Role of autophagy in therapeutic resistance of glioblastoma
Chia-Hung Chien, Wei-Ting Hsueh, Jian-Ying Chuang, Kwang-Yu Chang,4,5
1National Institute of Cancer Research, National Health Research Institutes, Tainan 70456, Taiwan.
2Center for Neurotrauma and Neuroregeneration, Taipei Medical University, Taipei 11031, Taiwan.
3The Ph.D. Program for Neural Regenerative Medicine, Taipei Medical University, Taipei 11503, Taiwan.
4Division of Hematology/Oncology, Department of Internal Medicine, National Cheng Kung University Hospital, Tainan 70403, Taiwan.
5Department of Oncology, School of Medicine, National Cheng Kung University, Tainan 70403, Taiwan.
6Department of Radiation Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan 70403, Taiwan.
Abstract Patients with glioblastoma (GBM), a malignant brain tumor, exhibit a mean survival of less than 1.5 years. Despite treatment, the disease eventually develops resistance, resulting in disease relapse. Autophagy is a process of degradation and clearance that is activated to maintain cellular homeostasis. Its roles in cancer disease course and the treatment response, however, are controversial. In GBM, accumulating evidence has indicated that autophagy can protect cells, especially those with stemness features, causing the development of cell resistance. In this review, we discuss the impact of the cell reaction to currently active treatments, including temozolomide, radiation,tumor treating fields, bevacizumab (Avastin), etoposide (VP-16), cisplatin (CDDP), and carmustine (BCNU). Most of these induce the up-regulation of autophagy through signaling pathways of DNA damage response, reactive oxygen species, hypoxia, retinoblastoma, AMP-activated protein kinase, AKT/mTOR and MST4 kinase affecting cell fate by altering cell metabolism, cell death, and DNA repair. Treatment-related autophagy may be modulated by combining autophagy inhibitors such as chloroquine or antioxidants to prevent the development of resistance,thus improving cancer treatment.
Keywords: Autophagy, resistance, glioblastoma
INTRODUCTION
The term autophagy is derived from Greek words, “auto” and “phagy”, meaning self-eating[1], representing a process of degradation and clearance that is activated in response to aggregated protein and dysfunctional organelles to maintain cellular homeostasis[2]. The autophagy pathway is initiated by a series of active proteins, such as autophagy-related (ATG) proteins, UVRAG, Beclin 1, PIK3C3, LC3 and p62[3]. Together,they contribute to the formation of double-membrane autophagosomes, and eventually, fusion with lysosomes for degradation of inner substances [Figure 1]. Hence, autophagy has been implicated in several diseases including cancer[4]. In the past, autophagy was believed to be related to apoptosis[5]. Recently, an opposite impact of autophagy, i.e., promoting cell survival, has been widely studied[6]. This “double-edged sword” effect in tumorigenesis varies depending on the cellular reaction to specific stimulation as well as in different cancer types. High levels of autophagy in the tumor environment (with limited nutrient and oxygen conditions) allow cancer cells to survive, resuming proliferation and initiation[7].
Glioblastoma (GBM) is a disease composed of extremely varied tumor microenvironments and heterogeneous cancer cells. It is a fatal disease known to have a poor prognosis, and is not considered curable. Despite advanced treatment strategies, a notable improvement was not observed in terms of the outcome. Hence, it is not surprising that the tumor cells in the apex of the hierarchy, or those that are capable of self-renewal and differentiation, display highly-activated autophagy signaling to survive and thrive from the given treatment, such as temozolomide (TMZ)[8]. Moreover, the molecular characters of GBM involved in the growth and survival via intracellular pathways are distinct genetic aberrations in epidermal growth factor receptor (EGFR), PTEN, TP53, IDH1 and so on[9]. Among these frequently reported genes in clinical disease, EGFR is well-known as a driving receptor tyrosine kinase (RTK) in GBM that dictates multiple oncogenic signaling[10]. The signaling amplification of EGFR accounts for approximately 60% of GBM cases. The mutant form EGFRvIII receptor, which is constitutively active that is independent of the ligand binding condition, is also common. In association with EGFR signaling pathway, it was found that levels of MDA-9, a protein related to tumor cell behavior and stemness, were increased in glioma stem-like cells to regulate the protective autophagy[11][Figure 1]. Moreover, it was found that STAT3 levels were related to beclin 1 expression via EGFR amplification[12,13][Figure 1]. Other RTKs in GBM, such as vascular endothelial growth factor receptor (VEGFR)[14], platelet-derived growth factor receptor (PDGFR)[15]and discoidin domain RTK 1 (DDR1)[16], also contributes to modulating the autophagy formation through AKT/mTOR[16,17], RAF/MEK[18]and HIF-1/BCL2 Interacting Protein 3[19-21]signalings [Figure 1]. As thus, therapeutic agents targeting these factors, for example bevacizumab[14]and PDGF neutralizing antibody[15], has been reported to enhance autophagy signaling [Figure 1]. Despite of the implications by disease- or treatment-related autophagic alterations, clinical trials with autophagy inhibitors, such as chloroquine (CQ) or its analogs, showed only limiting benefits[22]. So far, the exact role of autophagy in GBM remains ambiguous, especially regarding treatment resistance[23].
The effect of autophagy on tumors is not universal. On one hand, autophagy protects cells by clearing damaged organelles, thereby promoting cell survival. On the other hand, it achieves damage control by eliciting self-eating, leading to the process of autophagic cell death. The pros and cons of this biologic reaction does not have a clear boundary and may be dynamic. Regarding drug treatment, the appearance of autophagy also had divergent effect. For example, in a study of PI3K/mTOR inhibitor BGT226, application of the drug led to autophagic cell death in head and neck cancer cell lines[24]. In contrast, application of PI3K/AKT/mTOR inhibitors sensitized cells to radiotherapy as well as reduced autophagy formation[25].Various effects of autophagy in the cancer treatment have been demonstrated, making the reaction complicated in each circumstance. However, in the case of GBM, the appearance of autophagy during specific treatment seems to support the development of resistance[26]. In this article, we will discuss the molecular significance of autophagy in the current treatment of GBM. We will also discuss the formation of the reaction and the potential benefit of inhibiting autophagy along with the treatment.
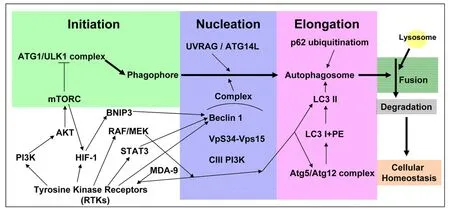
Figure 1. The schematic figure of the principal cell signaling pathways assocated with the autophagic process. The process of autophagy pathway begins from the initiation step to the nucleation step, the elongation step, and to the formation of mature autophagosome which subsequently fuses with lysosome to perform the degradation process. The commonly activated pathways related to aberrant RTKs in GBM are also shown. Their roles to affect the individual steps of the autophagic process are indicated. RTKs: receptor tyrosine kinases;GBM: glioblastoma; HIF-1: hypoxia-inducible factor-1
TMZ
Being the most standard chemotherapy for GBM, it is mandatory to understand how autophagy is induced by TMZ. The first-line drug acts by inducing lethal DNA damage and subsequent reactive oxygen species (ROS) production[27]. The effect is, however, mostly only transient and resistance is almost inevitable, with up to 90% of the patients experiencing early disease recurrence[28]. Development of TMZ resistance in GBM is complicated and less understood, but till date, clear factors leading to resistance are still limited to pre-existing O6-methylguanine-DNA methyltransferase (MGMT)[29]. According to previous studies, the DNA damage response (DDR) can induce autophagy in an yeast system[30]and the enhanced autophagy can increase DNA repair ability to cause chemo-resistance[31]. In GBM, TMZinduced autophagy in glioma cell lines is dependent on MGMT expression, mismatch repair system and Rad51-mediated homologous recombination[32]. The glioma cell lines resistant to TMZ cotreated with O6-benzylguanine, an O6-alkylguanine-DNA alkyltransferase (a DNA repair enzyme) inhibitor, can be resusceptive to TMZ via autophagy regulation[33]. The cascades of TMZ-induced autophagy showed that the phosphorylation of H2AFX at Ser139 following TMZ treatment initiates DDR [Figure 2], sequentially,leading to phosphorylation of PRKAA (Thr172), ULK1 (Ser555/575), MAPK14 (The180/Tyr182), RPTOR(Ser792) and suppressive phosphorylation of AKT (Ser473) and mTOR (Ser2448) to induce autophagy and inhibit apoptosis[34]. However, further investigation is needed to examine whether there are other signaling pathways involved between autophagy and DDR.
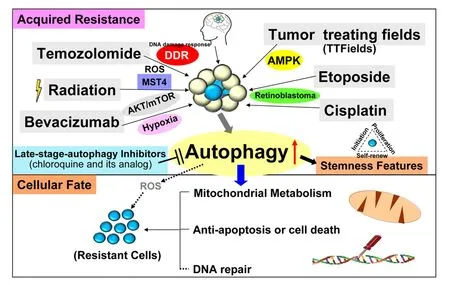
Figure 2. The schematic diagram demonstrates that in primary or recurrent GBM patients, the standard regimens consists of TMZ,radiation, TTFields, bevacizumab, etoposide and cisplatin, which enhance cell stemness, metabolism (energy), anti-apoptosis and DNA repair to acquire therapeutic resistance through the signaling pathways of DDR, ROS, hypoxia, AKT/mTOR, MST4 kinase, AMPK and RB.Nowadays, the inhibitors of late-stage autophagy, such as CQ or its analogs, have been tested in clinical trials of GBM patients. TMZ:temozolomide; GBM: glioblastoma; TTFields: tumor treating fields; ROS: reactive oxygen species; DDR: DNA damage response; AMPK:AMP-activated protein kinase; RB: retinoblastoma; CQ: chloroquine
In addition to MGMT that contributes to naïve TMZ resistance, the mechanism for tumor acquiring resistance is more complicated. In our previous studies, we identified transcriptional binding factor specific protein 1 and its downstream modulator, superoxide dismutase 2 (SOD2), as the critical factors in the tolerance of TMZ-induced ROS in models of resistance[35,36]. In fact, it has also been reported that TMZ can induce autophagy via ROS signaling[37][Figure 2]. The detailed mechanism between autophagy and ROS,however, needs further investigation. SOD2 is known to specifically function in mitochondria to regulate oxidative stress and energy metabolism[38]. Altered metabolic reprogramming by mitochondrial control in cancer may play a role in chemotherapy resistance[39]. For instance, increased autophagy derived from glucose starvation condition causes GBM cell quiescence, survival and chemoresistance[40]and complex I linked oxidative phosphorylation is required to maintain mitochondrial respiration (electron transfer system) through autophagy regulation[41]. Furthermore, presence of glioma stem-like cells may have crucial role in acquiring resistance[42]. An emerging concept in cancer biology suggests these specific subgroups with stemness features are responsible for acquisition of resistance[42]. The stem cells are characterized by self-renewal and multipotent abilities[43]. These allow cells to be resistant to standard therapy, and are associated with the treatment outcome[42]. In glioma stem-like cells, autophagy-associated proteins, i.e.,DNA Damage Regulated Autophagy Modulator 1 and p62, can regulate the cell migration and invasion by modulating energy metabolism (ATP) and affect mitochondrial morphology by regulating mitochondrial fusion[44]. Some studies have indicated that autophagy can regulate epithelial-to-mesenchymal transition[45]and the capabilities of migration and invasion in those cells under nutrient deprivation are damaged when autophagy is activated through regulation of ATG proteins or beclin 1[46]. However, it was found that TMZ treatment plus autophagy inhibitor could suppress cell migration and invasion[47], suggesting further investigation was needed to determine the role of TMZ-induced autophagy in this aspect. Moreover,stemness features in the glioma stem-like cells are reduced by autophagy inhibition and apoptosis is enhanced by blockage of autophagy[48]. Recently, it is found that the blockage of autophagy up-regulates the TMZ-sensitivity in these stem-like cells through metabolic dysfunction-related ferroptosis[49]in the stemlike cells, demonstrating the close correlation between autophagy and metabolism. In summary, application of these the explains how the disease is capable of surviving TMZ toxicity regardless of MGMT status.
RADIATION
Radiation can cause similar treatment response to TMZ. Radiation treatment in GBM cells can induce autophagy through the PI3K/AKT/mTOR pathway, ROS[50,51]and MST4 kinase-ATG4B signaling[52][Figure 2].Inhibition of autophagy makes these cells susceptible to stimulation[53,54]. Moreover, it is reported that GBM cells are susceptible to radiation following inhibition of MDA-9 expression[55]. Radiation has various effect to autophagy. Studies have indicated that the relatively radio-sensitive cells or small radiotherapy fraction (2 Gy) displays enhanced autophagic flux, while more radio-resistant cells or largeer radiotherapy fraction exhibited an inhibition of autophagic flux[56]. Regarding the glioma stem-like cells, it was reported the subsets were enhanced for autophagy after radiation to promote metabolism, anti-apoptosis and stemness[57], while the other reports showed that CD133+cells or sphere cells from one patient have a lower level of autophagy[58,59]. Notably, it is also found that CD133+glioma stem-like cells can be resensitized to radiation treatment by using autophagy inhibitors (bafilomycin) or down-regulation of ATG protein levels[60]. Though the exact effect remains unclear, the association between autophagy, radiation, and the stemness features is intriguing.
Whether targeting autophagy will benefit radiotherapy effect is also controversial. On one hand, a study has found that autophagy in malignant glioma cells is a transition status promoting apoptotic cell death following radiation, which can be reversed by suppressing autophagy function[61]. On the other hand, from the aspect of PI3K/AKT/mTOR signaling (it is active in most GBM patients[62]), Cernigliaet al.[63]and Gil del Alcazaret al.[64]have indicated that NVP-BEZ235, a dual PI3K/mTOR inhibitor, can promote GBM cells sensitive to radiation and up-regulate autophagy by directly affecting the function of DNA damage repair(DNA-dependent protein kinase catalytic subunit and ATM kinase) and autophagy related proteins (ATG5 and Beclin1), respectively. The results demonstrated that there are different responses after administrating the inhibitor and radiation leading to cell death. Furthermore, Zhuanget al.[65]also reported that rapamycin can lead to the differentiation of glioma stem-like cells, up-regulating the cell radiosensitivity and reducing tumorgenesis through activation of autophagy. However, the application of the autophagy inducers on mice experiment or clinical seems to be rare and limited in information[62,66]. In addition, most clinical trials nowadays prefer the combination of autophagy inhibitors with radiochemotherapy but not radiotherapy only[67]. Therefore, the directions of future research must focus on further exploration of how radiation induces autophagy (time point, tumor suppress genes, DNA damage repair and so on) and how the affected autophagy changes the cell fate.
TUMOR TREATING FIELDS
OPtune, a tumor treating fields (TTFields) device, is used as an advanced GBM treatment with only minor side effects noted in newly diagnosed or recurrent GBM patients. The device in combination with TMZ increased the median overall survival by almost 5 months compared to the TMZ alone group[68]. At 200 kHZ frequency, TTFields treatment has been reported to cause damaged mitosis and up-regulation of autophagy[69]. It is also found that the enhancement of autophagy is induced by TTFields treatment through AMP-activated protein kinase (AMPK) signaling[70][Figure 2] and down-regulation of the pathway inhibits the protective autophagy to reduce TTFields-induced acquired resistance. By combining with sorafenib,a multi-kinase inhibitor that can induce autophagy in prostate cancer cells through increased levels of LC3[71], the device exhibited enhanced autophagy and more inhibited tumor behavior to promote a better therapeutic strategy[72]. Regarding the mechanism, it was reported that TTFields can suppress DDR in nonsmall cell lung cancer cell lines[73]and the combination of radiation can inhibit DNA damage repair in glioma cell lines[74]. Furthermore, it is found that TTFields can cause ROS production to promote apoptosis in the cell line study[72]. This may have impact in inducing the clearance of damaged mitochondria(mitophagy)[75]. It is not clear, however, whether TTFields-increased autophagy is associated with inhibition of DNA damage repair. Further explorative experiments are thus needed to elucidate the mechanism and the role of autophagy in the disease-controlling mechanism of the device.
BEVACIZUMAB
Bevacizumab can directly promote apoptosis and antiangiogenesis by regulating the associated proteins in GBM cells[19,76]. This drug has been reported to cause resistance in the cells through suppressing AKT-mTOR signaling[76]or through inducing hypoxia-inducible factor-1α/AMPK pathway[19]to increase autophagy influx [Figure 2]. It was also reported that bevacizumab could cause a hypoxia microenvironment, which in turn, was related to enhanced autophagy[77,78]. Thus, inhibition of the autophagy process was postulated to resensitize bevacizumab to GBM cells.
Up-regulation of glucose metabolism before autophagy induction was observed in bevacizumab-treated GBM cell lines[79]. Further investigation is needed to link the mechanism between autophagy and the cell metabolism after the treatment. It was also noted that bevacizumab can induce autophagy in glioma stemlike cells by enhancing VEGF-independent angiogenesis (vasculogenic mimicry, an alternative vasculature)through KDR/VEGFR-2 phosphorylation, which the formation is closely related with GBM resistance[80].This highlights the therapeutic value of autophagy-inhibiting strategies in regard with the anti-angiogenesis therapy.
ETOPOSIDE
Etoposide (VP-16) treatment has been reported to induce autophagy, thereby increasing the therapeutic resistance in GBM cells[81,82]. The drug is a topoisomerase II inhibitor that also enhances autophagy by modulating though retinoblastoma protein (RB)[81][Figure 2]. Down-regulation of RB can inhibit autophagy by preventing autophagosomes fusion from lysosome and promoting cell death in GBM cells and glioma stem-like cells[81]. Furthermore, knockdown of RB can enhance apoptotic cell death and upregulate the immunostaining intensity of γ-H2AX (a DNA double-strand break marker) induced by etoposide[81]. Earlier studies have demonstrated a close link between etoposide-induced autophagy, RB, and DNA damage.
CISPLATIN
Cisplatin (CDDP) is a DNA alkylating agent that is widely used for human malignant tumors including GBM[83]. CDDP-associated resistance often develops through autophagy induction in multiple cancer cell types[84-87]. In cervical cancer cells, it is found that blockage of autophagy can increase CDDP-induced cytotoxicity via endoplasmic reticulum stress[88]. One study indicated that autophagy was associated with CDDP resistance in GBM and down-regulation of RB suppressed autophagy induced by CDDP[87][Figure 2]. Moreover,it was found that CDDP could induce long non-coding RNA to suppress CDDP-induced autophagy, which promoted apoptosis in GBM cells[89]. CDDP could also enhance the level of death receptor (DR5), directing glioma stem-like cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) apoptotic pathway[90]. TRAIL, on the other hand, was reported to enhance the expression of autophagy leading to resistance of breast cancer cells, and blockage of autophagy could reduce the resistance[91]. The association between autophagy and TRAIL in GBM has thus been inferred. In addition, the mechanism of DNA repair induced by CDDP could promote resistance of GBM cells via the Jun kinase/stress-activated protein kinase signaling pathway[92]. Further experiments are required to verify whether autophagy is related to the DNA repair induced by CDDP.
CARMUSTINE
Another DNA damaging nitrosoureas agent carmustine (BCNU) is reported to promote ROS-mediated autophagic cell death in solid tumor cells in combination with arsenic trioxide[93]. In terms of the role of ROS in the drug reaction, it was supportively reported that in human glioma cell line U98MG, the overexpression of Nrf 2, an anti-oxidant transcriptional factor, could reduce the carmustine-induced cytotoxicity[94]. How autophagy was utilized in carmustine-related oxidative stress and anti-oxidant reaction remains to be elucidated.
AUTOPHAGY INHIBITORS IN CLINICAL TRIAL
Traditionally, CQ is used for malaria treatment. Due to the basic property of CQ, it stays in lysosomes leading to enhanced lysosomal pH and inhibition of autophagy[95,96]. Hence, CQ has been investigated in clinical trials for cancer therapy including GBM. An early clinical trial of CQ in a small population of GBM patients (NCT00224978) showed that the median survival increased in patients receiving CQ in combination with standard regimens[97]. Currently, there are 3 ongoing clinical trials involving CQ in GBM or astrocytoma patients (NCT02378532 (Phase 1), NCT02432417 (Phase 2) and NCT03243461 (Phase 3).In addition to CQ, more candidate autophagy modulators are expected with or without the standard drugs to be investigated in clinical trials.
CONCLUSION
The current standard treatments of primary or recurrent GBM can cause autophagy induction and the enhanced autophagy through DDR, ROS, hypoxia, AKT/mTOR, AMPK and RB pathways may result in cell resistance, resulting in enhancement of stemness features, increased abilities of metabolism, anti-apoptosis,and DNA repair. Therefore, in order to reduce the resistance caused by treatments, the usage of standard treatment plus autophagy inhibitors and signaling inhibitors may be a potential strategy for GBM therapy.
DECLARATIONS
Authors’ contributions
Manuscript writing: Chien CH, Hsueh WT, Chuang JY, Chang KY Supervised the final version: Chang KY
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by grants from National Health Research Institutes, Taiwan (CA-107-PP-08) and the Ministry of Science and Technology, Taiwan (MOST 108-2314-B-400-026).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2019.
 Journal of Cancer Metastasis and Treatment2019年9期
Journal of Cancer Metastasis and Treatment2019年9期
- Journal of Cancer Metastasis and Treatment的其它文章
- AUTHOR INSTRUCTIONS
- Micromanaging autophagy with microRNAs to drive cancer metastasis
- Liquid biopsy in lymphomas: a potential tool for refining diagnosis and disease monitoring
- Membrane lipid binding molecules for the isolation of bona fide extraceIIuIar vesicIe types and associated biomarkers in Iiquid biopsy
- The immunological regulation of cancer cachexia and its therapeutic implications
