Exploring the efficacy of natural products in alleviating Alzheimer's disease
Prajakta Deshpande, Neha Gogia, Amit Singh,
1 Department of Biology, University of Dayton, Dayton, OH, USA
2 Premedical Program, University of Dayton, Dayton, OH, USA
3 Center for Tissue Regeneration and Engineering at Dayton (TREND), University of Dayton, Dayton, OH, USA
4 The Integrative Science and Engineering Center, University of Dayton, Dayton, OH, USA
5 Center for Genomic Advocacy (TCGA), Indiana State University, Terre Haute, IN, USA
Abstract Alzheimer's disease (hereafter AD) is a progressive neurodegenerative disorder that affects the central nervous system. There are multiple factors that cause AD, viz., accumulation of extracellular Amyloid-beta 42 plaques, intracellular hyper-phosphorylated Tau tangles, generation of reactive oxygen species due to mitochondrial dysfunction and genetic mutations. The plaques and tau tangles trigger aberrant signaling, which eventually cause cell death of the neurons. As a result, there is shrinkage of brain, cognitive defects, behavioral and psychological problems. To date, there is no direct cure for AD. Thus, scientists have been testing various strategies like screening for the small inhibitor molecule library or natural products that may block or prevent onset of AD. Historically, natural products have been used in many cultures for the treatment of various diseases. The research on natural products have gained importance as the active compounds extracted from them have medicinal values with reduced side effects, and they are bioavailable. The natural products may target the proteins or members of signaling pathways that get altered in specific diseases.Many natural products are being tested in various animal model systems for their role as a potential therapeutic target for AD, and to address questions about how these natural products can rescue AD or other neurodegenerative disorders. Some of these products are in clinical trials and results are promising because of their neuroprotective, anti-inflammatory, antioxidant, anti-amyloidogenic, anticholinesterase activities and easy availability. This review summarizes the use of animal model systems to identify natural products,which may serve as potential therapeutic targets for AD.
Key Words: Alzheimer's disease; amyloid-beta 42; natural products; Lunasin; neuroprotective; anti-inflammation;antioxidant; Drosophila; cell death; neurodegeneration
Introduction
In 1906, Dr. Alois Alzheimer, first described shrinkage of the brain in the autopsy of the patient who suffered from increasing short-term memory loss, paranoia and deteriorating cognitive abilities. In 1968, the damaged brain tissue in elderly subjects was measured using the newly developed cognitive measurement scales (Tomlinson et al., 1968). Alzheimer's disease (AD), a senile dementia, is an age-related neurodegenerative disorder. Based on the time and cause of onset of AD, epidemiologists have clinically divided AD into: the early onset before 65 years, late-onset group consist of patients with the age 65 or above and familial AD (Rajan et al., 2018). The prevalence of AD in 2017 is 44 million worldwide. It is estimated to be around 75 million worldwide in 2030 (Selkoe and Hardy, 2016). AD is the sixth leading cause of mortality. It is estimated that AD will be a global epidemic by 2050, when the number of individuals affected by AD will soar to 135 million (Alzheimer's Association, 2018). AD manifests in the patients as declining cognitive and mental abilities due to the loss of neurons in the hippocampus and the cortex (Hardy, 2009; Sarkar et al.,2016). Neurons communicate with each other via synapses,which makes it plausible to recall the memories. The loss of synapses in AD results in loss of learning and memory.Clinically, AD patients face difficulty in short term memory,word finding, and language, which results in memory loss and slow progression of cognitive impairment (Albert et al.,2011).
AD is caused by multiple mechanisms including excessive accumulation of extracellular amyloid-beta 42 (Aβ42)plaques, intracellular hyper-phosphorylated tau neurofibrillary tangles in the brain, oxidative stress due to mitochondrial dysfunction, and/ or genetic as well as the environmental factors (Figures 1 and 2) (Selkoe and Podlisny,2002; Hardy, 2009; Sarkar et al., 2016; Selkoe and Hardy,2016; Sierra-Fonseca and Gosselink, 2018). Genetically the mutation in apolipoprotein E, which regulates the lipid homeostasis and carries the cholesterol to neurons, is a risk factor for the late-onset of AD. Oxidative stress results from generation of reactive oxygen species (ROS) (Chen et al.,2012; Sarkar et al., 2016). The Aβ42 plaques can form due to inappropriate cleavage of Amyloid precursor protein(APP), a transmembrane glycoprotein, encoded by the APP gene on chromosome 21. Generally, APP is cleaved proteolytically by α-secretase and γ-secretase to form 40 amino acid long peptide, however, when miscleaved by β-secretase and γ-secretase results in Aβ42 polypeptides (Figure 3A)(O'Brien and Wong, 2010; Selkoe and Hardy, 2016). These misfolded Aβ42 polypeptides accumulate to form amyloid plaques, which triggers neurodegeneration by activating aberrant signaling (Figure 3B) (Shankar et al., 2008; O'Brien and Wong, 2010; Fernandez-Funez et al., 2013; Sarkar et al., 2016;Selkoe and Hardy, 2016). Hyper-phosphorylation of tau, a protein involved in the stability of microtubules, decreases tau's association to microtubules, results in accumulation of toxic insoluble intracellular neurofibrillary tangles (Figure 1)(Gendron and Petrucelli, 2009; Sierra-Fonseca and Gosselink,2018). The existing clinical regimen for AD offer transient symptomatic improvement but offer little or no benefit in terms of changing the overall course of disease. Lately, there have been disappointing results from AD drug trials (Goldman et al., 2018). These widespread AD drug trial failures are due to lack of diversity in novel targets and a lack of reliable and effective preclinical models. It is imperative that there is a need for a more robust, diversified drug development pipeline and screening animal models to find cures for AD.
Animal Models
The genetic machinery is highly conserved across the species.As a result, several animal models for AD have been developed to understand the mechanism of disease progression,and to test the potential targets of AD (Pandey and Nichols,2011; Fernandez-Funez et al., 2013; Saito and Saido, 2014;Sarkar et al., 2016). Among vertebrates, the mouse model has been used extensively since they have similarities with the human brain (Di Carlo, 2012; Sasaguri et al., 2017). Both loss-offunction and gain-of-function mouse models have been generated using the genes responsible for AD. Early AD mouse model was generated where mutant APP was expressed that progressively manifested AD neuropathology (Games et al.,1995). Later, a combination type model system including Tau and Psen1 was generated in APP mouse background (Oddo et al., 2003). It was modified further where human sequence was introduced into the APP mouse to better understand AD pathology in humans (Saito et al., 2014). These model systems helped in discerning the learning and memory defects associated with AD by using behavioral assays. One of the major challenges was lack of concordance between preclinical models and human clinical trials (LaFerla and Green, 2012). As a result the drugs, which successfully inhibited the amyloid plaques in mice, failed to cure AD in humans (King, 2018).Majority of the data generated in mouse was from young animals, when their immune system is robust unlike the old ones. Thus, mouse models reflects early stages of AD. The reason clinical trial are failing because drugs that target Aβ42 in mouse model most probably treat early plaque build-up process rather than late, which is unlike the stage when AD is detected in humans. Thus, those drugs might fit prevention than treatment paradigm (King, 2018).
The other common animal models to study underlying mechanisms of AD are Drosophila melanogaster (fruit fly),Caenorhabditis elegans (worm), Danio rerio (zebrafish), and sea urchins (Iijima and Iijima-Ando, 2008; Iijima-Ando and Iijima, 2010; Pandey and Nichols, 2011; Singh, 2012; Singh and Irvine, 2012; Fernandez-Funez et al., 2013; Prussing et al., 2013; Saito and Saido, 2014; Sarkar et al., 2016). Caenorhabditis elegans, a nematode, has short life-span, and has been established as a simple genetic model system (Di Carlo,2012; LaFerla and Green, 2012). Transgenic lines were generated in nematode to study Aβ toxicity by using learning and behavior assays (Steinkraus et al., 2008). Zebrafish is a good genetic model system due to its optical clarity during developmental process of embryogenesis (Di Carlo, 2012),and genome is sequenced. This zebrafish possess genes orthologous to those mutated in familial AD, which makes it a useful model system to study AD (Newman et al., 2014).
Drosophila melanogaster, an invertebrate, has developed into an excellent model for human disease. The entire genome of Drosophila is sequenced, and the genetic machinery is highly conserved between flies and humans (Lenz et al.,2013). The fly genome has less genetic redundancy as compared to the humans (Bier, 2005; Lenz et al., 2013). Nearly 70% of fly genes are closely related to the human disease genes (Bier, 2005; Singh and Irvine, 2012). Therefore, using fruit fly to model human disease is practically beneficial.In addition, they are smaller in size and thus can be stored efficiently, and are cost effective. Drosophila can produce two generations in a month as the life cycle is short, and has a repertoire of genetic tools (Sarkar et al., 2016). In addition, the transgenic system is well established, which allows introduction of genes from other organism in the flies. It makes Drosophila an ideal model for studying age related progressive diseases like AD. Several models of AD have been developed in Drosophila. These include transgenic flies misexpressing human Aβ42, human Aβ40, human APP,β-site APP-cleaving enzyme, β-secretase and Presenilin, and Familial AD (Cauchi and van den Heuvel, 2006; Cao et al.,2008; Iijima and Iijima-Ando, 2008; Tare et al., 2011; Fernandez-Funez et al., 2013). These flies display several aspects of clinical AD neuropathology and symptoms including the generation of amyloid aggregates, external morphological abnormalities, dramatic neuroanatomical changes and defects in motor reflex behavior and memory. As a proof of concept, treatment with an inhibitor of γ-secretase can suppress these neurodegenerative phenotypes. Drosophila has also been used to model other neurodegenerative diseases like Parkinson's disease, Huntington's disease, fronto-temporal lobar degeneration, and amyotrophic lateral sclerosis(Bier, 2005; Pandey and Nichols, 2011; Singh and Irvine,2012; Fernandez-Funez et al., 2013).
Lately, Drosophila eye has also been extensively used for modeling human disease (Singh and Irvine, 2012; Moran et al., 2013; Steffensmeier et al., 2013). Drosophila has a fully functional nervous system where function of vision, olfaction, learning and memory can be separated. Furthermore,the genes involved in eye development are structurally and functionally similar between flies and humans. The adult compound eye of Drosophila (Figure 4A), develops from a mono-layer epithelium housed in the larva and referred to as the eye-antennal imaginal disc (Ready et al., 1976; Singh and Choi, 2003; Singh et al., 2005a, 2012; Kumar, 2011; Tare et al., 2013a). The larval eye imaginal disc metamorphose into a highly organized compound eye comprised of photoreceptor cells, cone cells, and pigment cells (Ready et al., 1976;Kumar, 2011, 2013; Singh et al., 2012; Tare et al., 2013a).The precise nature of the structure of Drosophila eye makes it highly sensitive to genetic manipulations that can be easily screened using a stereo microscope (Tare et al., 2013b; Singh et al., 2005b), which allows quick screening of large sample size (Singh and Choi, 2003; Singh et al., 2005a, b, 2006; Tare et al., 2013a). The Drosophila eye allows direct visualization of the cellular and developmental defects. Therefore, Drosophila eye can be used to mimic many neurodegenerative disorders including AD (Bier, 2005; Tare et al., 2011; Singh and Irvine, 2012; Moran et al., 2013; Cutler et al., 2015; Sarkar et al., 2016). Since it is easy to visualize the phenotypes in Drosophila eyes caused by neurodegeneration (Tare et al.,2013a), the Drosophila eye model can be used to study the different signaling pathways causing AD or other neurodegenerative disorders, and to screen for the therapeutic targets.
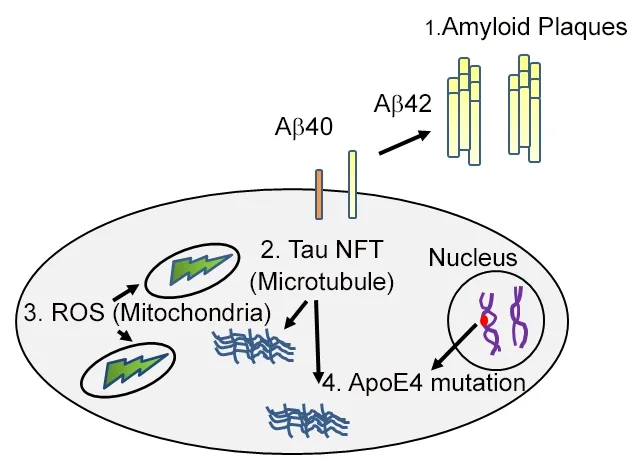
Figure 1 A cartoon showing the causes of Alzheimer's disease.Alzheimer's disease, a highly prevalent neurodegenerative disorder can be caused by: (i) Extra-cellular accumulation of amyloid-beta 42 (Aβ42)plaques; (ii) intra-cellular generation of hyper-phosphorylated Tau neurofibrillary tangles (NFT); (iii) generation of reactive oxygen species(ROS) in mitochondria; (iv) genetic basis of apolipoprotein E (ApoE4).

Figure 2 Schematic presentation of mechanisms by which natural products block Alzheimer's disease.Natural products can prevent neurodegeneration by reducing the oxidative stress, preventing the formation of neurofibrillary tangles(NFTs), or amyloid-beta 42 (Aβ42) plaque accumulation and thereby block Aβ42 mediated neurodegeneration.
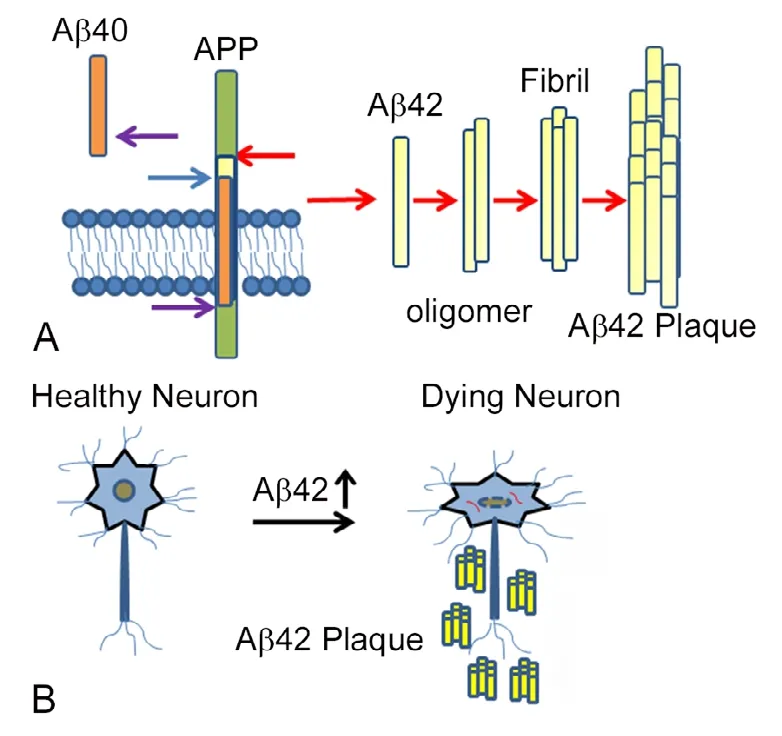
Figure 3 Accumulation of Aβ42 plaques triggers neurodegeneration.(A) Cartoon showing generation of amyloid-beta(Aβ)42 plaques by improper cleavage of a transmembrane protein, amyloid precursor protein (APP). Usually, APP is cleaved by α-secretase and γ-secretase to give Aβ40 peptide. If APP is cleaved by β-secretase and γ-secretase,then insoluble Aβ42 peptide is generated which forms Aβ42 oligomers and aggregate to Aβ42 fibrils and then amyloid plaques.(B) These Aβ42 get accumulated around the neurons, which triggers a neurodegeneration response. The molecular genetic mechanism of this process is not fully understood.
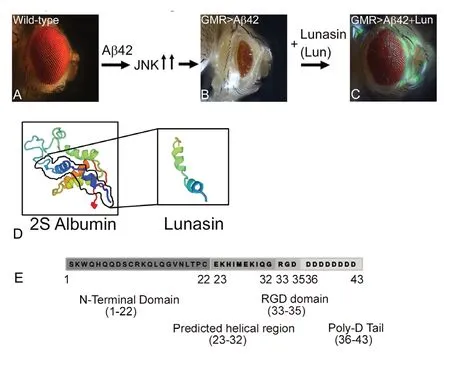
Figure 4 Novel neuroprotective response of soy protein Lunasin is mediated through downregulation of JNK signaling pathway.(A, B) Wild-type compound eye of adult fly (A), undergoes neurodegeneration upon misexpression of human Aβ42 polypeptide in the differentiating retinal neurons of the Drosophila eye using GMRGal4 driver (GMR>Aβ42) (B). Note that GMR>Aβ42 flies show highly reduced adult eye with glazed morphology. (C) Misexpression of soy protein Lunasin (Lun) along with human Aβ42 (GMR>Aβ42+Lun)in the developing Drosophila eye results in significant rescue of Aβ42 mediated neurodegeneration. Lunasin blocks the Jun-N terminal kinase (JNK) signaling pathway recovering the reduced eye phenotype caused by Aβ42 induction. (D) The ribbon structure of 2S Albumin and Lunasin has been predicted from Swiss model (https://swissmodel.expasy.org/). (E) Lunasin polypeptide is 43 amino acids long. Lunasin is the smaller subunit of 2S Albumin (158 amino acids). N-terminal domain of Lunasin spans from 1-22 with unknown function. The amino acid residues 23-32 have helical structure and binds to the chromatin. Arginine-glycine-aspartate (RGD) domain encompassing residues 33-35 have cell adhesion motif, which helps Lunasin to internalize into the cells. Poly-D tail follows the RGD domain.
The Drosophila model has a large repertoire of tools that allows expression of foreign genes along spatial and -temporal axes. Using Gal4/UAS (upstream activating sequence)transgenic systems (Brand and Perrimon, 1993), human Aβ42 was misexpressed in the differentiating photoreceptor neurons of the developing eye (Tare et al., 2011; Moran et al., 2013; Steffensmeier et al., 2013). It resulted in accumulation of extracellular amyloid plaques, as evidenced by immuno-histochemical approaches. These human Aβ42 expressing eyes exhibited progressive neurodegenerative phenotype in the Drosophila eye (Figure 4B) as compared to the wild-type adult compound eye (Figure 4A) (Tare et al., 2011; Sarkar et al., 2016). This Aβ42 mediated neurodegeneration phenocopies AD like neuropathology in terms of progression of phenotype (Figure 4A & B). Thus, Drosophila eye model of Aβ42 mediated neurodegeneration can be exploited for genome wide genetic screens or for screening chemical libraries for potential therapeutic targets.
Current Therapeutic Targets for AD
Presently, Food and Drug Administration approved drug regimen for AD help to treat the symptoms but do not cure the disease itself. The anti-inflammatory drugs (Aisen, 2000),like atorvastatin (Sparks et al., 2005), aspirin (AD2000 Collaborative Group et al., 2008), and rosiglitazone (Harrington et al., 2011) have not shown the complete cure in improving the cognitive decline in AD patients. The AD patients have less acetylcholine neurotransmitter in the brain that results in loss of neurons. Therefore, cholinesterase inhibitors have been approved by Food and Drug Administration for AD treatment regimen (Gomes et al., 2018). But these drugs have side-effects like nausea, vomiting, diarrhea, muscle cramps, fatigue indigestion, weight loss, confusion and headache, and do not sure AD (Colovic et al., 2013). Hence,there is a need of medication, which has less side effects and can treat the disease by preventing the neuronal cell death.The available medicines for AD ameliorate the symptoms but cannot cure the disease altogether. Lately, there have been disappointing results from AD drug trials due to widespread drug failures and lack of diversity of novel targets(Goldman et al., 2018). Efforts are directed towards search for alternative treatment approaches, and among them screening for natural product library for potential therapeutic targets is on the forefront.
Natural Products: Potential Targets for AD
The traditional herbal medicines that are compounds extracted from natural plant products can be less toxic, may have less side-effects and inexpensive than the synthetic drugs. Some plant products have medicinal properties for neurodegenerative diseases like anticholinesterase activities, anti-inflammatory, antioxidant, and neuroprotective function. Natural products like Lunasin, Polyphenols, Flavonoids, Alkaloids, and Tannins are potential therapeutic candidates for the AD (Zhang et al., 2008; Williams et al.,2011; Pany et al., 2014; Sarkar et al., 2018). Here we discuss some of the natural products that have demonstrated a potential in preventing neurodegeneration observed in AD in cell lines or in animal model systems.
Lunasin
Lunasin was first extracted in 1987 in Japan from soybean(Glycine max) seed, a staple in the diets of many cultures,while screening for the protease inhibitors. It is also found in other plants like wheat, barley, rye, amaranth and triticale. Lunasin, a thermostable protein, which should be ingested at optimum amount (Liu et al., 2014). Lunasin has antioxidant, anti-inflammatory and anti-cancer properties(Jones and Srivastava, 2014). Lunasin is the smaller subunit of the soybean 2S albumin protein (Figure 4D). The gene GM2S-1 encodes methionine rich protein, signal peptide, a linker peptide and Lunasin which are post-translationally processed (Jones and Srivastava, 2014). It has 43 amino acid residues, a molecular weight of 5.5 kDa, and eight negatively charged aspartate residue sequence at the carboxyl end. Lunasin has four motifs: N-terminal domain, predicted helical region, arginine-glycine-aspartate (RGD) domain and poly-D tail (Figure 4E). The function of N-terminal domain of Lunasin is unknown. The predicted helical domain binds to the chromatin. The poly D sequence acts as H3-H4 histone acetylation inhibitor. The amino acids preceded the poly D stretch are RGD, which helps in internalization of Lunasin into nucleus and in binding of Lunasin to the core histone proteins (Liu et al., 2014). The function of other peptides in Lunasin has not been defined.
Lunasin can inhibit mitosis by increasing the expression of cyclin dependent kinases inhibitors like p21 and p27 (Figure 5) (Dia and Gonzalez de Mejia, 2011; Jones and Srivastava, 2014). The cyclin dependent kinases play an important role in cell cycle and their misexpression results in cell cycle deregulation. Tumor suppressor genes regulate the cell cycle progression and are often downregulated in cancers. PTEN,a Tensin homolog and a tumor suppressor phosphatase, is mutated in cancer. Lunasin is found to increase the levels of PTEN in human breast cancer cells (MCF-7) inducing the apoptosis (Figure 5) (Pabona et al., 2012). Lunasin represses the expression of genes, which are involved in tumor suppression (Hsieh et al., 2017). Animal studies suggests that Lunasin has anti-inflammatory effects (Table 1 and Figure 5)by inhibiting cytokines such as tumor necrosis factor α and interleukin-6. It also inhibits the release of nitric oxide (Liu and Pan, 2010). Hence, Lunasin may be potential therapeutic target for the disease where inflammation is observed.
Drosophila eye model system has been used to study the effects of Lunasin in transgenic flies expressing high levels of human Aβ42 in differentiating retinal neurons of the eye(Sarkar et al., 2018). Targeted misexpression of Aβ42 in the developing retinal neurons of the flies showed glazed eye appearance and necrotic spots suggesting neurodegeneration(Sarkar et al., 2018). This study suggests that misexpression of Lunasin in the differentiating retinal neurons of the developing Drosophila eye can rescue Aβ42 mediated neurodegeneration (Figure 2) (Sarkar et al., 2018). Previously, it has been shown that activation of evolutionarily conserved Jun-N terminal kinase signaling pathway is involved in AD.Sarkar et al. (2018) reported that Lunasin downregulates Jun-N terminal kinase signaling to rescue Aβ42 mediated neurodegeneration. AD a neurodegenerative disorder exhibits neuro-inflammation due to production of the ROS(Figure 5). Lunasin can be used as a potential therapeutic target for AD and further studies on vertebrate models will be helpful to discern its medical properties on mammals.

Table 1 Natural products and their mode of action in neurodegeneration
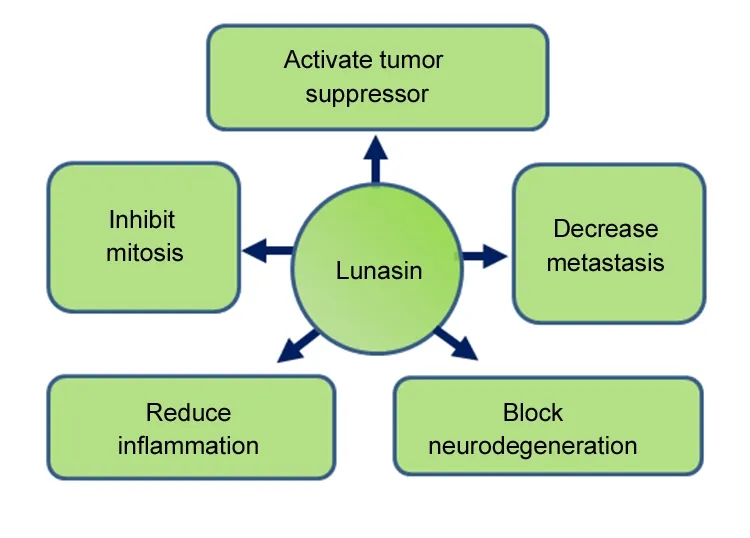
Figure 5 Schematic representation of functions of Lunasin.Lunasin has been reported to play role in tumors and invasiveness of tumors by activating tumor suppressor, inhibiting mitosis, and by decreasing metastasis. It has also been shown to reduce inflammation.Recent studies reported its role in blocking neurodegeneration caused by accumulation of amyloid-beta 42 plaques.
Polyphenols
Polyphenols derived from different plants are part of our daily diet, and have been reported to improve cognitive activities and reduce the neuropathology in animal models of AD (Wang et al., 2014). They have antioxidant property,which helps in scavenging ROS (Malar and Devi, 2014).Polyphenols can prevent formation of neurofibrillary tangles by preventing tau hyper-phosphorylation, which makes it potential target for AD (Figure 2 and Table 1).
Curcumin, a polyphenol, extracted from Curcuma longa and has been used in Ayurveda medicines. Curcumin has been shown to inhibit the generation of Aβ42 oligomers and the plaques (Figure 2 and Table 1) (Williams et al., 2011).Administration of curcumin in AD animal model prevented neuro-inflammation, tau hyper-phosphorylation and protein kinase B (Akt)/glycogen synthase kinase 3 signaling pathway (Hoppe et al., 2013). It also helps in decreasing ROS by regulating pathways mediated neurodegeneration(Figure 2) (Doggui et al., 2013).
Resveratrol, a polyphenolic compound, has found to play role in activation of SIRT1, SIRT2 and SIRT3 (Sirtuins),which plays role in neuronal cell survival and longevity(Bastianetto et al., 2000; Rahman et al., 2006; Schirmer et al.,2012). As a result, Sirtuins reduce the Aβ plaque aggregation in transgenic model of AD (Table 1) (Karuppagounder et al., 2009). Hyper-activation of Microglia triggers neuronal death. Resveratrol exhibits antioxidant effects in microglial cell line in hypoxia injury model. Thus, resveratrol may exhibit neuroprotective function by regulating microglia in brain (Song et al., 2014). Polyphenols have also been reported to lessen the mitochondrial dysfunction thereby preventing onset or delaying progression of neurodegeneration.
Combination of polyphenol treatment has been found to be the novel treatment target for AD (Auti and Kulkarni,2018). A combination of three polyphenols like grape seed extract, resveratrol and Concord grape juice extract have been tested in mouse model of AD. They found that combination treatment proved to be better than the individual treatment in reducing amyloid content in the brain and thereby improving the cognitive impairment in AD mouse model (Wang et al., 2014).
Flavonoids
Flavonoids are plant secondary metabolites, which are anti-oxidant, anti-amyloidogenic, anti-inflammatory and modulate cell-signaling pathways (Baptista et al., 2014; Bakhtiari et al., 2017). Approximately 128 flavonoids have been reported. They have acetylcholinesterase inhibitory activity proving to be a promising drug for AD (Auti and Kulkarni, 2018).Moreover, these phytochemicals can cross the blood-brain barrier, which has direct effect on the brain (de Andrade Teles et al., 2018). Flavonoids affect phosphatidylinositol 3-kinase/Akt pathway and mitogen activated protein kinase signaling pathway, which regulate expression of transcription factors involved in survival (Bakhtiari et al., 2017). Flavonoids reduce the toxic Aβ aggregation by inhibiting β-secretase and activate the- secretase activity (Figure 2) (Baptista et al.,2014). Additionally, it also prevents the formation of tau hyper-phosphorylation (Baptista et al., 2014). Another flavonoid,Quercitin (3,3′,4′,5,7-pentahydroxyflavone), can decrease the levels of ROS and inhibits apoptosis in young rats (Figure 2) (de Andrade Teles et al., 2018). Another study shows that quercitin blocks nuclear factor ĸB levels and hence reduces the inflammation (Pany et al., 2014). Catechin, derived from catechu, is a plant secondary metabolite. Catechins prevent Aβ42 accumulation and oxidative stress (Figure 2 and Table 1) (Rezai-Zadeh et al., 2005; Pervin et al., 2018). Kaempferol,a natural flavonoid, can be derived from variety of plants.Effect of Kaempferol on transgenic AD flies was found to decrease the oxidative stress and acetylcholinesterase activity(Table 1) (Beg et al., 2018).
Alkaloids
Alkaloids are organic compounds that contain basic nitrogen atoms. Most of them are acetyl-cholinesterase and butylcholinesterase inhibitors (Konrath et al., 2013). Huperzine A,isolated from Huperzia serrate, has been used for memory enhancement in ancient Chinese medicine. Huperzine A, a specific and selective acetylcholinesterase inhibitor, showed increase in acetylcholine in rat brain (Zangara, 2003). It is also known to reduce Aβ aggregates in brain (Zhang et al.,2008). Additionally, Huperzine A, an N-methyl-D-aspartic acid receptor antagonist, reduces glutamate neurotoxicity.Hence, it reduces the synaptic loss and cell death of neurons(Table 1) (Auti and Kulkarni, 2018).
Nicotine, 3-(1-methyl-2-pyrrolidinyl) pyridine, is obtained from Nicotiana tobacum. Nicotine patches as well as injections have reported to improve the learning abilities in animals as well as AD patients. Since nicotine has adverse effects,it is important to assess the efficacy in AD patients (Table 1)(Levin and Simon, 1998; Nordberg et al., 2002; Newhouse et al., 2012; Auti and Kulkarni, 2018). Berberine, isoquinoline,have been used in Chinese and Ayurvedic medicine regimens.It is anti-inflammatory, anti-oxidant, anti-cancer, anti-depressants, and prevents Aβ42 plaque formation (Figure 2,Table 1) (Durairajan et al., 2012; Cai et al., 2016).
Terpenes
Terpenes, aromatic molecules, are group of hydrocarbons that consist of repeats of isoprene units. Terpenes inhibit acetylcholinesterase activity and block the advanced glycation end product formation (Auti and Kulkarni, 2018).Triterpenes have anti-oxidative and anti-inflammatory activities. Gensenoside, which promotes health and longevity,have been studied for their effects on AD. Gensenoside prevents the Aβ42 levels by increasing Neprilysin gene expression (Figure 2 and Table 1) (Yang et al., 2009). Moreover,Ginseng improves learning ability in aging brains of rodents.
Ginkgolides, cyclic diterpenes, isolated from Ginkgo biloba.It has been shown that it promotes cell survival of neurons from synaptic damage by Synaptophysin, a presynaptic marker (Bate et al., 2008). It has been reported that Ginkgolide B can rescue Aβ induced apoptosis by promoting the synthesis of neurotrophic factor and reduces the cell death of neurons in the AD rats brains (Figure 2) (Serruys et al., 2011).
A triterpenoid, Platycodin D, which is present in the root extract of Platycodon Grandiflorus (balloon flower or Chinese bellflower) inhibits the Aβ42 plaques mediated neuronal loss(Figure 2). Using rat PC12 cell lines (derived from phenochromocytoma of the rat adrenal medulla cells), it was shown that Platycodon Grandiflorus extract from root contains Platycodin D, can increase synaptogenesis in the hippocampus by activating mitogen activated protein kinase/extracellular regulated protein kinase pathway. Platycodon Grandiflorus extract from root can be studied further on AD models to test its therapeutic potential efficiency (Kim et al., 2017).
Future of Natural Products As a Therapeutic Medicine
The use of medicinal plants as source of natural products to cure disease is a result of man experimenting by trial and error for hundreds of centuries, searching for available foods for the treatment of diseases. The natural products have been used as medicines throughout history in the form of traditional medicines, remedies, potions and oils. Before the discovery of chemical based drug formulations, majority of drug regimens were natural product based across the civilizations. The earliest records dates as early as Mesopotamia (2600 B.C.) where oils from Cupressus semperviviens(Cypress) and Commiphos species (myrrh) were used to treat cough and colds. These treatments are still in use today. In India, Curcurmin or Azadirachta indica (Neem), a member of Meliaceae family that has rich source of antioxidants has been extensively used in Ayurveda medicine. These natural products have been extensively used in Indian, Chinese and Unani medicine because of their property of scavenging ROS, and their pivotal role in anticancer management and other diseases. It has been shown to modulate various growth regulatory pathways including p53, phosphate and tension homology deleted on chromsome ten (pTEN), phosphatidylinositol 3-kinase/Akt, etc. (Alzohairy, 2016).
It is evident that discovery of natural products will be beneficial on multiple levels. Many of these bioactive natural products are still unidentified. Natural plant products are available in everyday diet and they are the part of the traditional medicines in Indian Ayurveda and Chinese medicines. The fruits, vegetables and grains prevent the onset or delay the onset of the disease and promote healthy aging.These natural products also reduce the risk of AD (Howes et al., 2003). The bioactive compounds with pharmacological effects have been extensively studied to cure various neurological diseases. These phytochemicals have better pharmacological effects and can cross the blood-brain barrier and high bioavailability. They are safe and effective as compared to other available drugs in the market. As a result, they might be considered to be an effective alternative to the current drugs.
Conclusions
The natural phytochemicals with pharmacological effects can serve as potential therapeutic treatments for AD. These natural products may reduce ROS, or prevent the toxic Aβ42 plaque production. Alternatively, these products may act downstream of Aβ42 plaque accumulation and modulate the aberrant signaling events that are triggered due to Aβ42 plaques in AD. Studies in animal model system strongly suggest that some of these natural products can cure and prevent AD effectively (Figure 2 and Table 1).
As the age expectancy will increase due to advancement in medical science, the prevalence of the neurodegenerative diseases will increase in aging population. It has been seen that changes in lifestyle, change in food habits, excessive stress due to work or environmental conditions have aided to increase in frequency of neurodegenerative disease like AD. The management or finding cures for AD is an issue to target multiple causative agents of the disease (Goldman et al., 2018). Understanding the molecular mechanism of the disease by using animal model system in vivo studies would be helpful in identifying the potential therapeutic targets. Various animal models are being used to study the mechanism of action of these products at molecular level. Scientists have turned to natural products for drug development for neurodegenerative diseases like AD, because of their medicinal properties. The phytochemicals seem to be promising and potential therapeutic targets for neurodegenerative diseases like AD. The strength of usage of natural products lie in their bioavailability. Thus,following a strict diet regimen with emphasis on natural products with potential medicinal properties have promising expectation for finding cures for AD.
Interestingly, in humans by the time AD is detected, the disease has progressed significantly. At this stage, a critical number of neurons are already lost, which manifests as the loss of cognitive functions in the patient. Since many of the neurons are post mitotic, they cannot be replaced by normal cell division. Therefore, efforts are being directed towards approaches to recover and regenerate the damaged or lost neurons to restore the lost cognitive functions (Felsenstein et al., 2014). Blocking Aβ42 mediated neurodegeneration at the time when AD is detected, will only prevent the further loss of neurons after that stage. However, it will not recover dead neurons and as a result, there will be no recovery of cognitive functions lost due to neurodegeneration. Thus, there is a need to utilize these established animal models to screen for potential therapeutic targets which can generate regeneration response in the neuronal population of AD patients.
Acknowledgments:We thank the Bloomington Stock Center for the Drosophila strains.
Author contributions:Conception and design of the manuscript: AS;manuscript writing and editing: AS and PD; figure preparation: AS, PD and NG.
Conflicts of interest:All authors disclose no conflicts of interest.
Financial support:Schuellein Chair Endowment Fund to AS supports PD and Graduate program of Biology supports NG. This work was supported by National Institute of General Medical Sciences (NIGMS) - 1 R15 GM124654-01, Schuellein Chair Endowment Fund to Amit Singh, STEM Catalyst Grant from University of Dayton and start-up support from UD to AS.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Yun-Bae Kim, Chungbuk National University,Republic of Korea.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Improvement of ataxia in a patient with cerebellar infarction by recovery of injured cortico-ponto-cerebellar tract and dentato-rubro-thalamic tract: a diffusion tensor tractography study
- Tandem pore TWIK-related potassium channels and neuroprotection
- Dendritic shrinkage after injury: a cellular killer or a necessity for axonal regeneration?
- Regenerative biomarkers for Duchenne muscular dystrophy
- Role of macrophages in peripheral nerve injury and repair
- Therapeutic strategies for peripheral nerve injury:decellularized nerve conduits and Schwann cell transplantation

