Treadmill training improves neurological deficits and suppresses neuronal apoptosis in cerebral ischemic stroke rats
Li-Mei Cao, Zhi-Qiang Dong, Qiang Li, Xu Chen
Department of Neurology, Shanghai No. 8 People's Hospital, Shanghai, China
Abstract Rehabilitation training is believed to be beneficial to patients with stroke, but its molecular mechanism is still unclear. Rat models of cerebral ischemic stroke were established by middle cerebral artery occlusion/reperfusion, and then received treadmill training of different intensities, twice a day for 30 minutes for 1 week. Low-intensity training was conducted at 5 m/min, with a 10-minute running, 10-minute rest, and 10-minute running cycle. In the moderate-intensity training, the intensity gradually increased from 5 m/min to 10 m/min in 5 minutes, with the same rest cycle as above. In high-intensity training, the intensity gradually increased from 5 m/min to 25 m/min in 5 minutes, with the same rest cycle as above. The Bederson scale was used to evaluate the improvement of motor function. Infarct volume was detected using 2,3,5-triphenyltetrazolium chloride staining. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining was applied to detect the apoptosis of nerve cells in brain tissue. Western blot assay was employed to analyze the activation of cyclic adenosine monophosphate (cAMP)/protein kinase A and Akt/glycogen synthase kinase-3β signaling pathways in rat brain tissue.All training intensities reduced the neurological deficit score, infarct volume, and apoptosis in nerve cells in brain tissue of stroke rats.Training intensities activated the cAMP/protein kinase A and Akt/glycogen synthase kinase-3 beta signaling pathways. This activation was more obvious with higher training intensities. These changes were reversed by intracerebroventricular injection of protein kinase A inhibitor Rp-cAMP. Our findings indicate that the neuroprotective effect of rehabilitation training is achieved via activation of the cAMP/protein kinase A and Akt/glycogen synthase kinase-3 beta signaling pathways. This study was approved by the Ethics Committee of Animal Experimentation in Shanghai No. 8 People's Hospital, China.
Key Words: nerve regeneration; ischemic stroke; treadmill training; neuronal deficit; apoptosis; cyclic adenosine monophosphate; protein kinase A; glycogen synthase kinase-3β; neuroprotective effect; neural regeneration
Graphical Abstract
The neuroprotective effect of treadmill training against neurological deficits might be mediated by activation of cAMP/PKA and Akt/GSK-3β signaling pathways

Introduction
Stroke is one of the top three leading causes of death worldwide, and poses a serious mental and physical burden (Joutel et al., 1996; Sajjad et al., 2013). Acute cerebral ischemic stroke, which is the most common neurologic diagnosis,results from abrupt interruption of focal cerebral blood flow(Brott and Bogousslavsky, 2000; Ghobrial et al., 2013; Li et al.,2017). A series of risk factors are involved in stroke, including advanced age, hypertension, diabetes mellitus, smoking, atrial fibrillation, and serum albumin levels (Rothwell et al., 2005;Carter et al., 2007). Stroke prevention programs can achieve a reduction in incidence and mortality of stroke (Wolf et al.,1992; Wardlaw et al., 2014; Qiu et al., 2016).
Physical training is a necessary and effective method for motor rehabilitation post stroke (Sun et al., 2014; Wang et al.,2017; Li, 2018). Treadmill training is an efficient rehabilitation training technique that promotes neuro-rehabilitation and recovery of motor functions (Wang et al., 2001; Alomari et al., 2013). Some research has found that functional motor training is not only neuroprotective, but can also activate brain networks underlying limb movement post stroke (Hosp and Luft, 2011; Mao et al., 2017). It has been suggested that exercise promotes neural activity, spatial learning, and memory formation, and can increase the level of hippocampal brain-derived neurotrophic factor (Alomari et al., 2013; Sun et al., 2014).
Various studies have shown that an exercise-induced effect occurs via cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)/cAMP-responsive element binding protein (CREB) and Akt/glycogen synthase kinase (GSK)-3β (Park et al., 2014; Song et al., 2014; Li et al., 2015b; Qian et al., 2015; Han et al., 2017). Song et al. (2014) showed that Chinese herbal medicine decreased neurological functional scores, apoptotic neurons, and activated the Akt/GSK-3β pathway in ischemic stroke rats, which suggests that the Akt/GSK-3β pathway plays an important role in ischemic stroke. Jiang et al. (2016) showed that cAMP/PKA signaling is involved in neuroprotection in cerebral ischemic stroke.This suggests that the cAMP/PKA and Akt/GSK-3β signaling pathways may have a protective effect on neuronal activity and motor function. However, the association of cAMP/PKA and Akt/GSK-3β signaling pathways with a rehabilitation training-mediated neuronal protective effect or motor recovery has not been studied.
This study was designed to detect the effect of rehabilitation training on neuronal protection and motor recovery, and its association with cAMP/PKA and Akt/GSK-3β signaling pathways. Rat models of stroke were induced using middle cerebral artery occlusion/reperfusion (MCAO/R) surgery.Ischemic stroke rats were pretreated with PKA inhibitor RpcAMP and/or received treadmill training at different intensities. The neuroprotective effect of rehabilitation training was assessed by detecting neuronal deficits, apoptosis, and activation of cAMP/PKA and Akt/GSK-3β signaling pathways.
Materials and Methods
Animals
One hundred and twenty specific-pathogen-free adult male Sprague-Dawley rats aged 2 months and weighing 280-320 g (license number: SCXK(Hu)2017-0005) were purchased from SLAC Laboratory Animal Co., Ltd., Shanghai, China.All rats were housed for 7 days with free access to food and water, followed by a method of accommodation (Sun et al.,2014). All animal experimental procedures were approved by the Ethics Committee of Animal Experimentation in Shanghai No. 8 People's Hospital and performed according to the National Institutes of Health guidelines for the Care and Use of Laboratory Animals (Publication No. 85-23, revised 1996).
MCAO animal model establishment
The MCAO animal model was established as previously described (Sun et al., 2014). A total of 100 rats were randomly subjected to the MCAO/R surgeries for ischemic stroke (n= 85) or sham surgeries (sham control, n = 15) (Longa et al.,1989; Sun et al., 2014). An intracerebroventricular injection of Rp-cAMP (40 nM; Sigma, St. Louis, MO, USA) was administered to experimental rats (n = 20), which then immediately underwent MCAO/R surgery.
Rehabilitation training
Ischemic stroke rats with motor impairment and a Bederson's behavioral score of 1-3 at 6 hours post-surgery were included (Bederson et al., 1986; Jiang et al., 2016). Two rats with a Bederson's behavioral score of 0 or 4 were excluded,leaving 18 rats in the Rp-cAMP group. The other 65 rats received MCAO/R surgery, after which one more rat with a post-surgery Bederson's score of 0 was excluded. In total,64 rats were randomly assigned to one of the four following groups: a no training group (NTG, n = 16); a low-intensity training group (LIT, n = 16; a velocity of 5 m/min, with a 10-minute running: 10-minute rest: 10-minute running cycle for 30 minutes with assistance if necessary); a moderate-intensity training group (MIT, n = 16; the velocity gradually increased from 5 m/min to 10 m/min in 5 minutes,with the same rest cycle over 30 minutes); and a high-intensity training group (HIT, n = 16; the velocity gradually increased from 5 m/min to 25 m/min in 5 minutes, with the same rest cycle over 30 minutes). In addition, rats pretreated with an intracerebroventricular injection of Rp-cAMP received HIT (HIT + Rp-cAMP, n = 18). Training was performed 2 times/day. Immediately after the last training (at 1, 3, or 7 days after the first training), animals were deeply anesthetized (10% chloral hydrate, 3.5 mL/kg; intraperitoneally) and sacrificed. Brain tissues were isolated and prepared for further experiments.
Locomotor function scoring
Neurological deficit and locomotor function were assessed at 1, 3, and 7 days post the first training using a five-point scale(0-4) with the Bederson's test (Bederson et al., 1986). The Bederson's test and neurological evaluation were performed by a blinded researcher.
2,3,5-Triphenyltetrazolium chloride (TTC) staining
TTC staining was performed to evaluate the infarct volumes of the brain in MACO rats (Jiang et al., 2016; Zhang et al.,2017) 7 days after surgery. Briefly, the isolated brain tissues were washed with phosphate-buffered saline, sectioned into 2 mm-thick slices, which were then treated with 2% TTC(Sigma) in phosphate-buffered saline at 37°C for 15 minutes. Next, the slices were fixed in 4% paraformaldehyde for 2 minutes. An Olympus digital camera (fe-240; Pooher Photoelectric Technology Co., Ltd., Shanghai, China) was used to capture images. Image Pro Plus 6.0 software (Media Cybernetics, Inc., Bethesda, MD, USA) was used to analyze the infarction ratio (Jiang et al., 2016).
Double immunofluorescence of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining and NeuN to visualize neuronal apoptosis
Neuronal apoptosis in rat brain tissues was detected 72 hours after model establishment using the double immunofluorescence of staining TUNEL and neuronal nuclei antigen (NeuN) (Zhang et al., 2017). In brief, an In Situ Cell Apoptosis Detection kit from Roche Diagnostics (Shanghai,China) was applied. For immunofluorescence analysis, after incubation with 0.4% Triton X-100 for 20 minutes at 37°C,sections were washed and blocked with 10% normal donkey serum for 90 minutes. Samples were then incubated with primary antibody of mouse anti-NeuN antibody (ab177487,1:500; Abcam, Cambridge, UK) at 4°C overnight. Samples were then incubated with fluorescein isothiocyanate-conjugated donkey anti-mouse IgG (1:100; Proteintech, Wuhan,China) for 90 minutes at 37°C in the dark. The apoptotic neurons and nuclei were stained with NeuN (red; 1:50) and DAPI (blue), respectively. A laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany) was used to count apoptotic cells. The average numbers of TUNEL- and NeuN-positive cells per mm2in five non-overlapping fields in the penumbra regions at 200× magnification were calculated.
Western blot assay
For the assessment of cAMP-PKA and Akt-GSK-3β activation, western blot assay was used to detect the expression of phospho (p)-GSK-3β (Ser9) (1:1000), GSK-3β (1:2000),p-Akt (Ser473) (1:1000), Akt, p-AMPKα (Thr172) (1:2000),AMPK (1:1500), p-PKA (Ser96) (1:1000), and PKA-Cα(1:1500) proteins 72 hours after model establishment. The expression of apoptosis-related factors Bax (1:1500), Bcl-2(1:2000), and cleaved caspase-3 (1:1000) was also detected.Goat anti-rabbit/mouse IgG (1:20,000) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) or Cell Signaling Technology, Inc. (Danvers, MA, USA). β-Actin (1:2000; Cell Signaling Technology, Beverly, MA, USA)was used as the internal reference control protein. Immunoblot analyses of these proteins were made using the standard methods with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and polyvinylidene fluoride membranes.In brief, membranes were blocked with 5% skimmed milk(Sigma), and incubated with primary anti-rabbit antibodies at 4°C overnight and then with horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG (1:20,000, Santa Cruz Biotechnology, Inc.) at room temperature for 40 minutes.Enhanced chemiluminescent reagents (Amersham, Arlington Heights, IL, USA) were used to visualize protein expression.
Statistical analysis
Data are presented as the mean ± SD. Statistical analyses were performed using GraphPad Prism version 6.0 (Graph-Pad, San Diego, CA, USA). Between-group differences in neurologic deficit scores were assessed using the Kruskal-Wallis test. Variables between-group differences were assessed using a one-way analysis of variance with Newman-Keuls post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Treadmill training exhibits a protective effect on ischemic stroke-induced neurological deficits
A total of 82 ischemic stroke model rats were successfully obtained after excluding 3 rats with Bederson's scores of 0 or 4 at 6 hours post MACO/R surgery. During the 7-day experimental period, another 5 rats were lost, including 2 with subarachnoid hemorrhage (1 in the ischemic stroke group and 1 in the HIT + Rp-cAMP group), 2 without pathological changes in histology (1 in the LIT group and 1 in the HIT+ Rp-cAMP group), and 1 death (in the HIT + Rp-cAMP group). Finally, one rat from the MIT group and one from the HIT group were randomly omitted to reach a uniform (n= 15 per group) (Table 1).
The neurological deficit scores were significantly reduced after treadmill training in the training group compared withthe no training group (P = 0.03; Figure 1). The neurological deficit scores of ischemic stroke rats at 3 and 7 days after treadmill training in the LIT group were significantly higher than those of the MIT group (P = 0.021) and HIT group(P = 0.009), respectively. However, the rats in the HIT +Rp-cAMP group that received pre-administration of PKA inhibitor Rp-cAMP showed significantly higher neurological deficit scores at 3 and 7 days post training than the HIT group (P = 0.04).
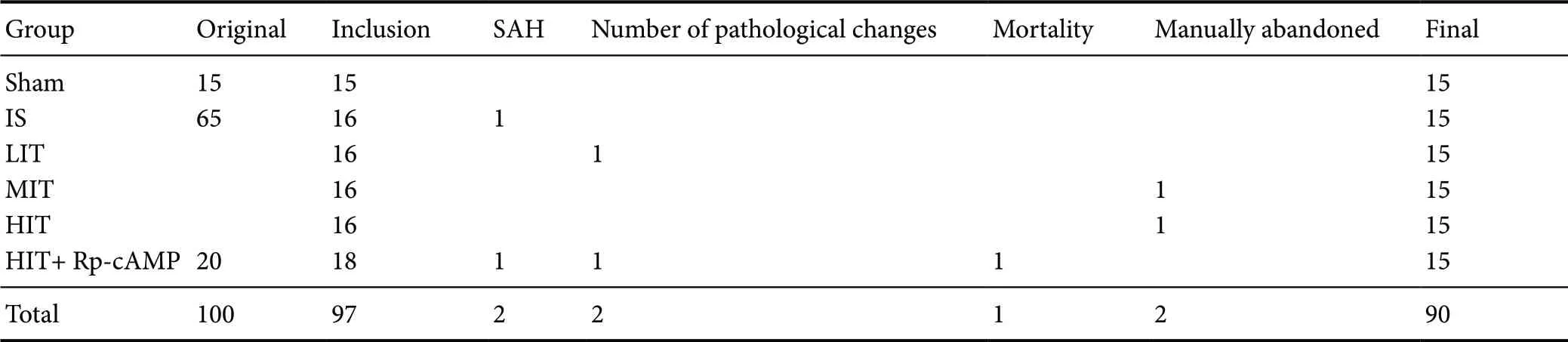
Table 1 Exclusion and mortality of animals in each group
Treadmill training reduces infarct volume
According to the TTC assay, the percentage of infarct volume in ischemic stroke rat brains was reduced by treadmill training in an intensity-dependent manner. The infarct volume ratio of the MIT and HIT groups was significantly lower than that of the ischemic stroke rats (P = 0.019 and P =0.004, respectively; Figure 2). In contrast, the rats pretreated with PKA inhibitor (Rp-cAMP) exhibited a higher infarct volume ratio than the HIT group (P = 0.035).
Treadmill training activates the cAMP-PKA and Akt-GSK-3β signaling pathways
Western blot assay results showed a downregulation of p-AMPK, p-PKA, p-GSK-3β, and p-Akt in the ischemic stroke rat brains. The expression levels of the p-AMPK,p-PKA, p-GSK-3β, and p-Akt proteins were significantly higher in the brains of mice in the LIT, MIT, and HIT groups compared with those the IS group (P = 0.033, 0.004,0.005; Figure 3). The upregulation of these factors was training intensity-dependent. However, pre-administration of the PKA inhibitor in HIT + Rp-cAMP group significantly downregulated these factors compared with the MIT and HIT groups (P = 0.024 and P = 0.001), which suggests that PKA inhibition prevented the treadmill training-induced activation of the cAMP-PKA and Akt-GSK-3β signaling pathways.
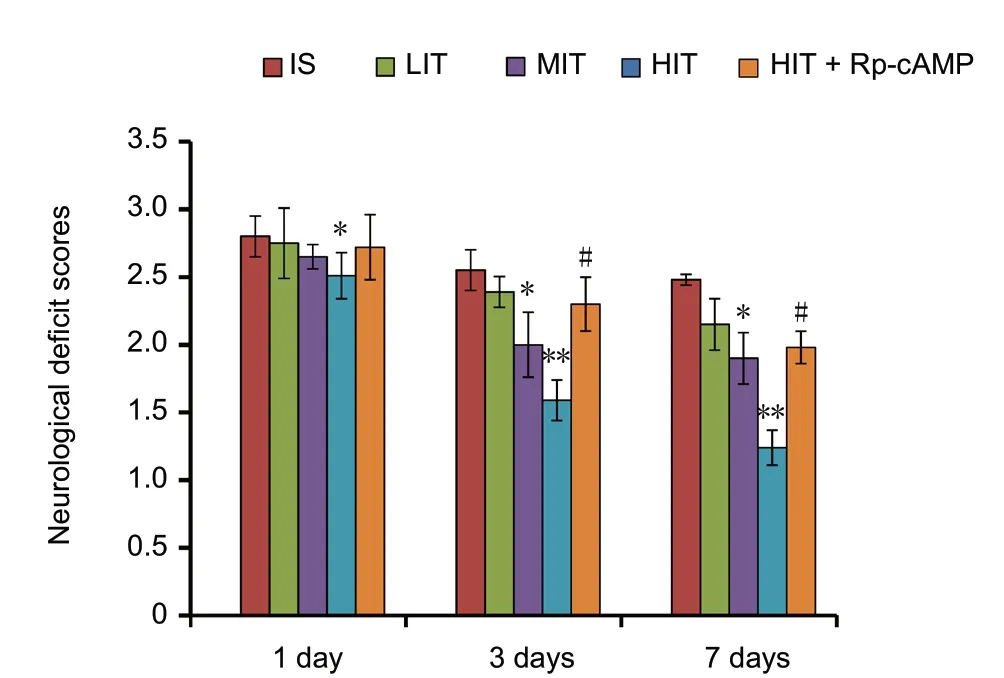
Figure 1 Neurological deficit scores of cerebral ischemic stroke rats subjected to treadmill training.Neurological deficit scores were evaluated at 1, 3, and 7 days during the intervention. The protein kinase A inhibitor Rp-cAMP was intracerebroventricularly injected into the rat brain. Data are presented as the mean ± SD (Kruskal-Wallis test). *P < 0.05, **P < 0.01, vs. IS group;#P < 0.05, vs. HIT group. LIT: Low-intensity training; MIT: moderate-intensity training; HIT: high-intensity training; IS: ischemic stroke;cAMP: cyclic adenosine monophosphate.
Treadmill training suppresses neuronal apoptosis via activation of cAMP-PKA signaling
The number of TUNEL and NeuN co-expressed positive cells was upregulated in the ischemic stroke rat hippocampus compared with the sham group (P < 0.05; Figure 4A and 4B), which were then decreased by treadmill training compared with the control group in an intensity-dependent way. In the MIT and HIT groups, the number of apoptotic neurons in the hippocampus were significantly lower than that in the ischemic stroke group (P = 0.044 and P = 0.009,respectively). The cAMP-PKA inhibitor obviously reversed this change, resulting in a higher apoptotic neuron number in the hippocampus than exhibited in the HIT group (P =0.014). The expression of apoptosis-related proteins Bax and cleaved caspase-3 proteins were downregulated, while Bcl-2 protein was upregulated in the ischemic stroke rat brain,which was then reversed in rats that received treadmill training (Figure 4C). However, the pre-administration of cAMP-PKA inhibitor reversed the treadmill training-induced changes in these protein patterns, which suggests that treadmill training attenuated ischemic stroke-induced neuronal apoptosis via inhibition of the cAMP-PKA-mediated Bax/Bcl-2/caspase-3 pathway.
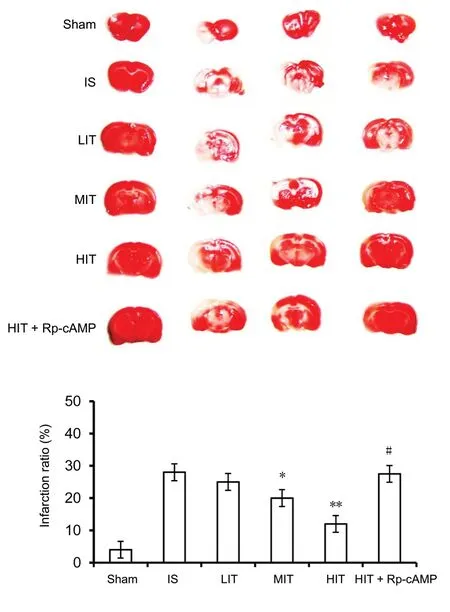
Figure 2 Infarction ratio of brains in treadmill training rats 72 hours after surgery.The infarction ratio of the IS rat brains was detected using a TTC assay.Data are presented as the mean ± SD (one-way analysis of variance with Newman-Keuls post hoc test). *P < 0.05, **P < 0.01, vs. IS group; #P <0.05, vs. HIT group. LIT: Low-intensity training; MIT: moderate-intensity training; HIT: high-intensity training; IS: ischemic stroke; cAMP:cyclic adenosine monophosphate; Rp-cAMP: protein kinase A inhibitor.
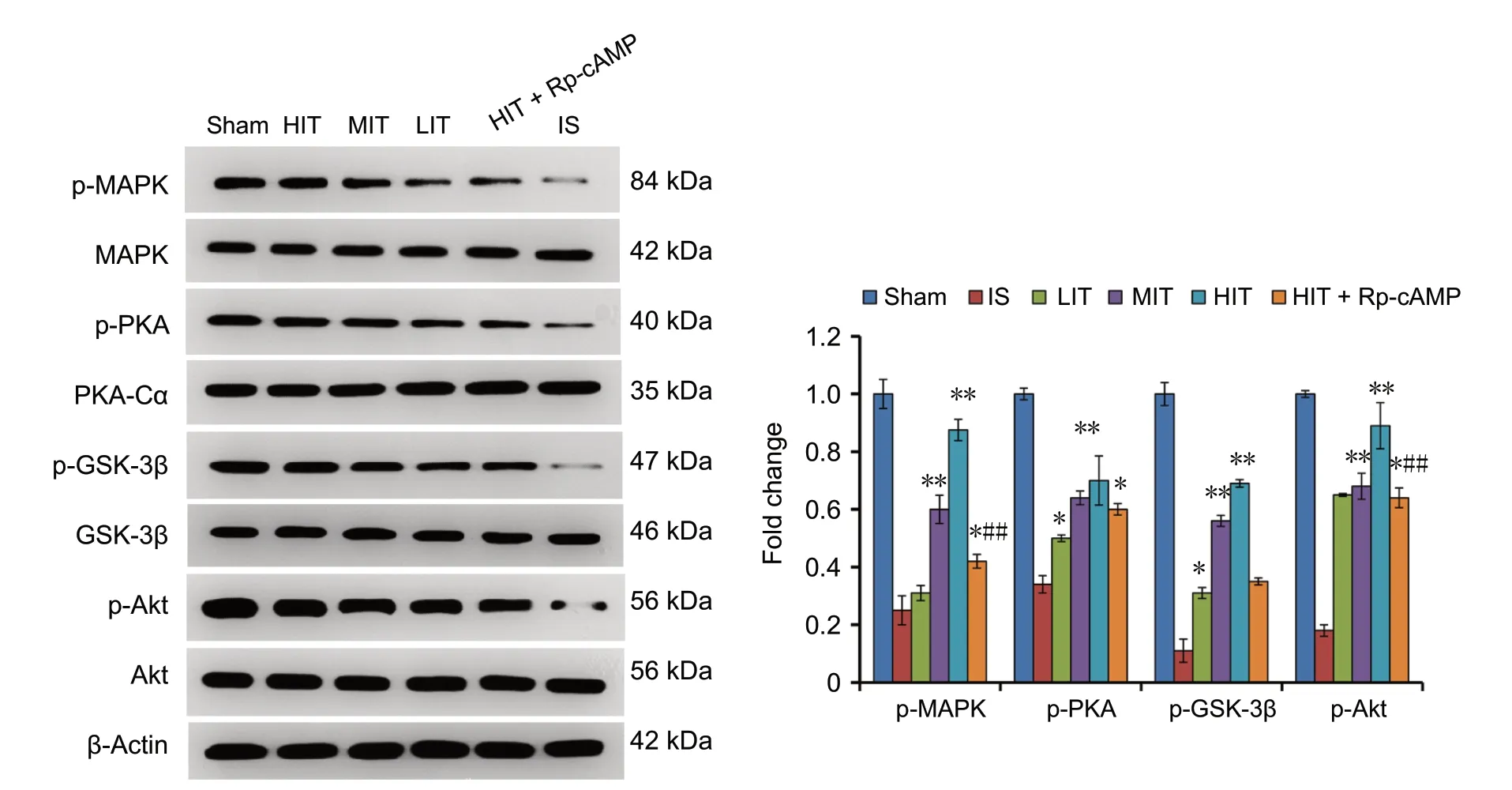
Figure 3 Expression of cAMP-PKA and Akt-GSK-3β signaling factors in the cerebral ischemic stroke rats subjected to treadmill training 72 hours after surgery.A western blot assay was used to detect protein expression. Data are presented as the mean ± SD (one-way analysis of variance with Newman-Keuls post hoc test). *P < 0.05, **P < 0.01, vs. IS group; #P < 0.05, ##P < 0.01, vs. HIT group. LIT: Low-intensity training; MIT: moderate-intensity training; HIT: high-intensity training; IS: ischemic stroke; cAMP: cyclic adenosine monophosphate; Rp-cAMP: protein kinase A inhibitor.
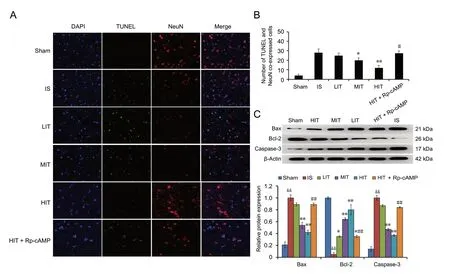
Figure 4 Detection of neuronal apoptosis and apoptosis-related proteins in IS rats subjected to treadmill training 72 hours after surgery.(A) Apoptotic neurons were detected using the TUNEL (green) and NeuN (red) immunofluorescence technique. Apoptotic neurons underwent double fluorescence labeling. Original magnification, 200×. (B) The number of apoptotic neurons was averaged from five random 200-fold fields. (C)Expression of apoptosis-related proteins. Data are presented as the mean ± SD (one-way analysis of variance with Newman-Keuls post hoc test). *P< 0.05, **P < 0.01, vs. IS group; #P < 0.05, ##P < 0.01, vs. HIT group; &&P < 0.01, vs. sham group. LIT: Low-intensity training; MIT: moderate-intensity training; HIT: high-intensity training; IS: ischemic stroke; cAMP: cyclic adenosine monophosphate; TUNEL: double immunofluorescence of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; Rp-cAMP: protein kinase A inhibitor.
Discussion
Neurological deficit after ischemic stroke has been reported to be affected by the intensity of treadmill training (Shimada et al., 2013; Sun et al., 2014). Our results suggest that the treadmill training intensity had a protective effect on ischemic stroke-induced neurological deficit. Using an MCAO/R-induced rat model of ischemic stroke, we confirmed that treadmill training intensity was associated with the neurological deficit scores, ratio of infarct size, and neuronal apoptosis. In addition, treadmill training intensity was strongly associated with the expression change of cAMPPKA and Akt-GSK-3β signaling proteins. This suggests that rehabilitation training reversed the neurological deficit by activating the cAMP-PKA and Akt/GSK-3β signaling pathways.
The hippocampal level of brain-derived neurotrophic factor represents neural activity (Alomari et al., 2013; Sun et al., 2014). A previous study suggested that exercise training increases hippocampal brain-derived neurotrophic factor levels and promotes spatial learning and memory formation(Alomari et al., 2013). Sun et al. (2014) found that a gradual increase in treadmill training intensity was the optimal method for motor recovery. Physical training has been reported as a necessary and effective method for motor and memory rehabilitation post stroke (Sun et al., 2014). In our study, we trained ischemic stroke rats at a constant running speed of 5 m/min (LIT group), gradually increased the speed to 10 m/min in 5 minutes (MIT group), and gradually increased the speed from 5 m/min to 25 m/min in 5 minutes(HIT group) over 30 minutes. The HIT group showed the best recovery, as shown by the lowest neurological deficit scores, ratio of infarct size, and apoptotic neurons. This suggests that treadmill training has a protective effect against motor deficits post cerebral ischemic stroke.
Motor and learning deficits associated with cerebral diseases has been associated with neuronal activity and damage(King et al., 2013; Drui et al., 2014; Stoica et al., 2014; Zhang et al., 2017). It has been reported that Akt-mediated inactivation of the GSK-3β pathway is protective for ischemic injury in the brain and heart (Li et al., 2015b). The cAMP-PKA pathway is crucial for neuronal activity and learning and memory (Kawahata et al., 2013; Bollen et al., 2014). Some studies have demonstrated that the neuroprotective effect was realized by preventing neuronal apoptosis via inactivation of the Akt-mediated GSK-3β and cAMP/PKA pathways (Huang et al., 2016; Han et al., 2017). Li et al. (2015b)demonstrated the neuroprotective effect of granulocyte-colony stimulating factor in a rat model of ischemic stroke,whereby it attenuated neuro-inflammation and blood-brain barrier disruption via inactivating the Akt-mediated GSK-3β pathway. However, some reports have demonstrated the neuroprotective effect of Akt and p-GSK-3β activation on axonal damage and regeneration (Park et al., 2014; Li et al., 2015a; Qian et al., 2015). Qian et al. (2015) and Park et al. (2014) found that maslinic acid and cordycepin administration prevented axonal damage by increasing Akt and p-GSK-3β expression. Li et al. (2015a) found a cardioprotective effect of exogenous hydrogen sulfide administration via the upregulation of p-Akt and p-GSK-3β. Song et al. (2014)showed the protective effect of the Chinese herbal formula Qi-Lian-Gui-Shou Tang against neurological function deficits via activation of the Akt/GSK-3β pathway.
Similar to the effects seen after administration of traditional Chinese medicines, including maslinic acid, cordycepin, and Qi-Lian-Gui-Shou Tang (Park et al., 2014; Song et al., 2014; Qian et al., 2015), exercise training has been shown to exert a beneficial effect on health. However, the underlying mechanisms have yet to be uncovered. There must be exercise that promote the activation of cAMP/PKA, Akt, and GSK-3β (Zhang et al., 2016). In the present study, the Akt/GSK-3β and cAMP/PKA signaling pathways were upregulated in the brains of rats that received treadmill training, which was accompanied with fewer apoptotic neurons. By contrast, the inhibition of PKA signaling by Rp-cAMP reversed these effects. Our results are consistent with those of Zhang et al. (2016), who found that treadmill exercise increased the expression of p-GSK-3β, p-CREB,and p-Akt in a rat model of Alzheimer's disease. Our results demonstrate the neuroprotective effect of treadmill training against cerebral ischemic stroke-induced neurological deficits through activation of the cAMP/PKA, Akt, and GSK-3β signaling pathways.
In summary, there is a close relationship between treadmill training and ischemic stroke rehabilitation, whereby training can recover and protect stroke-induced neurological deficit. Exercise can promote the activation of cAMP/PKA, Akt, and GSK-3β via the Akt/GSK-3β and cAMP/PKA signaling pathways in the brains of rats. The specific mechanism of this effect needs further research.
Author contributions: Experimental implementation and manuscript writing: LMC; experimental implementation and data analysis: ZQD,QL; study design, data analysis and manuscript revision: XC. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that they have no conflict of interest.
Financial support:This study was supported by Clinical Study on Treatment of Cerebral Small Blood Vessel Disease by Integrated Traditional Chinese and Western Medicine, No. ZHYY-ZXYJHZX-201625.The funder had no role in the study design, data collection or analysis;the decision to publish; or the preparation of the manuscript.
Institutional review board statement:This study was approved by the Ethics Committee of Animal Experimentation in Shanghai No. 8 People's Hospital, China.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers: Cheung-Ter Ong, Chia-Yi Christian Hospital,China; Juliana Dalibor Neves, Universidade Federal do Rio Grande do Sul Instituto de Ciencias Basicas da Saude, Brazil.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Improvement of ataxia in a patient with cerebellar infarction by recovery of injured cortico-ponto-cerebellar tract and dentato-rubro-thalamic tract: a diffusion tensor tractography study
- Tandem pore TWIK-related potassium channels and neuroprotection
- Dendritic shrinkage after injury: a cellular killer or a necessity for axonal regeneration?
- Regenerative biomarkers for Duchenne muscular dystrophy
- Exploring the efficacy of natural products in alleviating Alzheimer's disease
- Role of macrophages in peripheral nerve injury and repair

