Rac GTPases: domain-specific functions in neuronal development
Understanding fundamental mechanisms governing axon outgrowth and guidance can inform the development of therapeutic strategies to restore neuronal function damaged though injury or disease. Axons navigate the extracellular environment by responding to guidance cues that bind to cell surface receptors to relay information intracellularly via Rho GTPase family members, including the Rac GTPases. Rac GTPases act as master switches to regulate diverse signalling pathways to control the actin cytoskeleton-a major driver of axon outgrowth. However, scant evidence exists concerning how different domains within Rac GTPases regulate specific neurodevelopmental events, such as axon outgrowth and guidance.
In a recent study, we showed that distinct domains within a single Rac GTPase (CED-10/Rac1) regulates axon guidance and outgrowth in a context-specific manner (Norgaard et al.,2018). This reveals previously unappreciated domain-specific functions within Rac GTPases in controlling neuronal development. In our publication, we also identified molecules (NAB-1/Neurabin I and SYD-1/RhoGTPase-like activator) that modify Rac GTPase-directed axon outgrowth activity, thereby revealing novel controllers of CED-10/Rac-1-directed axon outgrowth. Further analysis of molecular networks that control axon outgrowth will potentially facilitate the development of strategies to repair neuronal injury.
A new mutant revealing domain-specific function of a Rac GTPase: Multiple genetic model systems have been used to elucidate molecular mechanisms that control axon outgrowth and guidance. The nematode Caenorhabditis elegans has been particularly useful in this regard and includes the discovery of the classical UNC-6/Netrin guidance cue (Hedgecock et al.,1990; Ishii et al., 1992). Genetic tractability and the ability to visualize neurons and their axonal processes at single cell resolution in vivo in C. elegans, continues to provide outstanding opportunities to identify genes required for correct development of the nervous system.
The use of C. elegans in deciphering protein function in the nervous system is exemplified by our revelation that distinct domains of a single protein, CED-10/Rac1, differentially regulate specific developmental decisions (Norgaard et al., 2018).Rac GTPases comprise three highly-conserved domains:Switch 1, Switch 2 and membrane-targeting region. The switch domains interact with upstream regulators and downstream effector molecules to control signalling, while the membrane-targeting region directs Rac GTPases to the plasma membrane(Etienne-Manneville and Hall, 2002). Serendipitously, we identified a spontaneous mutation in ced-10 that causes severe axon outgrowth and guidance defects in a subset of neurons(Figure 1). We showed that the genetic lesion causes a single amino acid substitution in the Switch 1 Domain of the CED-10/Rac1 protein. Surprisingly, other alleles of CED-10/Rac1 were reported to only cause minor neuronal defects, supposedly due of redundancy between Rac GTPases (Lundquist et al.,2001). However, we showed that the Switch 1 Domain of CED-10/Rac1 confers a partially non-redundant function in axon outgrowth. This prompted us to utilise this allele to further elucidate the CED-10/Rac1-controlled molecular network that directs axon outgrowth.
We assayed development of diverse morphological classes of neurons in our newly isolated allele (Switch 1 mutation) and in previously isolated ced-10 alleles with mutations in either the Switch 2 or the membrane-targeting regions. Interestingly, these genetic lesions cause differential effects on axon guidance and outgrowth defects in distinct neurons, revealing neuron-specific functions of the CED-10/Rac1 domains. For example, Switch 1 mutants exhibit a highly penetrant PVQ axon outgrowth defects and minor defects in PLM neuronal guidance. Whereas, this pattern is reversed in Switch 2 mutants. Similar patterns of allelic variation were observed between domain-specific mutations for other neuron subclasses. Therefore, the differences in penetrance observed are not likely due to changes in the degree of CED-10/Rac1 signalling strength between the alleles, but a true reflection of CED-10/Rac1 domain-specific functions during development (Figure 2). We also observed such domain-specific functions in other CED-10/Rac1-regulated developmental processes. For example, the Switch 2 and membrane targeting alleles cause severe defects in the phagocytosis of apoptotic cells in larvae, whereas the Switch 1 allele has no detectable role in this process.
The structure of the Switch 1 and Switch 2 domains (fully conserved from worms to humans) is important for coordinating interactions between Rac GTPases and their regulatory and effector molecules (Vetter and Wittinghofer, 2001). Therefore,our data suggest that specific molecules interact with these domains to drive distinct developmental processes. Our findings support this notion and provided us with a platform to decipher which interacting molecules are important for the specific developmental decision of axon outgrowth.

Figure 1 A CED-10 Switch 1 mutation causes axon guidance and outgrowth defects.Ventral views of animals expressing green fluorescent protein (GFP) in the PVQ neurons (PVQR and PVQL). The PVQs are a pair of bilateral interneurons extending in the ventral nerve cord from the tail (right in figure) to the head (left in figure) of the animal. Wild-type (top image),ced-10 Switch 1 mutant animals with axon guidance defect (middle image-white arrow) and axon outgrowth defect (bottom image-red arrow). Scale bar: 20 μm.
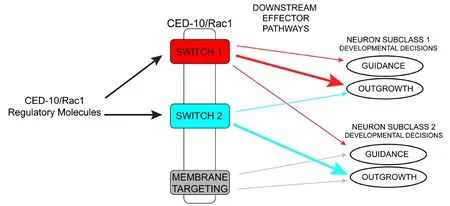
Figure 2 Conceptualization of how CED-10/Rac1 domains differentially contribute to distinct aspects of neuronal development.Upstream regulators control CED-10/Rac1 activity. Different domains of the CED-10/Rac1 protein regulate distinct developmental outcomes depending on the context, presumably through specific interactions with downstream effector pathways. Arrows depict the importance of a signal from a specific domain towards a particular developmental outcome in each neuronal subclass (thicker arrow = more important).
Identification of axon outgrowth regulators:Initial studies recognized that Rho GTPases are imperative for regulation of the actin cytoskeleton (Hall, 1998). Subsequent studies in mammalian systems demonstrated how Rac1 is a fundamental regulator of both axon outgrowth and guidance and how Rac1 function is required at the growth cone, the leading edge of the axon, to promote outgrowth (Kuhn et al., 1999; Briancon-Marjollet et al., 2008). Additionally, other work revealed how mutations in a TRIO, a Rac activator, is associated with intellectual disability in humans (Ba et al., 2016).
Studies in higher organisms are often complicated by Rho GTPase redundancy. The C. elegans genome contains fewer Rho GTPases which simplifies functional analysis. However,null mutants of CED-10/Rac1 are embryonic lethal (Lundquist et al., 2001). Therefore, our newly identified viable ced-10 allele, which reveals domain-specific functions of CED-10/Rac1,provided a unique opportunity to perform an unbiased genetic forward suppressor screen to identify molecules specifically required for axonal outgrowth. We isolated four independent alleles that suppress the outgrowth defects observed in the CED-10/Rac1 Switch 1 mutant. One of the alleles resulted in a premature stop codon in nab-1 (ortholog of mammalian Neurabin-1). Our subsequent analysis revealed how NAB-1 acts with SYD-1 (RhoGAP-like, Rac inactivator) to inhibit Rac activity and control axon outgrowth. This role is supported by previous studies in a mammalian model system that revealed how Neurabin 1 levels are important for Rac1 activation and neurite outgrowth (Causeret et al., 2007). Intriguingly, only axon outgrowth was suppressed in the isolated suppressor alleles, whereas axon guidance was unaffected. This provides further credence to the concept that Rac GTPase domains control distinct neurodevelopmental events through different effector molecules. We are currently in the process of identifying the genetic lesions in the other three alleles isolated from our genetic screening approach.
In our subsequent studies, we aim to further elucidate the differences between mechanisms that govern axon guidance and axon outgrowth. Are they two sides of the same coin?Or are they two distinct processes? One could envision that outgrowth defects are caused by misguidance, however, we found that severely misguided neurons continue to extend. We also showed that guidance defects are not a prerequisite for outgrowth defects; hinting that these processes may be at least partially uncoupled. This is further supported by our suppressor screen in which alleles that suppress outgrowth defects had no effect on guidance. Validation of this hypothesis could be provided through the identification of mutant alleles that supress guidance defects and not outgrowth defects in the CED-10/Rac1 Switch 1 mutant. Using high-throughput screening approaches in the C. elegans model may enable such a discovery in the future.
Conclusion and perspectives:Our research has revealed how distinct CED-10/Rac1 domains confer context-specific functions during development. Our data suggest how the regulation of distinct signalling pathways through Rac GTPase domains can drive diverse developmental outcomes. It will be intriguing if further examples of domain-specific functions of Rho GTPases are identified in other systems. Ultimately, a broader understanding of genetic networks controlling neurite outgrowth will lead to a framework from which therapeutic strategies to alleviate nerve injuries could be devised.
This work was supported by a grant from an NHMRC Project Grant (GNT1105374), NHMRC Senior Research Fellowship(GNT1137645), and a Victorian Endowment for Science, Knowledge and Innovation Fellowship (VIF23) (to RP).
Steffen Nørgaard, Roger Pocock*
Development and Stem Cells Program, Monash Biomedicine Discovery Institute and Department of Anatomy and
Developmental Biology, Monash University, Melbourne,
Victoria, Australia (Nørgaard S, Pocock R)
Biotech Research and Innovation Centre, University of
Copenhagen, Copenhagen, Denmark (Nørgaard S, Pocock R)
*Correspondence to: Roger Pocock, PhD, roger.pocock@monash.edu.orcid: 0000-0002-5515-3608 (Roger Pocock)
Received: November 21, 2018
Accepted: December 24, 2018
doi: 10.4103/1673-5374.253515 Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.Plagiarism check: Checked twice by iThenticate.Peer review: Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer: Yen-Chung Chang, National Tsing Hua University,Taiwan, China
- 中国神经再生研究(英文版)的其它文章
- Improvement of ataxia in a patient with cerebellar infarction by recovery of injured cortico-ponto-cerebellar tract and dentato-rubro-thalamic tract: a diffusion tensor tractography study
- Tandem pore TWIK-related potassium channels and neuroprotection
- Dendritic shrinkage after injury: a cellular killer or a necessity for axonal regeneration?
- Regenerative biomarkers for Duchenne muscular dystrophy
- Exploring the efficacy of natural products in alleviating Alzheimer's disease
- Role of macrophages in peripheral nerve injury and repair

