Assessment of cerebrovascular reserve impairment using the breath-holding index in patients with leukoaraiosis
Ying Bian , Jin-Chun Wang, Feng Sun, Zi-Yi Sun, Yu-Jiao Lin, Yang Liu Bin Zhao, Li Liu, Xiao-Guang Luo
1 Department of Neurology, the First Affiliated Hospital of China Medical University, Shenyang, Liaoning Province, China
2 Department of Neurology, the Fifth People's Hospital of Shenyang, Shenyang, Liaoning Province, China
3 Department of Endocrinology, Shengjing Hospital, China Medical University, Shenyang, Liaoning Province, China
Abstract Many studies have demonstrated that leukoaraiosis is associated with impaired cerebrovascular reserve function. However, the definitive hemodynamic changes that occur in leukoaraiosis are not clear, and there are many controversies. This study aimed to investigate hemodynamic changes in symptomatic leukoaraiosis using transcranial Doppler ultrasonography and the breath-holding test in a Chinese Han population, from northern China. A total of 203 patients who were diagnosed with ischemic stroke or clinical chronic progressive ischemic symptoms were enrolled in this study, including 97 males and 106 females, with an age range of 43-93 years. The severity of leukoaraiosis was evaluated according to the Fazekas grading scale, and patients were divided into four groups accordingly. Grade 0 was no leukoaraiosis, and grades I, II, and III were mild, moderate, and severe leukoaraiosis, respectively, with 44, 79, 44, and 36 cases in each group. Transcranial Doppler ultrasonography and the breath-holding test were performed. The mean blood flow velocity of the bilateral middle cerebral artery was measured and the breath-holding index was calculated. The breath holding index was correlated with leukoaraiosis severity and cognitive impairment. Patients with a low breath holding index presented poor performance in the Montreal Cognitive Assessment (MoCA) and executive function tests. That is, the lower the breath holding index, the lower the scores for the MoCA and the higher for the trail-making test Parts A and B. These results indicate that the breath-holding index is a useful parameter for the evaluation of cerebrovascular reserve impairment in patients with leukoaraiosis. In addition, the breath-holding index can reflect cognitive dysfunction, providing a new insight into the pathophysiology of leukoaraiosis. This study was approved by the Ethics Committee of the Fifth People's Hospital of Shenyang, China (approval No. 20160301) and registered in the Chinese Clinical Trial Registry (registration number:ChiCTR1800014421).
Key Words: nerve regeneration; cerebral small vascular disease; white matter hyperintensities; cerebral hemodynamics; cerebral hypoperfusion;middle cerebral artery; blood flow velocity; breath-holding test; breath-holding index; cognitive function; neural regeneration
Graphical Abstract
Introduction
The incidence of ischemic cerebrovascular disease, and especially cerebral small vessel disease, is increasing year by year,and is a global health issue. Leukoaraiosis (Hachinski et al.,1987), also known as white matter hyperintensity and white matter lesions (Wardlaw et al., 2013), is the most common imaging sign of subcortical small vessel disease (Wallin et al.,2018), and has attracted much attention from researchers.A large number of studies have been carried out to explore the pathogenesis of leukoaraiosis, especially hemodynamic changes. Investigators have used various neuroimaging modalities, including positron emission tomography/computed tomography, single-photon emission computed tomography, and functional magnetic resonance imaging (MRI) (van der Veen et al., 2015; Cheng et al., 2017). Arba et al. (2017)observed intracranial hypoperfusion in patients with leukoaraiosis using computed tomography (CT) and MRI, and found that this hypoperfusion was correlated with the severity of leukoaraiosis (Arba et al., 2017). In addition, some researchers have found a relationship between white matter disconnection and cognitive impairment in leukoaraiosis patients using magnetic resonance diffusion tensor imaging.Based on these studies, it has been proposed that diffusion tensor imaging parameters may be potential predictors for cognitive function impairment, and that cognitive function changes are related to impaired white matter integrity rather than reduced cerebral blood supply (Wang et al., 2017; Yuan et al., 2017; Zhong et al., 2017).
The hemodynamic changes observed in patients with leukoaraiosis are yet to be elucidated. Transcranial Doppler ultrasonography is a non-invasive and low-cost method for hemodynamic monitoring. In previous studies, Turk et al.(2016a) found that patients with leukoaraiosis had a higher pulsatility index and higher resistance index compared with normal controls. Additionally, the blood flow velocity(BFV) of the middle cerebral artery was reduced with carotid atherosclerosis progression (Turk et al., 2016a). However,current studies are mainly limited to measuring hemodynamic features in the resting state. As a crucial metabolic factor, carbon dioxide can stimulate vasoconstriction and vasodilatation. Thus, we speculated that the measurement of hemodynamic parameters during the breath-holding test may be a sensitive indicator for cerebrovascular reactivity.Some researchers have investigated the correlation between the breath-holding index (BHI) and cognitive impairment in patients with multiple cerebral infarction and Alzheimer's disease (Matteis et al., 1998). However, there is currently no uniform standard for testing hemodynamic impairment in leukoaraiosis. To the best of our knowledge, this is the first transcranial Doppler ultrasonography study to reveal hemodynamic changes during the breath-holding test in symptomatic leukoaraiosis patients of Han ethnicity.
This study will therefore evaluate the relationship between the BHI and image performance in patients with leukoaraiosis of Han ethnicity in northern China, and explore the BHI's relationship with cognitive and executive functions.From a hemodynamics perspective, this study aims to analyze the pathogenesis of patients with symptomatic leukoaraiosis, and to explore the simple and convenient method of intracranial hypoperfusion in leukoaraiosis, to provide a good theoretical basis for clinical diagnosis and treatment.
Participants and Methods
Design
This was a cross-sectional study.
Participants
A total of 203 patient s of Han ethnicity were enrolled from the Department of Neurology in the Fifth People's Hospital of Shenyang, China, between November 2016 and October 2017. This study was approved by the Ethics Committee of the Fifth People's Hospital of Shenyang, China (approval No.20160301) and registered in the Chinese Clinical Trial Registry(registration number: ChiCTR1800014421). Written informed consent was obtained from each participant.
Inclusion criteria
(1) Patients were aged 43-93 years, and were either diagnosed with ischemic stroke or showed clinical chronic progressive ischemic symptoms including dizziness, headache,aphasia, numbness, and memory loss (Podgorska et al.,2002; Jokinen et al., 2009; Debette and Markus, 2010; Brickman et al., 2011). (2) Depression (Longstreth et al., 1996;Teodorczuk et al., 2010) and gait instability (Longstreth et al., 1996; Baezner et al., 2008; Srikanth et al., 2009). Leukoaraiosis was diagnosed based on brain MRI. Patients agreed to undergo MRI, transcranial Doppler ultrasonography, and the breath-holding test. (3) There was no more than 50%stenosis of the middle cerebral artery, vertebrobasilar artery,or intracranial or external carotid artery. (4) Leukoaraiosis was diagnosed based on brain MRI (Fazekas et al., 1987).
Exclusion criteria
(1) Contraindications to brain MRI; (2) severe cerebrovascular disease sequelae, such as a disturbance of consciousness or visual impairment meaning that the patient cannot complete behavioral assessments; (3) severe respiratory, cardiovascular, or hepatorenal disease; (4) white matter damage or disease, such as subcortical arteriosclerotic encephalopathy,autoimmune white matter demyelination, hypoxic encephalopathy, normal intracranial pressure hydrocephalus, hypoglycemia- or intoxication-induced encephalopathy, multiple sclerosis, or hereditary white matter disease; (5) a disability preventing the Valsalva maneuver; (6) an intracranial infarction lesion with diameter > 1.5 cm; or (7) moderate or severe stenosis (> 50%) of the intracranial or extracranial carotid artery, middle cerebral artery, or vertebrobasilar artery.
The individual medical profiles were documented, including age, gender, and history of smoking and drinking,hypertension, diabetes, coronary heart disease, and previous stroke. Additionally, multiple laboratory examinations were performed, including routine blood and urine tests, as well as tests for liver and renal functions, thyroid function, and immunity. An electrocardiography, carotid artery ultrasound, head CT, and head MRI were performed.
Radiological evaluation
Brain MRI, including T1-weighted, T2-weighted, fluid attenuated inversion recovery, and magnetic resonance angiography sequences, was performed in all patients using a General Electric 1.5T MRI scanner (GE Healthcare, Waukesha, WI, USA). The MRI data were independently reviewed by two radiologists and one neurologist. The clinical severity of leukoaraiosis was evaluated according to the Fazekas scale: no leukoaraiosis (grade 0), mild leukoaraiosis (grade I),moderate leukoaraiosis (grade II), and severe leukoaraiosis(grade III) (Fazekas et al., 1987).
Cognitive assessment
Individual cognitive function was assessed using identical versions of the Montreal Cognitive Assessment (MoCA),and cognitive impairment was defined as a score of < 26(Pendlebury et al., 2010, 2012). Executive function was assessed using the trail-making test Part A (TMTA) and Part B(TMTB) (Reitan, 1955). TMTA and TMTB were measured in seconds, and tests were interrupted after 6 minutes if they were not completed. Double-blind assessments were independently performed by two neurologists.

Table 1 Clinical characteristics and laboratory examination results of leukoaraiosis patients
Transcranial Doppler ultrasonography
Conventional transcranial Doppler ultrasonography tests were performed to exclude patients with moderate or severe intracranial vascular stenosis (> 50%). Transcranial Doppler ultrasonography was performed before the breath-holding test. Blood flow signals were detected using a 2.0 MHz pulse probe at a depth of 50-60 mm via the left temporal window.When the unilateral common carotid artery was compressed along the anterior border of the sternocleidomastoid muscle,the ipsilateral middle cerebral artery BFV was obviously decreased. The baseline BFV (BFVbaseline) was measured in the resting state, and the mean BFVbaselineof the bilateral middle cerebral artery was calculated. Afterwards, the patient was told to take a deep breath and then hold the breath for at least 20 seconds. The breath-holding time and the mean BFV after the breath-holding test (BFVafter) of the bilateral middle cerebral artery were documented. The BHI was calculated by (BFVafter-BFVbaseline) / (BFVbaseline× breath-holding time) × 100.
Statistical analysis
SPSS 24.0 software (IBM, Armonk, NY, USA) was used to perform statistical analyses. Enumeration data are presented as percentages and were analyzed using the chi-square test, while non-normally distributed data are expressed as the median.Quantitative data with non-normal distributions and heterogeneity of variance were compared using the Kruskal-Wallis test. P values ≤ 0.05 were considered statistically significant.
Results
Clinical characteristics and laboratory parameters
The patients' ages ranged from 43 to 93 years. Among the four groups, as classified according to the Fazekas grading system, there was no significant difference in gender(P = 0.056), smoking history (P = 0.314), hypertension (P= 0.448), or diabetes (P = 0.918); however, older age (P =0.006), coronary heart disease (P = 0.000), and stroke history(P = 0.004) were identified as risk factors for leukoaraiosis.
Among the four groups, there were no differences in the levels of serum total cholesterol (P = 0.090), triglyceride (P= 0.551), low-density lipoprotein (P = 0.522), high-density lipoprotein (P = 0.317), apolipoprotein A/B (P = 0.307),apolipoprotein A1 (P = 0.670), glycosylated hemoglobin (P= 0.825), or homocysteine (P = 0.708). The statistical results are summarized in Table 1.
Cognitive functions
Individual MoCA scores ranged from 9 to 29 [median/interquartile range (Q3-Q1): 26/9]. Pairwise comparisons revealed no differences in MoCA scores between the leukoaraiosis grade II and grade III groups (P = 0.473) or between the grade 0 and grade I groups (P = 1.000). The MoCA scores in the grade 0 group were significantly higher than in the grade II(P = 0.000) and grade III (P = 0.000) groups, and the MoCA scores in the grade I group were also significantly higher than the grade II (P = 0.000) and grade III (P = 0.000) groups.
Individual TMTA scores ranged from 30 to 170 [median/interquartile range (Q3-Q1):57/47]. Individual TMTB scores ranged from 40 to 390 [median/interquartile range(Q3-Q1):128/129]. Pairwise comparisons showed no difference in the TMTA or TMTB scores between grade II and grade III groups (TMTA: P = 0.685; TMTB: P = 0.364) or between grade 0 and the grade I groups (TMTA: P = 1.000;TMTB: P = 1.000). However, TMTA and TMTB scores in the grade 0 group were significantly lower than in the grade II (P = 0.000) and grade III (P = 0.000) groups, and TMTA and TMTB scores in the grade I group were also significantly lower than in the grade II (P = 0.000) and grade III (P =0.000) groups. The cognitive evaluation results are summarized in Table 2.
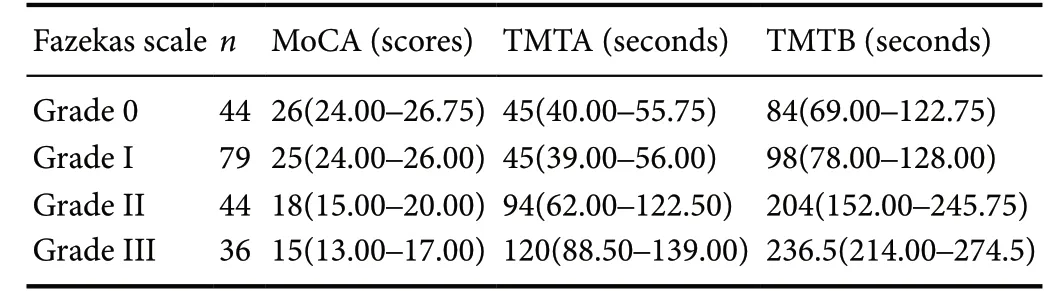
Table 2 Cognitive evaluation results of leukoaraiosis patients
Transcranial Doppler ultrasonography examination
Pairwise comparisons showed no difference in BHI between the grade II group and the grade III group (P = 0.924) or between the grade 0 group and the grade I group (P = 0.244).The BHI in the grade 0 group was significantly higher than in the grade II (P = 0.000) and grade III (P = 0.000) groups.The BHI in the grade I group was significantly higher than in the grade II (P = 0.000) and grade III (P = 0.000) groups.The results are displayed in Figure 1.
To analyze any correlation of the BHI with cognitive and executive functions (i.e., the MoCA, TMTA, and TMTB scores) in patients with leukoaraiosis, the average BHI value(0.84) was used as a reference value and the patients were divided into two groups. The lower BHI group comprised patients with a BHI ≤ 0.84, and the higher BHI group comprised patients with a BHI > 0.84. Nonparametric tests revealed that BHI was negatively correlated with leukoaraiosis severity (P < 0.05) and significantly correlated with cognitive impairment (P < 0.05).
Discussion
In this study, the breath holding test was used to observe that as the degree of leukoaraiosis was aggravated, the BHI decreased, and the hemodynamics of leukoaraiosis changed. Our results demonstrated that symptomatic leukoaraiosis showed intracranial hypoperfusion, thus indicating that the BHI can be used to assess impaired brain reserve function in leukoaraiosis. However, currently the BHI is only an indicator used by scholars to explore the dynamic changes of blood flow. There is no uniform diagnostic standard for leukoaraiosis.
Recently, several researchers have suggested that transcranial Doppler ultrasonography can be used as a non-invasive and sensitive approach to evaluate the functional status of intracranial vessels (both large arteries and downstream microvessels) (Fisse et al., 2016; Malojcic et al., 2017). Relevant studies in vascular dementia patients have also noted that cognitive dysfunction may be related to impaired cerebrovascular reserve function (Tsivgoulis et al., 2014). These findings indicate a potential use for hemodynamic monitoring in the risk assessment of leukoaraiosis and cognitive impairment. The BHI reflects the regulatory function of cerebral vessels, but at present, BHI changes in leukoaraiosis patients are controversial.
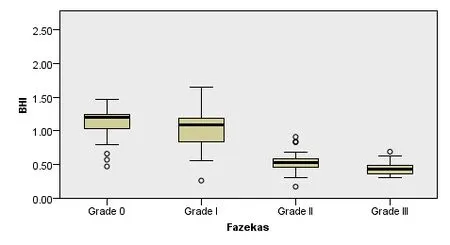
Figure 1 Comparison of breath-holding index (BHI) between each leukoaraiosis grade.**P < 0.01, vs. Grade I and II groups. Non-normally distributed data are expressed in lower quartile, median, and upper quartile. Quantitative data were compared using the Kruskal-Wallis test.
Zavoreo et al. (2010) enrolled 60-70-year-old elderly patients with mild cognitive impairment, and compared them with healthy young (30-40 years) and elderly (60-70 years)controls. Results showed that there were no statistically significant differences in BHI between the three groups.However, the regression analysis demonstrated that BHI was strongly correlated with MoCA score after controlling for age and the risk factors of cerebrovascular disease. It was therefore suggested that the BHI be used as a parameter of impaired cerebrovascular reactivity for small vessel cerebrovascular disease in the early stages of cognitive dysfunction(Zavoreo et al., 2010). In addition, Peisker et al. (2010) conducted a breath-holding test in people of different ages. They observed that as age increased, the regulation of cerebral blood vessels decreased, and correlated with leukoaraiosis severity. In addition, leukoaraiosis was associated with the resistance index. Decreasing BHI can indicate a patient's changed cerebral blood flow and brain reserve dysfunction(Peisker et al., 2010). More recently, Inhalakham et al. (2017)performed a breath-holding test in 15 patients with lacunar infarction to observe changes in BFV, heart rate, and blood pressure in the middle cerebral artery. The results showed that sympathetic nerve function in patients with ischemic cerebral infarction is decreased (Intharakham et al., 2017).Whether this phenomenon is due to the pathogenesis of lacunar infarction or hypertension still needs further research.However, there was no significant difference in the history of hypertension between the four groups in this study.Therefore, the decrease in BHI is not associated with hypertension, but it is independently associated with the decrease in brain reserve function in leukoaraiosis.
However, conflicting results have also been reported.To verify the ability of the autonomic nervous system in maintaining a steady cerebral physiological function in the elderly, Bohr et al. (2015) enrolled 45 elderly patients (75-89 years old) to perform the Valsalva maneuver, and used twoway echo functional MRI to evaluate the effective transverse relaxation rate (R2) and calculate the volume of leukoaraiosis. Although there was an overlap between the leukoaraiosis area and the excitatory deoxidization area, there was no significant correlation between these independent variables after statistical analyses. The authors suggested that transient-repeated hypoxia does not give a high signal of white matter in the brain, so the study does not support the cumulative effect of autonomic dysfunction on the pathogenesis of white matter hyperintensity (Bohr et al., 2015). In addition,Fisse et al. (2016) identified patients with autosomal dominant cerebral arterial disease with subcortical infarction and leukoencephalopathy by recording transcranial Doppler ultrasonography parameters after a breath-holding test. There was no significant difference between pulsatility index, resistance index, and BHI in the normal control group. In addition, Birns et al. (2009) observed changes in blood pressure and BFV in the middle cerebral artery in 64 patients with small vessel disease (lacunar infarction and leukoaraiosis)after inhalation of 8% CO2. The results demonstrated that cerebral autoregulation function and CO2reactivity are two different processes that are not related to white matter lesions, but instead are related to blood pressure levels and the course of hypertension. Combined with previous methods,MRI and gas inhalation are not feasible, and the measurement criteria are inconsistent. In contrast, transcranial Doppler ultrasonography and the breath-holding test are simple and easy. Therefore, this study was designed to assess the relationship between BHI and leukoaraiosis.
The current study is innovative for a number of reasons.We enrolled patients with symptomatic leukoaraiosis, including acute ischemic stroke and chronic progression of ischemic symptoms. The subjects were restricted to the category of small vessel disease (lesion diameter less than 1.5 cm). In contrast, previous international studies included community health groups, lacunar infarction, or mild cognitive impairment patients. In addition, in the present study, patients with leukoaraiosis were grouped using a semiquantitative classification in an attempt to match the confounding factors of each group, so that these factors did not influence the conclusion. However, some international studies did not match all confounding factors.
This study demonstrates that the BHI is negatively correlated with leukoaraiosis severity, suggesting that cerebrovascular reserve is impaired in patients with leukoaraiosis.When the subject takes a deep breath, oxygen is consumed in the body and the arterial carbon dioxide partial pressure increases, leading to vasodilatation and increased BFV (Ju et al., 2017). It has been suggested that these hemodynamic changes lead to intracranial hypoperfusion (Mok et al., 2012;Poels et al., 2012; Nasel et al., 2017), resulting in cognitive dysfunction (Lin et al., 2017). Indeed, statistical analyses confirmed that the severity of leukoaraiosis was strongly correlated with MoCA and executive function scores. These results are consistent with the findings of Sivakumar et al.,who observed a correlation between leukoaraiosis severity and the level of subcortical cognitive impairment following stroke (Sivakumar et al., 2017).
In the present study, there were acute stroke episodes and chronic ischemic progression, and the study group was based on the imaging of leukoaraiosis. Leukoaraiosis is a subtype of small vessel disease often accompanied by lacunar infarction, and presents with multiple clinical manifestations (Nam et al., 2017). When patients with leukoaraiosis experience long-term hypertension, diabetes, and other cerebrovascular diseases, arterial hardness increases (Hainsworth et al., 2017), and cerebrovascular arterial stenosis results in impaired cerebrovascular autoregulation. Many previous studies showed that the hemodynamic changes in leukoaraiosis were modified; the BFV of the middle cerebral artery decreased, while the pulsatility index value and resistance index value increased (Turk et al., 2016a), whether it was acute (Arba et al., 2017), subacute (Webb et al., 2012), or chronic (Turk et al., 2016a). BHI is strongly associated with BFV. When blood vessels are stimulated by systolic and diastolic stimulation,the cerebral BFV changes, and the cerebrovascular reactivity is determined by the degree of these changes (Smolinski and Czlonkowska, 2016). In the present study, there was no significant difference in BHI and cognitive function when there was no image change or only a mild lesion of white matter(i.e., grades 0 or I). Nevertheless, when there were early confluent and flaky confluent lesions (i.e., grades II and III), the patients' cognitive and executive function impairments were obvious, and there was a significant difference in the BHI between grades 0, I and grades II, III. One possible explanation for this result is that tissue structure damage of small vessel disease is homogeneous. When the white matter lesion is mild, the changes in abnormal and normal structures are less obvious. However, as leukoaraiosis gradually worsens, intracranial tissue structure and cerebrospinal fluid permeability increases, and then the blood brain barrier is damaged (Valdes Hernandez et al., 2017), leading to vascular endothelial injury (Tan et al., 2017), oxidative stress (De Silva and Miller,2016), cerebral arteriosclerosis (Hainsworth et al., 2017), and intracranial hypoperfusion (Maccarrone et al., 2017). Therefore, our results suggest that the pathological mechanism of leukoaraiosis is multivariate (Traylor et al., 2016). Small spot lesions are associated with confounding etiology, and leukoaraiosis with confluent lesions is related to small vessel disease (Traylor et al., 2016). However, the exact pathogenesis and pathological changes are still unclear. It is necessary to expand the sample size for further clinical and basic research in the future.
Additionally, Lin et al. (2017a) proposed that subependymal leukoencephalopathy is pathologically characterized by ependymal damage and might be caused by chronic ischemia of arterioles. Recent studies also demonstrate hemodynamic changes in patients with leukoencephalopathy(Turk et al., 2016a,b). It is well understood that cerebral blood flow is regulated by blood pressure, as well as neurogenic and metabolic factors (Hamel, 2006). In populations with hypertension, diabetes, or cerebrovascular diseases, arteriosclerosis and/or arteriostenosis can reduce cerebrovascular reactivity, and thus increase the risk for leukoaraiosis(Hainsworth et al., 2017). Our study demonstrates that older age, coronary heart disease, and previous stroke history were strongly correlated with leukoaraiosis severity in this sample of patients, which is consistent with the above hypothesis.
There are some limitations to the present study. One limitation is the sample size, which was relatively small. Some patients could not cooperate with the breath-holding test and neurological assessment, for reasons such as poor penetration of the temporal window, aphasia, poor hearing or vision, or severe cognitive impairment. In addition, some patients could not undergo MRI scanning, for various reasons. Another limitation of our study is that the leukoaraiosis grading method was only semi-quantitative; quantitative morphometry was not performed. An additional limitation is that the patients' age and risk factors for cerebrovascular disease, such as stroke history and coronary heart disease,were not completely matched to eliminate the effects of various confounding factors. Finally, this study had a cross-sectional design, and there were no long-term clinical observational data. Therefore, further clinical studies with a large cohort that include long-term longitudinal observations are needed to provide a reliable theoretical basis for the clinical prevention and treatment of leukoaraiosis.
The BHI is an indicator reflecting the impairment of cerebrovascular reserve in patients with leukoaraiosis, and the BHI is associated with cognitive dysfunction. This study provides a new insight into the pathophysiology of leukoaraiosis.
Acknowledgments: The authors thank all of the patients for their trust and cooperation, thank Xiu-Ying Wang from the Department of Neurology, the Fifth Hospital of Shenyang, China for assistance in clinical data collection.
Author contributions:YB drafted the manuscript. YB, BZ, LL, and YJL collected and analyzed the data. ZYS reviewed the literatures and organized the data. XGL, FS, and JCW conceived and designed the study.YJL performed the transcranial Doppler ultrasonography. All authors have read and approved the final version of the paper.
Conflicts of interest: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support:None.
Institutional review board statement:The study was approved by the Ethics Committee of the Fifth People's Hospital of Shenyang, China(approval No. 20160301). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the forms the patients or their legal guardians have given their consent for patients' images and other clinical information to be reported in the journal. The patients or their legal guardians understand that the patients' names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of the First Affiliated Hospital of China Medical University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures,and appendices) will be in particular shared. Study protocol form will be available. The data will be available immediately following publication without end date. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Improvement of ataxia in a patient with cerebellar infarction by recovery of injured cortico-ponto-cerebellar tract and dentato-rubro-thalamic tract: a diffusion tensor tractography study
- Tandem pore TWIK-related potassium channels and neuroprotection
- Dendritic shrinkage after injury: a cellular killer or a necessity for axonal regeneration?
- Regenerative biomarkers for Duchenne muscular dystrophy
- Exploring the efficacy of natural products in alleviating Alzheimer's disease
- Role of macrophages in peripheral nerve injury and repair

