和季节性缺氧湖泊[47-48].此外,最近在淡水湖,半咸水水域沉积物中也发现电缆细菌的存在[50-51](表1),有关水源水库沉积物电缆细菌的研究未见报道.
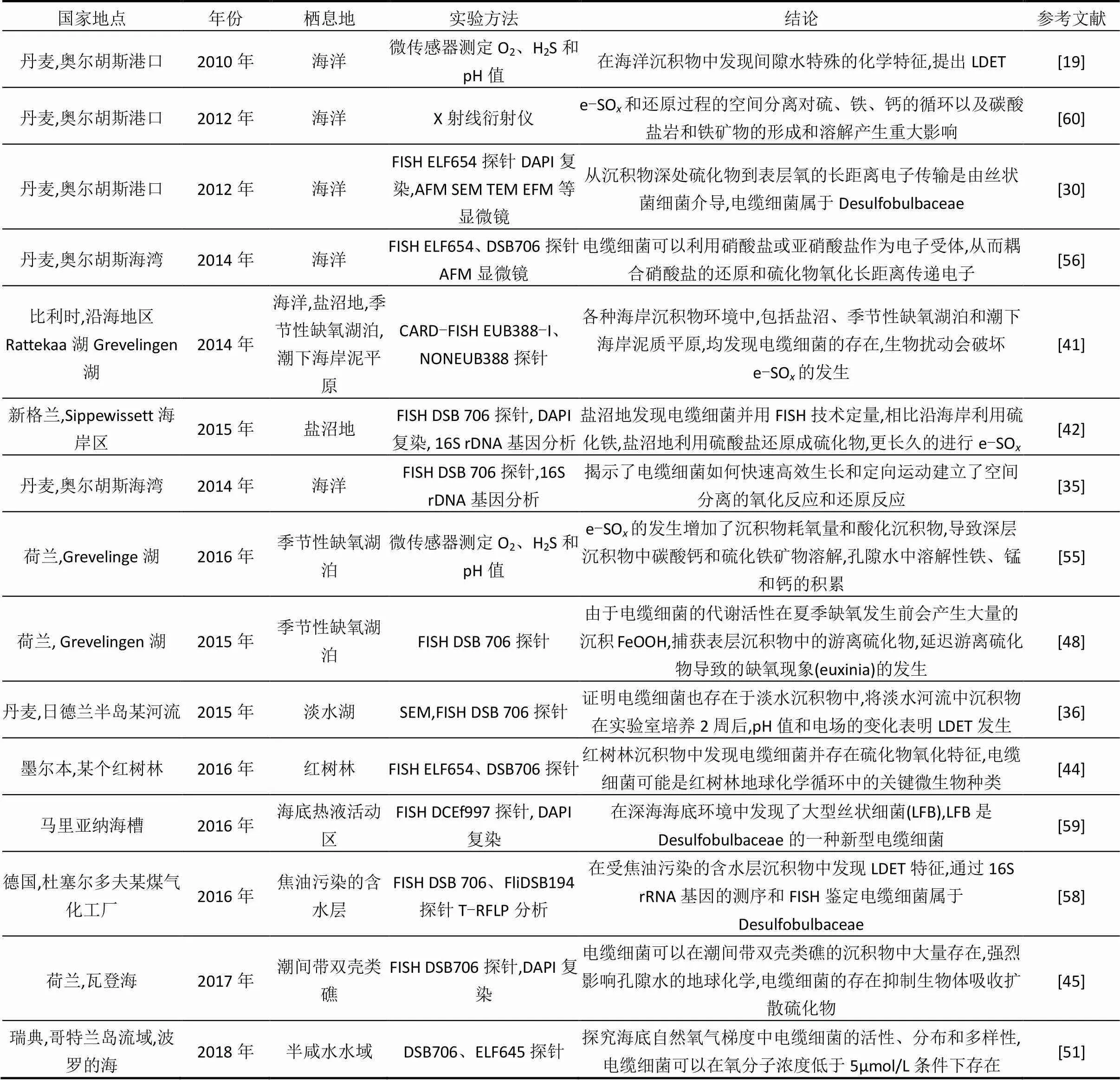
表1 电缆细菌栖息环境
4 电缆细菌对生物地球化学循环的影响
4.1 电缆细菌对沉积物中铁锰钙元素影响
e-SO对水体-沉积物生物地球化学循环有显著影响,电缆细菌通过调控硫循环[46,49]、磷循环[52]、锰循环和铁循环[47,53],从而影响季节性缺氧环境的生态系统功能.主要机理为e-SO生成硫酸盐同时生成质子H+,导致沉积物缺氧层中硫化铁和碳酸钙溶解,Ca2+、Fe2+和SO42-向孔隙水流动,由于沉积物中40%以上的氧分子参与质子H+的电化学反应,阴极部分有氧区呈碱性,所以Fe2+向上扩散到有氧区形成FeOOH,Ca2+在氧区沉淀为CaMg(1-x)CO3[60]. e-SO导致溶解的Mn2+和Fe2+在深处释放,而锰和铁的氧化物富集在沉积物表面附近.电缆细菌的代谢造成表层沉积物中形成FeOOH,随后作为夏季沉积物中游离硫化物的缓冲物,延迟euxinia在水底的发生,类似“防火墙”的作用[54].电缆细菌的种群动态变化,也会诱导沉积物铁−磷的季节性变化.春季,在电缆细菌的作用下铁氧结合磷大量存在,夏季缺氧,氧化铁耦联的磷被释放出来,水中磷含量大大增加[52].
4.2 电缆细菌对沉积物中微量元素循环的影响
e-SO导致孔隙水中pH值偏移,从而影响对pH值敏感的矿物质(硫化物和碳酸盐)循环[60].此外, e-SO对沿海沉积物中微量金属的早期成岩作用也有影响.微量元素砷和钴可吸附在硫化物沉淀上,当e-SO使孔隙水酸化后,硫化铁的溶解使微量元素释放出来向上扩散,在上覆水富集[61].沉积物中的钼主要来源有:铁氧化物和氢氧化物颗粒表面富集携带;上覆水中在硫化物作用下形成的硫代钼酸盐的释放[62-64].电缆细菌的代谢引起锰碳酸盐和FeS的溶解,从而间接影响钼动力学,扩大了钼的季节性变化[62].
4.3 电缆细菌对污染物迁移转化的影响
电缆细菌不仅改变水-沉积物的生物地球化学特征,参加元素循环,在微生物修复和污染物迁移转化方面也值得深入研究.Malkin等[45]证明蓝贝、牡蛎等生物依靠微生物e-SO来阻止硫化物在鳃表面的吸收扩散,因此,电缆细菌可能是双壳类礁沉积物解毒的关键.硫酸盐是沉积物中重要氧化剂,石油烃类在硫酸盐还原作用下降解.在受污染的海洋沉积物缺氧区,通过电缆细菌的作用硫化物氧化形成硫酸盐促进石油烃类氧化生物降解[57].综上,电缆细菌对生物地球化学循环的影响与硫化物的溶解、沉淀有密切联系.
5 电缆细菌在污染沉积物修复中的应用
沉积物中的污染物可作为电缆细菌的代谢过程中的电子供受体,从而降解污染物,驱动沉积物生物修复[65-66].电缆细菌利用氧分子和硝酸盐作为电子受体,硫化物是e-SO过程中的电子供体.有毒硫化物损害植物根系,在电缆细菌的代谢活动下硫化物浓度降低,改善海洋沉积物植物根系的环境[58];在季节性缺氧环境中,电缆细菌参与铁锰循环,在沉积物表层形成”保护壳”,延缓硫化物的释放,增强化学自养菌群活性[37],此外,铁锰氧化物形成减少沉积物向水中铁锰磷的释放通量[55];电缆细菌调控硫循环参与烃类污染物(甲苯、石油)的降解[57].微生物利用电子受体大量繁殖,电缆细菌也可以将硝酸盐的还原与硫化物的氧化耦合[56],可通过生物刺激方式激活电缆细菌,促进污染物的降解速率.
目前,由于电缆细菌研究尚在探索阶段,沉积物电缆细菌还未成功富集分离纯培养;硝酸盐循环机制未明确;电缆细菌与其他微生物是否存在促进或竞争关系等问题,均限制了电缆细菌在污染沉积物生物修复工程中的应用.
6 展望
目前,对水环境沉积物电缆细菌的研究尚在初期阶段,还有颇多未知问题有待深入探索:(1)有关沉积物电缆细菌菌株基因组的测定;(2)电缆细菌如何在运动中协调氧分子和硫化物代谢转化;(3)上覆水中氧分子浓度的准确阈值与菌株丰度的关系;(4)电缆细菌在深水型(高静水压)贫营养水源水库沉积物中是否存在,及其在水源水库水质演变过程中的代谢调控机制尚不明确(尤其是季节性物理热分层的作用机制);(5)人工外源增氧(如扬水曝气技术)调控水源水库“沉积物-水”界面驱动表层沉积物中电缆细菌丰富、组成代谢活性机制尚需探索.综上,在水环境微生物生态研究和污染生态修复工程领域,对特殊生境沉积物中电缆细菌的深度研究,将驱动沉积物微生物生态学发展,并且为污染沉积物微生物菌剂修复领域提供崭新的方向.
[1] Donoghue P C J, Antcliffe J B. Early life: Origins of multicellularity [J]. Nature, 2010,466(7302):41-42.
[2] De M S, Rainey P B. Nascent multicellular life and the emergence of individuality [J]. Journal of Biosciences, 2014,39(2):237-248.
[3] Bonner J T. The origins of multicellularity [J]. Integrative Biology Issues News and Reviews, 2015,1(1):27-36.
[4] Grosberg R K, Strathmann R R. The evolution of multicellularity: a minor major transition [J]. Annual review of ecology enolution and systematics, 2007,38(38):621-654.
[5] Basu S, Gerchman Y, Collins C H, et al. A synthetic multicellular system for programmed pattern formation [J]. Nature, 2005,434(7037): 1130-1133.
[6] 潘怡然,崔康平,张 硕,等.颗粒活性炭促进高温厌氧消化的研究[J]. 中国环境科学, 2018,38(4):1324-1328. Pan Y R, Cui K P, Zhang S, et al. The study on granular activated carbon to promote thermophilic anaerobic digestion [J]. China Environmental Science, 2018,38(4):1324-1328.
[7] 汪明霞,王 娟,司友斌.MR-1异化还原Fe(III)介导的As(III)氧化转化[J]. 中国环境科学, 2014,34(9):2368-2373. Wang M X, Wang J, Si Y B. As(III) oxidization coupled to Fe(III) reduction byMR-1 [J]. China Environmental Science, 2014,34(9):2368-2373.
[8] Kato S, Hashimoto K, Watanabe K, et al. Microbial interspecies electron transfer via electric currents through conductive minerals [J]. Proceedings of the National Academy of Sciences, 2012,109(25): 10042-10046.
[9] Marsili E, Baron D B, Shikhare I D, et al.secretes flavins that mediate extracellular electron transfer [J]. Proceedings of the National Academy of Sciences, 2008,105(10):3968-3973.
[10] Roden E E, Kappler A, Bauer I, et al. Extracellular electron transfer through microbial reduction of solid-phase humic substances [J]. Nature Geoscience, 2010,3(6):417-421.
[11] 杨 超,何小松,席北斗,等.填埋初期水溶性有机物结构受电子转移的影响 [J]. 中国环境科学, 2017,37(1):229-237. Yang C, He X S, Xi B D, et al. Effect of electron transfer on the structure of dissolved organic matter during initial landfill stage [J]. China Environmental Science, 2017,37(1):229-237.
[12] Hartshorne R S, Reardon C L, Ross D, et al. Characterization of an electron conduit between bacteria and the extracellular environment [J]. Proceedings of the National Academy of Sciences of the United States of America, 2009,106(52):22169-22174.
[13] Liu X, Tremblay P L, Malvankar N S, et al. Astrain expressing pseudomonas aeruginosa type IV pili localizes OmcS on pili but is deficient in Fe(III) oxide reduction and current production [J]. Applied and Environmental Microbiology, 2014,80(3):1219-1224.
[14] Reguera G, Mccarthy K D, Mehta T, et al. Extracellular electron transfer via microbial nanowires [J]. Nature, 2005,435(7045):1098- 1101.
[15] Eaktasang N, Kang C S, Lim H, et al. Production of electrically- conductive nanoscale filaments by sulfate-reducing bacteria in the microbial fuel cell [J]. Bioresour Technol, 2016,210:61-67.
[16] Kumar S S, Basu S, Bishnoi N R. Effect of cathode environment on bioelectricity generation using a novel consortium in anode side of a microbial fuel cell [J]. Biochemical Engineering Journal, 2017,121: 17-24.
[17] Cologgi D L, Lampa-Pastirk S, Speers A M, et al. Extracellular reduction of uranium viaconductive pili as a protective cellular mechanism [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011,108(37):15248-15252.
[18] 马 晨,周顺桂,庄 莉,等.微生物胞外呼吸电子传递机制研究进展[J]. 生态学报, 2011,31(7):2008-201. Ma C, Zhou S G, Zhuang L, et al. Electron transfer mechanism of extracellular respiration: a review [J]. Acta Ecologica Sinica, 2011, 31(7):2008-2018.
[19] Nielsen L P, Risgaard-Petersen N, Fossing H, et al. Electric currents couple spatially separated biogeochemical processes in marine sediment [J]. Nature, 2010,463(7284):1071-1074.
[20] Borch T, Kretzschmar R, Kappler A, et al. Biogeochemical redox processes and their impact on contaminant dynamics [J]. Environmental Science and Technology, 2009,44(1):15-23.
[21] Small G E, Cotner J B, Finlay J C, et al. Nitrogen transformations at the sediment–water interface across redox gradients in the Laurentian Great Lakes [J]. Hydrobiologia, 2014,731(1):95-108.
[22] Weber K A, Urrutia M M, Churchill P F, et al. Anaerobic redox cycling of iron by freshwater sediment microorganisms [J]. Environmental microbiology, 2006,8(1):100-113.
[23] Duan Y, Gan Y, Wang Y, et al. Arsenic speciation in aquifer sediment under varying groundwater regime and redox conditions at Jianghan Plain of Central China [J]. Science of the Total Environment, 2017, 607-608:992-1000.
[24] 董 军,赵勇胜,张伟红,等.垃圾渗滤液中重金属在不同氧化还原带中的衰减 [J]. 中国环境科学, 2007,27(6):743-747. Dong J, Zhang Y S, Zhang W H. Attenuation of heavy metals in landfill leachate at different redox zones [J]. China Environmental Science, 2007,27(6):743-747.
[25] Robinson G, Caldwell G S, Wade M J, et al. Profiling bacterial communities associated with sediment-based aquaculture bioremediation systems under contrasting redox regimes [J]. Scientific Reports, 2016,6(1):38850.
[26] Thompson A, Chadwick O A, Rancourt D G, et al. Iron-oxide crystallinity increases during soil redox oscillations [J]. Geochimica et Cosmochimica Acta, 2006,70(7):1710-1727.
[27] Jacinthe P A, Groffman P M. Microbial nitrogen cycling processes in a sulfidic coastal marsh [J]. Wetlands Ecology and Management, 2006, 14(2):123-131.
[28] 倪吾钟,沈仁芳,朱兆良.不同氧化还原电位条件下稻田土壤中15N标记硝态氮的反硝化作用 [J]. 中国环境科学, 2000,20(6):519-523. Ni W Z, Shen R F, Zhu Z L. Denitrification of15N labeled nitrate-N in rice field soil under different redox conditions [J]. China Environmental Science, 2000,20(6):519-523.
[29] Hojsak I, Abdović S, Szajewska H, et al.GG in the prevention of nosocomial gastrointestinal and respiratory tract infections [J]. Pediatrics, 2010,125(5):1171-1177.
[30] Pfeffer C, Larsen S, Song J, et al. Filamentous bacteria transport electrons over centimetre distances [J]. Nature, 2012,491(7423):218- 221.
[31] Gorski C A, Edwards R, Sander M, et al. Thermodynamic characterization of iron oxide-aqueous Fe2+redox couples [J]. Environmental Science and Technology, 2016,50(16):8538-8547.
[32] Flynn T M, O'Loughlin E J, Mishra B, et al. Sulfur-mediated electron shuttling during bacterial iron reduction [J]. Science, 2014,344(6187): 1039-1042.
[33] Bjerg J T, Boschker H T S, Larsen S, et al. Long-distance electron transport in individual, living cable bacteria [J]. Proceedings of the National Academy of Sciences current issue, 2018,115(22):5786- 5791.
[34] Jiang Z X, Zhang S, Klausen L H, et al. In vitro single-cell dissection revealing the interior structure of cable bacteria [J]. Proceedings of the National Academy of Sciences current issue, 2018,115(34):8517- 8522.
[35] Schauer R, Risgaard-Petersen N, Kjeldsen K U, et al. Succession of cable bacteria and electric currents in marine sediment [J]. The ISME Journal, 2014,8(6):1314-1322.
[36] Risgaard-Petersen N, Damgaard L R, Revil A, et al. Mapping electron sources and sinks in a marine biogeobattery [J]. Journal of Geophysical Research: Biogeosciences, 2015,119(8):1475-1486.
[37] Vasquez-Cardenas D, van de Vossenberg J, Polerecky L, et al. Microbial carbon metabolism associated with electrogenic sulphur oxidation in coastal sediments [J]. The ISME Journal, 2015,9(9):1966- 1978.
[38] Malkin S Y, Meysman F J. Rapid redox signal transmission by "cable bacteria" beneath a photosynthetic biofilm [J]. Applied and Environmental Microbiology, 2015,81(3):948-956.
[39] Bjerg J T, Damgaard L R, Holm S A, et al. Motility of electric cable bacteria [J]. Applied and Environmental Microbiology, 2016,82(13): 3816-3821.
[40] Trojan D, Schreiber L, Bjerg J T, et al. A taxonomic framework for cable bacteria and proposal of the candidate genera electrothrix and electronema [J]. Systematic and Applied Microbiology, 2016,39(5): 297-306.
[41] Malkin S Y, Rao A M, Seitaj D, et al. Natural occurrence of microbial sulphur oxidation by long-range electron transport in the seafloor [J]. The ISME Journal, 2014,8(12):1843-1854.
[42] Larsen S, Nielsen L P, Schramm A. Cable bacteria associated with long distance electron transport in New England salt marsh sediment [J]. Environmental Microbiology Reports, 2015,7(2):175-179.
[43] Rao A, Risgaard-Petersen N, Neumeier U. Electrogenic sulfur oxidation in a northern saltmarsh (St. Lawrence Estuary, Canada) [J]. Canadian Journal of Microbiology, 2016,162(6):530-537.
[44] Burdorf L D W, Hidalgo-Martinez S, Cook P, et al. Long-distance electron transport by cable bacteria in mangrove sediments [J]. Marine Ecology Progress, 2016,545:1-8.
[45] Malkin S Y, Seitaj D, Burdorf L D W, et al. Electrogenic sulfur oxidation by cable bacteria in bivalve reef sediments [J]. Frontiers in Marine Science, 2017,4:28.
[46] Velde S V D, Ludovic L, Laurine D W B, et al. The impact of electrogenic sulfur oxidation on the biogeochemistry of coastal sediments: A field study [J]. Geochimica et Cosmochimica Acta, 2016, 194:211-232.
[47] Sulu-Gambari F, Seitaj D, Behrends t, et al. Impact of cable bacteria on sedimentary iron and manganese dynamics in a seasonally-hypoxic marine basin [J]. Geochimica et Cosmochimica Acta, 2016,192:49-69.
[48] Seitaj D, Sulu-Gambari F, Burdorf L D W, et al. Sedimentary oxygen dynamics in a seasonally hypoxic basin [J]. Limnology and Oceanography, 2016,62(2):452-473.
[49] Burdorf L D W, Tramper A, Seitaj D, et al. Long-distance electron transport occurs globally in marine sediments [J]. Biogeosciences, 2017,14(3):683-701.
[50] Risgaard-Petersen N, Kristiansen M, Frederiksen R B, et al. Cable bacteria in freshwater sediments [J]. Applied and Environmental Microbiology, 2015,81(17):6003-6011.
[51] Marzocchi U, Bonaglia S, van de Velde S, et al. Transient bottom water oxygenation creates a niche for cable bacteria in long-term anoxic sediments of the Eastern Gotland Basin [J]. Environmental Microbiology, 2018,20(8):3031-3041.
[52] Sulu-Gambari F, Seitaj D, Meysman F J, et al. Cable bacteria control iron-phosphorus dynamics in sediments of a coastal hypoxic basin [J]. Environmental Science and Technology, 2015,50(3):1227-1233.
[53] Meysman F J R, Risgaard-Petersen N, Malkin S Y, et al. The geochemical fingerprint of microbial long-distance electron transport in the seafloor [J]. Geochimica et Cosmochimica Acta, 2015,152(52): 122-142.
[54] Seitaj D, Schauer R, Sulugambari F, et al. Cable bacteria generate a firewall against euxinia in seasonally hypoxic basins [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015,112(43):13278-13283.
[55] Rao A M F, Malkin S Y, Hidalgomartinez S, et al. The impact of electrogenic sulfide oxidation on elemental cycling and solute fluxes in coastal sediment [J]. Geochimica et Cosmochimica Acta, 2016,172: 265-286.
[56] Marzocchi U, Trojan D, Larsen S, et al. Electric coupling between distant nitrate reduction and sulfide oxidation in marine sediment [J]. The ISME Journal, 2014,8(8):1682-1690.
[57] Matturro D, Cruz Viggi C, Aulenta F, et al. Cable bacteria and the bioelectrochemical snorkel: the natural and engineered facets playing a role in hydrocarbons degradation in marine sediments [J]. Frontiers in Microbiology, 2017,8:952.
[58] Müller H, Bosch J, Griebler C. Long-distance electron transfer by cable bacteria in aquifer sediments [J]. The ISME Journal, 2016, 10(8):2010-2019.
[59] Kato S, Yamagishi A. A novel large filamentous deltaproteobacterium on hydrothermally inactive sulfide chimneys of the Southern Mariana Trough [J]. Deep Sea Research Part I: Oceanographic Research Papers, 2016,110:99-105.
[60] Risgaard-Petersen N, Revil A, Meister P, et al. Sulfur, iron-, and calcium cycling associated with natural electric currents running through marine sediment [J]. Geochimica et Cosmochimica Acta, 2012,92:1-13.
[61] Velde S V D, Callebaut I, Gao Y, et al. Impact of electrogenic sulfur oxidation on trace metal cycling in a coastal sediment [J]. Chemical Geology, 2017,452:9-23.
[62] Sulu-Gambari F, Roepert A, Jilbert T, et al. Molybdenum dynamics in sediments of a seasonally-hypoxic coastal marine basin [J]. Chemical Geology, 2017,466:627-640.
[63] Tribovillard N, Algeo T J, Lyons T, et al. Trace metals as paleoredox and paleoproductivity proxies: An update [J]. Chemical Geology, 2006,232(1/2):12-32.
[64] Zheng Y, Anderson R F, Van Geen A, et al. Authigenic molybdenum formation in marine sediments: a link to pore water sulfide in the Santa Barbara Basin [J]. Geochimica et Cosmochimica Acta, 2000, 64(24):4165-4178.
[65] 孔冠楠,许玫英,杨永刚.基于直接接触的微生物胞外电子传递 [J]. 微生物学报, 2017,57(5):643-650. Kong G N, Xu M Y, Yang Y G. Direct contact-dependent microbial extracellular electron transfer [J]. Acta Microbiologica Sinica, 2017, 57(5):643-650.
[66] 许玫英,虞志强,杨永刚,等.微生物厌氧呼吸与有机污染水体沉积物修复 [J]. 微生物学杂志, 2017,37(2):2-11. Xu M Y, Yu Z A, Yang Y G. Microbial anaerobic respiration and remediation of aquatic sediments contaminated by refractory organic pollutants [J]. Journal of Microbiology, 2017,37(2):2-11.
Research progress of cable bacteria in sediment.
ZHANG Hai-han*, WANG Yue, HUANG Ting-lin, HE Hui-yan, WANG Yue, ZHANG Meng-yao
(Key Laboratory of Northwest Water Resources and Environment, Ministry of Education, Shaanxi Key Laboratory of Environmental Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China)., 2019,39(7):3048~3055
Recently, a novel multicellular filamentous microbe named as cable bacteria was observed in sediments, which belong to Desulfobulbaceae of proteobacteria. Cable bacteria conducedted complete redox reaction by coupling oxygen reduction on sediment surface with sulfide oxidation in deep anoxic layer through long-distance electron transport. Cable bacteria had three connection modes and surface ridge structure for electronic transport. It existed widely in natural environment rich in sulfate and seasonal periodic variation. This new multicellular cooperation mode had a significant impact on the biogeochemical cycle of sediments, which promoted the dissolution of iron and manganese in sulphide areas of sediments, and inhibited the release of sulfur and phosphorus. Moreover, cable bacteria was involved in the degradation of sulfide and hydrocarbon pollutants, and providing a new research direction in the field of bioremediation of contaminated sediments. Here, the discovery, physiological metabolism, habitat environmental conditions and biogeochemical cycle of cable bacteria were reviewed systematically.
cable bacteria;long-distance electron transport;redox reaction;biogeochemical cycles
X524
A
1000-6923(2019)07-3048-08
张海涵(1981-),男,陕西西安人,副教授,博士,主要研究方向为水环境微生物群落结构.发表论文50余篇.
2018-12-16
国家重点研发计划(2016YFC0400706);陕西省国际科技合作计划项目(2018KW-011);国家级大学生创新创业项目(201809023)
* 责任作者, 副教授,zhanghaihan@xauat.edu.cn

