Role and prospects of regenerative biomaterials in the repair of spinal cord injury
Shuo Liu, Yuan-Yuan Xie, Bin Wang
Clinical Stem Cell Center, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, Jiangsu Province, China
Abstract Axonal junction defects and an inhibitory environment after spinal cord injury seriously hinder the regeneration of damaged tissues and neuronal functions. At the site of spinal cord injury, regenerative biomaterials can fill cavities, deliver curative drugs, and provide adsorption sites for transplanted or host cells. Some regenerative biomaterials can also inhibit apoptosis, inflammation and glial scar formation, or further promote neurogenesis, axonal growth and angiogenesis. This review summarized a variety of biomaterial scaffolds made of natural, synthetic, and combined materials applied to spinal cord injury repair. Although these biomaterial scaffolds have shown a certain therapeutic effect in spinal cord injury repair, there are still many problems to be resolved, such as product standards and material safety and effectiveness.
Key Words: nerve regeneration; spinal cord injury; regenerative biomaterials; scaffolds; tissue engineering;regeneration; transplantation; combination; functional recovery; repair strategy; microenvironment; neural regeneration
Introduction
Spinal cord injury (SCI) is considered to be one of the most challenging central nervous system injuries. With the rapid growth of the global economy in recent years, the incidence rate of SCI has been rising worldwide (Singh et al., 2014;Furlan et al., 2016). SCI can result in permanent voluntary motor dysfunction and sensation loss. In addition, SCI brings great economic and psychological burdens to the patients and their families (Cannon, 2013). Therefore, there is a critical need to develop suitable and effective therapeutic strategies for SCI (Tator, 2006).
Complex pathological and physiological changes occur in the damaged spinal cord tissue after SCI (Figure 1). During the early phase of SCI, the initial injury leads to the rupture of spinal cord nerve tracts, apoptosis, neuronal necrosis,and destruction of blood vessels in the tissue (Furlan et al.,2016). Subsequently, secondary injury brings a series of complications that continue to cause invasive damage to spinal cord tissue, including oxidative stress and infiltration of a large number of inflammatory cells (Tator et al., 1991).For example, edema also occurs simultaneously and disrupts the spinal cord blood supply, aggravating ischemia in the injured tissue (Kabu et al., 2015). The cavity formation can cause more apoptosis of residual cells and functional loss.Astrocytes rapidly proliferate at the injury site and then form glial scars, which can further impede axonal regeneration (Agbay et al., 2016). In short, there is an inhibitory microenvironment that impedes nerve regeneration, formed by a lack of nutritional factors and myelin protein, inflammatory responses, blood flow disruption, and other adverse elements in the lesion (Fujita et al., 2014; Liu et al., 2015;Tsintou et al., 2015).
In recent years, the repair strategies for SCI have been focused on overcoming one or more of the regeneration inhibitors after SCI. These strategies mainly include providing regeneration support and guidance for the damaged neurons by neural scaffolds, inhibiting the formation of glial scars,antagonizing myelin inhibitory signals, and combining cells,drugs or other substrates (Figure 2). The development of tissue engineering technology has opened up new avenues for treating SCI (Dietz et al., 2006; Furlan et al., 2016; Chen et al., 2018; Yao et al., 2018). The main strategy of tissue engineering is to inoculate living cells on extracellular matrix substitutes that can provide a physical structure for cell growth and differentiation, guide the growth of transplanted cells, and promote axonal regeneration of residual neurons. In general, these complexes of cells and substitutes are formed after a period of co-culture, and are then transplanted within the injured tissue to enhance tissue repair. As extracellular matrix substitutes for SCI, biomaterial scaffolds ought to meet certain requirements. Firstly, they need to keep a balance of softness and mechanical strength, to avoid crushing the surrounding residual tissues and to maintain local structure (Rooney et al., 2008). Secondly, the biomaterials should have appropriate apposite porosity, permeability, surface topography, and good biocompatibility for cells (Wang et al., 2015). In addition, their degradation rate should coordinate with the regeneration rate of axons and tissues (Kubinovà and Sykovã, 2010). This review summarized the progress and challenges in regenerative biomaterial scaffolds for SCI repair. We hope to provide new insights into developing novel composite functional repair scaffolds to mimic a better regenerative microenvironment in SCI repair and achieve better treatment effects.
The philosophy of developing regenerative biomaterials is to mimic the physiological extracellular matrix of the spinal cord and reconstruct a favorable regenerative niche for SCI repair. A schematic of the intact spinal cord microenvironment is shown in Figure 3. Oligodendrocytes and astrocytes are the two main supportive cells in spinal cord tissue. Oligodendrocytes encapsulate axons and form an insulating myelin structure. Astrocytes attach to adjacent neurons and capillary walls through their end feet. The neural extracellular matrix is comprised of a variety of factors, each responsible for different physiological functions. Hyaluronic acid is the main component and forms a huge molecule-sieving mesh to regulate the growth and migration of cells. The lecticans behave as extracellular anchors through binding with secreted cell-surface receptors, hyaluronic acid, and other extracellular matrix partners. Link proteins of the extracellular matrix play a pivotal part in axon guidance and synapse formation. Trophic factors support the growth and functional integrity of neurons. Tenascin accelerates the growth of neural stem cells (NSCs) and reconcile the growth factor signaling system. Proteoglycan regulates the growth and regeneration of nerve cells.
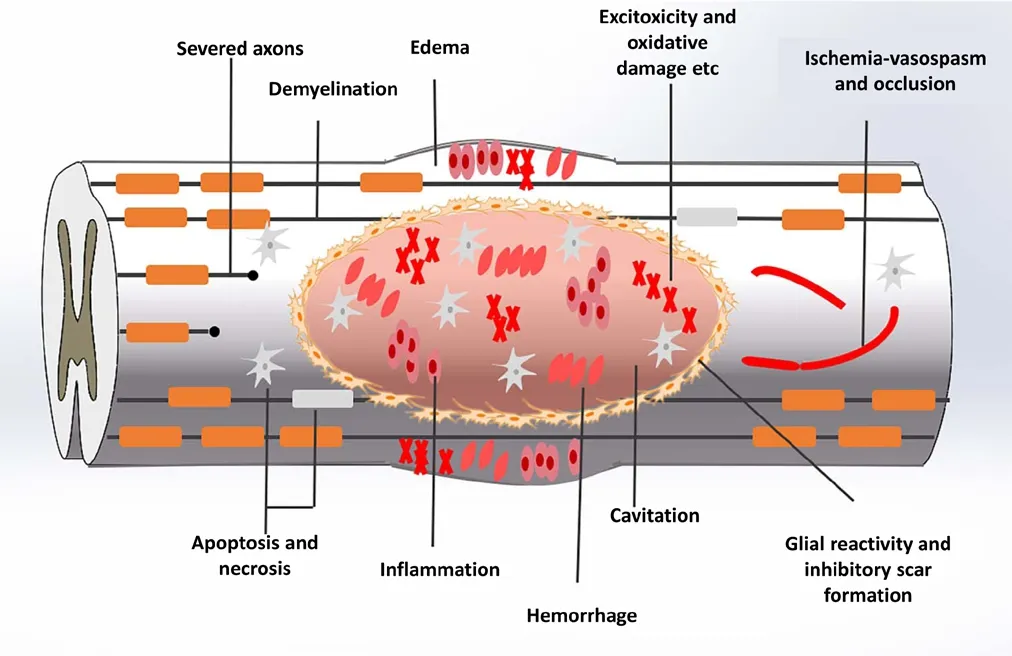
Figure 1 Pathological and physiological changes of spinal cord injury.The initial trauma leads to the rupture of spinal cord nerve tracts,apoptosis, necrosis of neurons, and destruction of blood vessels in the tissue. Secondary injury brings a series of complications, including oxidative stress, inflammatory cell infiltration, spinal cord edema, liquid-filled cavity and glial scars.
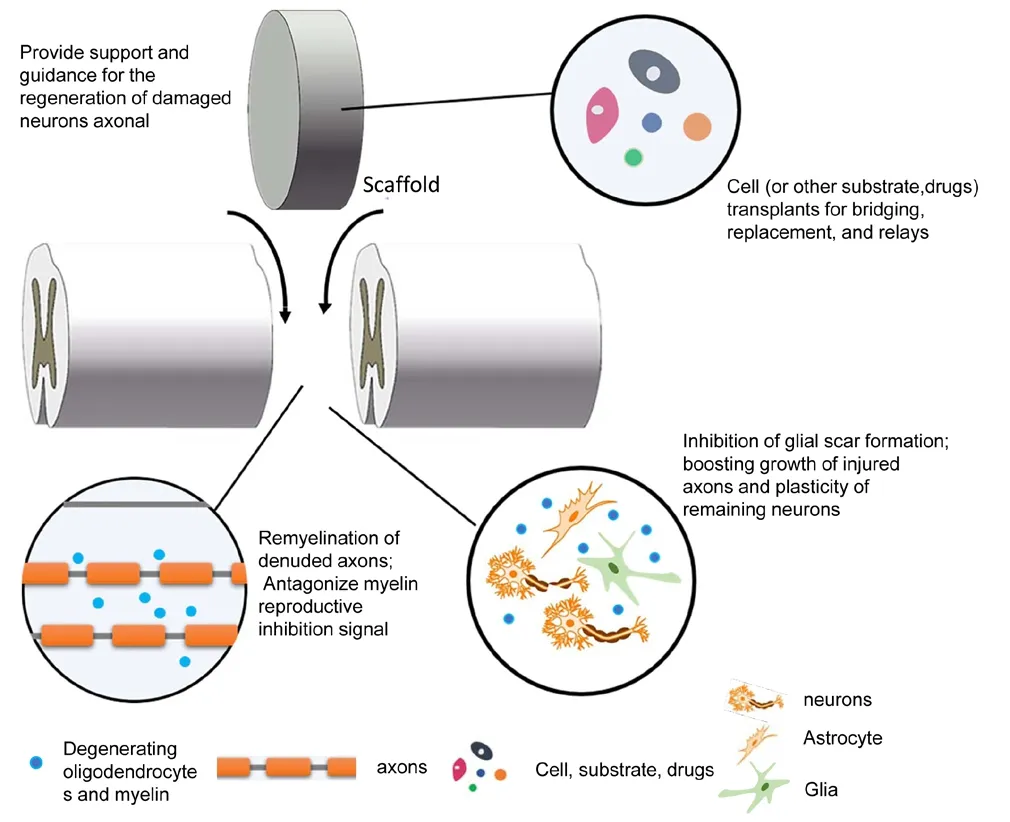
Figure 2 Experimental strategies to stop injury progression and promote the repair of spinal cord injury.
At present, natural, synthetic, and combined materials are used to fabricate biomaterial scaffolds for SCI repair based on their different characteristics. In this review, we summarized the current regenerative biomaterials used for SCI repair to provide new insights into biomaterial fabrication for achieving successful SCI repair.
Search Strategy
The articles included in this review were retrieved using the search terms “spinal cord injury, biomaterials, scaffolds, tissue engineering”. An electronic search of the Medline database for literature describing animal models of SCI from 1946 to 2018 was performed using the following conditions: SCI AND Models, Animal. The results were further screened by title and abstract to only present rats, mice, canines and non-human primates. Non-SCI experiments were also excluded.
In addition, an electronic search of the Medline database for regenerative biomaterials application in SCI repair was completed. This included publications before October 2018, with the following search criteria: “spinal cord injury(SCI), collagen, chitosan, hyaluronic acid, alginate, gelatin,agarose, fibrin, self-assembling peptides, acellular scaffold,poly-ε-caprolactone, poly(lactic acid), poly(lactic-co-glycolic acid), poly-β-hydroxybutyrate, polyethylene glycol, poly 2-hydroxyethyl methacrylate (pHEMA) and poly N-2-hydroxypropyl methacrylamide (PHPMA) and polyvinyl alcohol”. Subsequent searches relevant to clinical application were completed with terms of biomaterials.
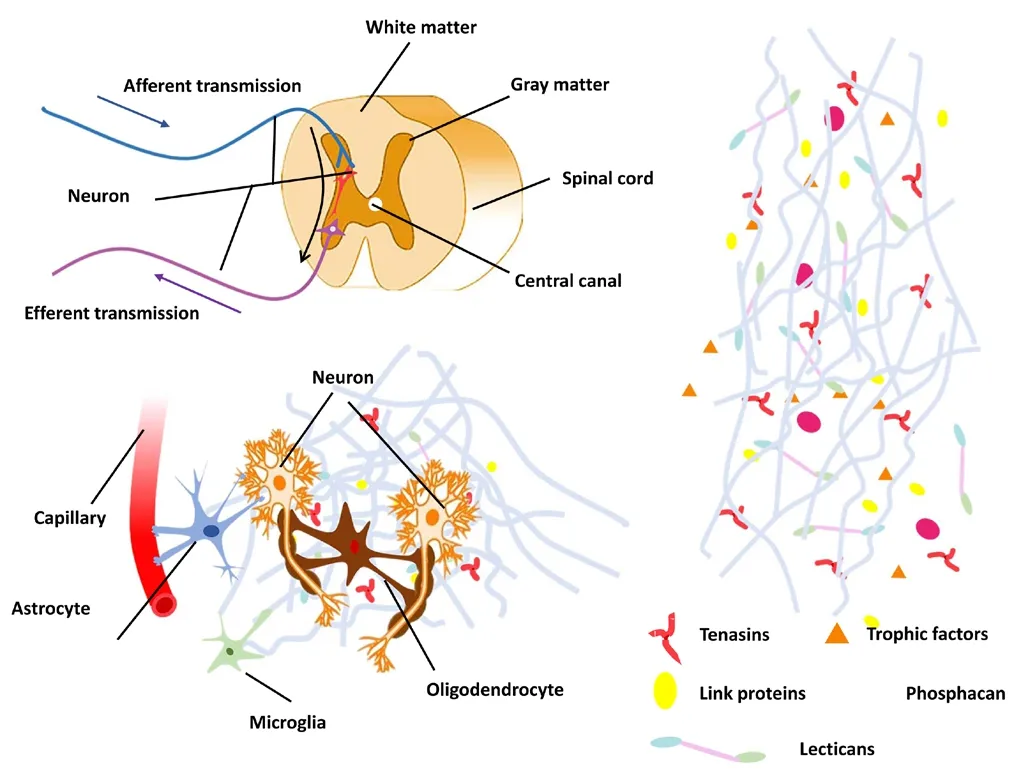
Figure 3 Microenvironment of the spinal cord.(A) Nerve transmission in the spinal cord. Enlargement of boxed area in A is shown in (B). Oligodendrocytes encapsulate axons. Some astrocyte end feet attach to adjacent neurons and capillary walls. (C) Predominant components of the neural extracellular matrix. Hyaluronic acid is the main component of the neural extracellular matrix. Lecticans can bind hyaluronic acid and secrete extracellular matrix proteins and cell-surface receptors. Link proteins play an important role in axon guidance and synapse formation. Trophic factors, tenascin and proteoglycan have a positive effect on the growth of nerve cells.
Natural Materials
Natural materials have been widely used to develop various forms of regenerative scaffolds for SCI repair because of a variety of advantages, such as good biocompatibility,biodegradability, low toxicity, intrinsic cellular interaction,and biological functionality (Libro et al., 2017). According to the previous reports, collagen, chitosan, hyaluronic acid,alginate, gelatin, agarose, and fibrin are the common natural materials applied in SCI repair (Table 1).
Collagen
Collagen is an abundant protein of the extracellular matrix in body tissue and is highly conserved between species. Thus,collagen is very easy to obtain and has low immunological rejection response after transplantation. Importantly, collagen can provide binding sites to support adhesion, migration, proliferation, and even differentiation of cells (Guan et al., 2013; Murphy et al., 2017). Because of the above advantages, collagen has become one of the most popular natural biological materials for treating SCI (Yoshii et al., 2010). In the early days, collagen scaffolds were designed as carriers of neurotrophic factors to promote SCI repair (Houweling et al., 1998a, b). Collagen materials are fabricated into multiple forms of scaffolds, such as sponge, hydrogel, and guidance conduit, to deliver cells, drugs, and proteins into the injured site. For example, an injectable collagen hydrogel (Breenet al., 2016) was developed to deliver neurotrophin-3 (NT-3)directly into the rat spinal cord hemisection injury site; it led to a good outcome including increased neurons, glial scar inhibition, and decreased collagen deposition. In another study, a collagen microfiber scaffold (Sugaiet al., 2015) was seeded with neural stem/progenitor cells and transplanted into a mouse transected SCI model. The microfiber scaffold enhanced the survival of the transplanted cells, but failed to establish complete nerve connection at the injured site and recovery of motor function. In canines with a complete spinal cord transection, linear ordered collagen scaffold (Han et al., 2018) loaded with human mesenchymal stem cells de-rived from placenta had a positive effect on neuronal regeneration and motor function improvement. To enhance the delivery efficiency, neurotrophic factors can be modified to specifically bind to the scaffold. Brain-derived neurotrophic factor has been linked with the collagen-binding domain containing the peptide TKKTLRT derived from collagenase, which was used to bind to the linear ordered collagen scaffold to treat transected spinal cord in canine (Han et al., 2014, 2015). The results showed that this approach can facilitate nerve growth and remarkably improve behavioral and electrophysiological recovery. EphA4 and PlexinB1 proteins can neutralize ephrinB3 and sema4D, respectively,to promote the recovery of SCI. In a previous study, EphA4 and PlexinB1 linked ligand-binding domain were imbedded into a transplanted collagen sponge scaffold (Li et al., 2015)and greatly facilitated axonal regrowth and locomotion recovery in a transacted spinal cord rat model. Additionally,a multichannel collagen conduit (Yao et al., 2013) was used to deliver the NT-3 encoding gene into the completely transected rat thoracic spinal cord, and aligned axon regeneration occurred after a month. In summary, collagen scaffolds have been widely investigated and have shown promise for improving recovery in SCI treatment.

Table 1 Natural regenerative biomaterials for treatment of spinal cord injury
Hyaluronic acid
Hyaluronic acid (also called hyaluronan) is a large natural polysaccharide composed of two disaccharide units including N-acetyl-glucosamide and D-glucuronic acid.Hyaluronic acid has a high concentration in the nervous system, especially in the central nervous system, so it is particularly beneficial for designing scaffolds for SCI repair.Moreover, hyaluronic acid reduces the formation of glial scars by inhibiting the migration, chemotaxis, and proliferation of lymphocytes (Liu et al., 2016b; Sensharma et al.,2017). Hyaluronic acid hydrogel has been found to have neuroprotective effects by relieving secondary injury after SCI (Kushchayev et al., 2016). Furthermore, a combination of peptides, growth factors and stem cells can improve the repair performance of hyaluronic acid scaffolds.
In a previous study, hyaluronic acid scaffolds (Li et al.,2017) were seeded with mesenchymal stem cells (MSCs) and implanted into transected spinal cords of rats. Hyaluronic acid scaffolds and MSCs functioned in synergy and promoted spinal cord tissue repair by inhibiting inflammation and formation of glial scars. In addition, a composite scaffold (Li et al., 2018) of brain-derived neurotrophic factor, genetically modified MSCs and polypeptide-modified hyaluronic acid also effectively alleviated inflammatory response, inhibited glial scar formation, and improved spinal tissue integrity.However, hyaluronic acid materials have some disadvantages such as poor water solubility and cell adhesion capacity(Wang et al., 2012). To overcome these weaknesses, hyaluronic acid can be modified with the integrin binding peptide arginine-glycine-aspartic acid (Zaviskova et al., 2018);such modification enhances the cell adsorption capacity and promotes cell growth on the scaffold. Furthermore, the same effect can be achieved by blending it with other biomaterials. For example, a collagen-hyaluronic acid (Wang et al.,2012) blended scaffold with desired mechanical properties was shown to be beneficial for the differentiation of NSCs in vitro and nerve regeneration after SCI. Other polymers can also be incorporated with hyaluronic acid, enhance its mechanical properties and orchestrate the degradation rate and regeneration rate of transplanted cells and local tissues.
Chitosan
Chitosan is a copolymer composed of N-glucosamine and N-acetyl-glucosamine, and is obtained by the deacetylation of chitin; it is widely found in cell walls of crustaceans, bacilli,and fungi (Patel et al., 2011; Ikeda et al., 2014). Chitosan has been demonstrated to be effective for nerve tissue regeneration in traumatic SCI due to its neuroprotective effect (Cho et al., 2010; Gnavi et al., 2013; Zheng et al., 2017). When implanted into rat spinal cord after a bilateral dorsal hemisection, a chitosan scaffold (Chedly et al., 2017) was shown to reduce fibrous glial scarring, promote the reconstitution of vasculature and spinal tissue, and modulate the inflammatory response in SCI. Another study (Sun et al., 2017) has shown that chitosan scaffold-loaded human umbilical cord-derived MSCs and NT-3 could reduce inflammation and enhance the recovery of motor function after being transplanted in a complete spinal cord transection mouse model. In a rat SCI model, in which the exposed spinal cord was stroked by an NYU impactor, transplantation of chitosan scaffolds (Zhang et al., 2016) enhanced cell viability of transplanted human dental pulp stem cells and neuronal differentiation, and increased production of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, NT-3 and beta nerve growth factor. Jian et al. (2015) transplanted a complex chitosan scaffold with SB216763 (a GSK-3 antagonist) and glioma extracellular matrix into lateral hemisected spinal cord in rats, which enhanced the differentiation of NSCs into neurons, oligodendrocytes and astrocytes. Although chitosan-based scaffolds have made progress in improving functional recovery, there remains a long way to go to treat SCI patients successfully.
Gelatin
Gelatin belongs to a macromolecule hydrophilic colloid. It is the hydrolysate of collagen in connective tissue (Pearlman et al., 1996). This form of biomaterial is easily combined with growth factors and cells for SCI. It was reported that porous gelatin microspheres (Lanet al., 2017) loaded with a basic fibroblast growth factor resulted in neuroprotective effects in a rat spinal cord hemisection model due to a reduction in necrosis, infiltration of leukocytes, and apoptotic cells by sustained release of basic fibroblast growth factor at the transplanted sites. A reduction of cavity area, collagen deposition and inflammation were observed when a gelatin methacrylate hydrogel (Fanet al., 2018) was combined with induced pluripotent stem cell-derived NSCs and then transplanted into a mouse spinal cord transection model. Further,another study showed that a gelatin sponge scaffold (Lai et al., 2016) combined with TrkC (NT-3 receptor) gene-modified NSCs (TrkC-NSCs) and NT-3 gene-modified Schwann cells (NT-3-SCs) created a favorable microenvironment for axonal regeneration and synaptogenesis of NSC-derived neurons in a transected rat spinal cord after 8 weeks. Thus,gelatin-based biomaterial scaffolds have the characteristics of filling the cavity, inhibiting inflammation, and decreasing necrosis and apoptosis when used for SCI repair.
Agarose
Agarose is a kind of linear polymer extracted from seaweed.A previous study in vitro has shown that agarose can be used to guide cell adsorption and neurite outgrowth (Luo et al.,2004). Agarose, along with its derivatives and blends, has been widely applied in tissue engineering and regenerative medicine (Zarrintaj et al., 2018). Han et al. (2018) transplanted a scaffold made of agarose and matrigel into the injured site of transected rat spinal cord, which successfully re-established descending motor projections from motor cortex. Matrigel in this scaffold promoted cell proliferation and axonal regeneration. In addition, agarose scaffolds (Gao et al., 2013) can be manufactured into precise linear arrays to support and guide motor axon regeneration. Further,these agarose scaffolds seeded with syngeneic bone marrow stromal cells and brain-derived neurotrophic factor showed a notable increase in linear growth of long-tract axons across the lesion in a spinal cord transection rat model. It was demonstrated that a combination of agarose, carbomer, arginine-glycine-aspartic acid, three-dimensional (3D)extracellular matrix and human MSCs immunomodulated the proinflammatory environment in a mouse SCI model obtained by an aneurysm clip (Caronet al., 2016), and increased the quantity of M2 macrophages and facilitated a regenerative environment in situ.
Alginate
Alginate is a natural polysaccharide carbohydrate extracted from kelp. It has good biocompatibility and is a popular biomaterial used to fill injured spinal cord (Suzuki et al.,2002). Transplantation of an alginate 3D scaffold (Mojtaba et al., 2016) cultured with NSCs into an aneurysm clip rat model of SCI reduced inflammation scores, lesion sizes, and caspase-3 activity (for apoptosis evaluation) after 12 weeks.To achieve a better repair effect, alginate is often combined with other natural materials and growth factors. For example, a previous study transplanted a silk fibroin/alginate scaffold (Jiao et al., 2017) loaded with nerve growth factor into the central transected spinal cord lesion region of rats. The sustained release of nerve growth factor from the biomaterial was beneficial for the growth and regeneration of neurons,and led to the improvement of motor function after 8 weeks.
Fibrin
Fibrin is mainly derived from plasma proteins and has significant blood or tissue compatibility, without toxic side effects or other adverse reactions to the recipient. In recent years, fibrin-based biomaterials have been widely used in the field of tissue regeneration. Yao et al. (2018) implanted a 3D aligned fibrin hydrogel into a rat dorsal hemisected SCI model, and reconstruction of nerve fibers and blood vessels was observed at the site of injury after 4 weeks. Eight weeks later, new axon regrowth had penetrated throughout the injured site. In another study of transected SCI in rats,adipose-derived MSCs were combined with a fibrin matrix(Mukhamedshina et al., 2018). In addition to the positive effect of fibrin on SCI repair, adipose-derived MSCs also reduced the area of cavities and enhanced tissue retention,jointly contributing to the regeneration of nerve tissue and the recovery of motor function.
Self-assembling peptides
Self-assembling peptides, consisting of short and repeating amino acid units, have been widely applied to SCI regeneration as a biomaterial scaffold. Self-assembling peptide-based scaffolds also have good biocompatibility. An NSCs/self-assembling peptides scaffold (Ye et al., 2016) transplanted into a rat SCI model established by NYU MASCIS impactor improved the motor function of the hind limbs. Ando et al.(2016) found that a self-assembling peptide gel 178 scaffold increased mRNA expressions of nerve growth factor and glial cell line-derived neurotrophic factor, and decreased glial scaring in rats with hemisected SCI. In another study, an FGLmx peptide hydrogel scaffold was prepared by mixing peptide RADA16and FGL (the motif from neural cell adhesion molecule) (Wang et al., 2015). This scaffold promoted NSC proliferation and migration. These results in vitro suggest that this kind of scaffold has great potential for SCI repair.
Acellular scaffolds
Acellular scaffolds have been increasingly applied in tissue engineering, and are fabricated by removing cells from the matrices (namely decellularization), leaving its 3D structure and the remnant extracellular matrix (Nishio, 2009).Thus, this form of acellular scaffold can provide a skeleton structure for the adhesion, proliferation, migration, and differentiation of seeding cells. Acellular spinal cord scaffolds co-cultured with adipose-derived stem cells contributed to functional recovery through facilitation of axonal regeneration and reduction of reactive gliosis in a rat spinal cord hemisection model (Hong et al., 2018). Another study of a rat spinal cord hemisection model demonstrated that bone marrow mesenchymal stem cells seeded into an acellular spinal cord scaffold resulted in remarkable improvement of motor function, and a reduction in the recruitment of macrophages and T lymphocytes around the SCI site (Wang et al., 2017).
Other materials
In addition to the natural regenerative biomaterials listed above, nanomaterials are also used in the diagnosis and treatment of neurological diseases (Zhang et al., 2018).Because of their unique properties and structure, nanomaterials can be designed as drug delivery vectors to cross the blood-brain barrier and deliver specific molecules to target cells with better efficacy and safety than traditional neurological systems (Siddiqi et al., 2018). Yang et al. (2018) developed a biodegradable nanoscaffold by layer self-assembly of MnO2nanosheets. After binding to laminin, drugs or cells,the nanoscaffold was transplanted into a hemisected SCI model; the scaffold facilitated proliferation and neuronal differentiation of transplanted NSCs and reduced the formation of glial scars. The degradation rate of the scaffold was adjusted by changing the number of layers of the assembled MnO2nanosheets. However, the potential neurotoxic effects of nanoparticles limit their application in the treatment of SCI (Sun et al., 2011; Fujioka et al., 2014; Valdiglesias et al.,2015).
These natural materials have a variety of advantages for use as biomaterial scaffolds for SCI repair, but they possess inevitable disadvantages because of their intrinsic properties.For example, the mechanical properties of scaffolds made of pure natural materials are not easy to control. In contrast to synthetic materials, some chemical modifications are more difficult to carry out on natural regenerative biomaterials.Therefore, it remains a big challenge to obtain ideal spinal cord repair scaffolds using these natural materials alone.
Synthetic Materials
Synthetic materials have a long list of advantages for use as scaffolds in regenerative medicine, including low inflammatory response, well-controlled biodegradability, low or non-toxicity, controllable porosity, and customized physicochemical and mechanical properties (Subramanian et al.,2009). Different kinds of synthetic polymers can be mixed together to form a new type of biomaterial with unique characteristics (Subramanian et al., 2009). Thus, synthetic material scaffolds can be applied for adjuvant treatment of injuries in the central nervous system and produce good therapeutic outcomes. Various polymeric regenerative biomaterials have been used in the repair and treatment of SCI to date (Table 2).
Poly-ε-caprolactone
Poly-ε-caprolactone is made of biocompatible and bioresorbable aliphatic polyester, with a good mechanical property but a slow degradation rate (Bechara et al., 2010; Patel et al., 2011). Shahriari et al. (2017) developed a form of porous poly-ε-caprolactone microtubes and reported linear axon growth within both the microtubes and the interstitial space between the tubes, after implanting the poly-ε-caprolactone microtubes into rat transected spinal cords. Moreover, the cell adhesion of poly-ε-caprolactone can be improved by adjusting porosity. Another patterned poly-ε-caprolactone scaffold (Donoghue et al., 2013) in an SCI model showed that soluble factor(s) secreted by astrocytes plated on the scaffold delayed oligodendrocyte differentiation, but did not prevent myelination. In another study, electrospun poly-ε-caprolactone scaffolds (Terraf et al., 2016) with human endometrial stem cells were implanted into the hemisected spinal cords of rats. Observations showed that the scaffold-stem cell restored the continuity of the injured spinal cord and decreased cavity formation.

Table 2 Synthetic regenerative biomaterials for the treatment of spinal cord injury
Poly(lactic acid)
Poly(lactic acid) is a polymer obtained by polymerizing lactic acid, with a good biodegradability. Poly(lactic acid)scaffolds can be prepared into various forms for the regeneration of SCI, including micro- or nanofibers, hydrogels, microporous sponges containing inner channels, and multiwall conduits (Deng et al., 2006; Li et al., 2007; Caiet al., 2010;Aet al., 2011; Hurtado et al., 2011). Aligned poly(lactic acid)microfibers enhanced axonal extension in vitro by providing directional guidance (Corey et al., 2007; Han et al., 2009; Liu et al., 2012) and reduced cystic cavity volume in SCI models(Hurtado et al., 2011; Colello et al., 2013). Poly(lactic acid)scaffolds also could be used as a drug carrier. Electrospun poly(lactic acid) microfibers carrying paclitaxel promoted neurite extension in a growth-conducive and inhibitory environment after SCI (Roman et al., 2016).
Poly(lactic-co-glycolic acid)
Poly(lactic-co-glycolic acid) is a degradable functional organic polymer and is formed by random polymerization of lactic acid and glycolic acid. The physicochemical properties of poly(lactic-co-glycolic acid) can be modulated by adjusting monomer ratios (de Ruiter et al., 2010). Poly(-lactic-co-glycolic acid) has been approved by the Food and Drug Administration as a cell delivery vehicle (Nejatikoshki et al., 2017).
In previous studies, poly(lactic-co-glycolic acid) hydrogels,microspheres, and nanoparticles have been used for spinal cord regeneration and functional recovery (Burdick et al.,2006; Goraltchouk et al., 2006; Stanwick et al., 2012). It has been confirmed that poly(lactic-co-glycolic acid) binding to cells is an effective strategy to improve therapeutic benefits in animal models (Yi et al., 2012). In one study, human NSCs were seeded on poly(lactic-co-glycolic acid) scaffolds(Kimet al., 2010) and then transplanted into hemisected canine spinal cord. The scaffold bridged the damaged spinal cord, and the grafted NSCs showed migratory behavior to the original spinal cord. In another study of a hemisection of segmental thoracic spinal cord in the African green monkey,poly(lactic-co-glycolic acid) scaffolds (Pritchard et al., 2010)combined with human NSCs implanted into the injured site showed potential for clinical application.
Poly-β-hydroxybutyrate
Poly-β-hydroxybutyrate is biopolyester with a high molecular weight, existing in many bacterial cytoplasms. poly-β-hydroxybutyrate degrades slowly after being implanted in the human body and converts into nontoxic metabolites secreted in the urine. Poly-β-hydroxybutyrate has been widely used as a biomaterial for years and also has been approved by the Food and Drug Administration (Sensharmaet al.,2017). poly-β-hydroxybutyrate-HV scaffolds are prepared by freeze-drying poly3-hydroxybutyrate-co-3-hydroxyvalerate. Poly-β-hydroxybutyrate-HV scaffolds demonstrate good tissue compatibility, low immunogenicity, and no tissue necrosis or abscess formation in vivo (Silvina et al., 2013).In addition, Xu et al. (2010) showed that poly-β-hydroxybutyrate-HHx nanofiber scaffolds (copolymer of 3-hydroxybutyrate and 3-hydroxyhexanoate) facilitated the growth and differentiation of NSCs, indicating that poly-β-hydroxybutyrate has potential for treating injuries in the central nervous system. However, because of the fragile mechanical properties of poly-β-hydroxybutyrate, it is not preferred for nerve regeneration. The properties of poly-β-hydroxybutyrate still need to be improved to meet the requirements of SCI repair.
Polyethylene glycol
Polyethylene glycol is a nonionic water-soluble polymer of ethylene oxide hydrolysate. It has an immune-protective effect by preventing cell penetration in the injection area.Polyethylene glycol rapidly reseals the damaged neuronal membrane and decreases mitochondria-derived oxidative stress to protect the injured spinal cord (Nehrt et al., 2010;Kouhzaei et al., 2013; Shi, 2013). Liu et al. (2016a) found that polyethylene glycol mediated by upconversion nanoparticles and combined with photodynamic therapy promoted functional recovery in rats by reducing the formation of glial scars and promoting remyelination of injured axons.A poly(N-isopropylacrylaminde)-g-polyethylene glycol scaffold with brain-derived neurotrophic factor increased the recovery rate of fine motor function (Grous et al., 2013).Brain-derived neurotrophic factor loaded in this scaffold promoted the growth of rubrospinal tract axons into the lesion of rat hemisected spinal cord. In a study by Pang et al. (2016), induced pluripotent stem cells were differentiated into neural precursor cells and grafted to a poly(lactic-co-glycolic acid)/polyethylene glycol nanofiber scaffold.The scaffolds greatly promoted the adhesion, proliferation and differentiation of induced pluripotent stem cell-derived neural precursor cells, and the scaffold combined with cells showed a better therapeutic effect in transected rat spinal cords.
Poly(2-hydroxyethyl methacrylate) and poly[N-(2-hydroxypropyl) methacrylamide]
The Poly(2-hydroxyethyl methacrylate) (pHEMA) hydrogels consist of a network of interconnected hydrophilic copolymers, swelling in water, and provide a 3D matrix for cell adhesion and growth. The pHEMA liquids have low interfacial tension and pHEMA hydrogels possess similar mechanical properties to the spinal cord (Bakshi et al., 2004; Hejčl et al.,2009). The pHEMA or p(HEMA-MMA) hydrogel was shown to be beneficial for the axonal regeneration of brainstem motor neurons in a transection SCI model (Tsai et al., 2004).Moreover, after combining with poly-ε-caprolactone and other neurotrophic factors, pHEMA or p(HEMA-MMA)hydrogels have a more appropriate mechanical strength to guide the directional outgrowth of neuronal tissues (Nomura et al., 2006; Tsai et al., 2006). In addition, pHEMA hydrogels modified with the peptide SIKVAV allowed bridging of the two ends of transected spinal cord (Kubinová et al., 2015).However, obvious functional recovery was not observed after the pHEMA hydrogel was implanted in the SCI site. In addition, non-biodegradability is a disadvantage of pHEMA applied in SCI repair.
As a hydrophilic copolymer, poly[N-(2-hydroxypropyl)methacrylamide] is similar to pHEMA in terms of physicochemical properties. Notably, poly[N-(2-hydroxypropyl)methacrylamide] hydrogels could improve the motor and neurophysiological recovery in SCI repair (Vet al., 2013).This recovery may be due to a more conducive environment to regenerate axons and/or prevent secondary injury and glial scar formation. A form of poly[N-(2-hydroxypropyl)methacrylamide] hydrogel (Woerly et al., 2001) with arginine-glycine-aspartic acid modification has been developed to treat transected SCI of rats, and is commercialized under the name of NeuroGelTM.
Polyvinyl alcohol
Polyvinyl alcohol is a hydrophilic polymer without toxicity.Polyvinyl alcohol has enough mechanical strength for tissue engineering, but does not have satisfactory biocompatibility.Therefore, polyvinyl alcohol should be combined with other polymers to improve its overall physicochemical and biological properties for SCI repair.
Although researchers have made great progress in synthetic materials used for regenerative medicine, especially in central nervous system injuries, some intrinsic physicochemical properties, such as relatively low biocompatibility and slow or no degradability impede their application in SCI repair. Thus, attention is shifting to composite scaffolds made of natural and synthetic materials.
Combination of Natural and Synthetic Materials
To improve the efficacy of SCI repair, combining natural and synthetic biomaterials can improve the performance of scaffolds. For instance, an anti-Nogo receptor antibody was modified on a hyaluronic acid hydrogel scaffold (Wen et al.,2016) and further mixed with poly(lactic-co-glycolic acid)microspheres containing brain-derived neurotrophic factor and vascular endothelial growth factor (hyaluronic acid +poly(lactic-co-glycolic acid)). When this composite scaffold was transplanted to a rat dorsal hemisection of the spinal cord,therapeutic outcomes were observed, including remarkable angiogenesis, neural regeneration and locomotor function recovery. A scaffold synthesized with a tubular prosthesis of poly-ε-caprolactone and aligned collagen (Maturana et al., 2013) directed aligned axonal growth of nerve fibers. In another study, gelatin sponge-electrospun poly(lactic-co-glycolic acid)/ polyethylene glycol nanofibers (Pang et al., 2016)loaded with neural precursor cells promoted functional recovery in rat transected spinal cords. Xu et al. (2017) prepared a composite scaffold by combining acellular spinal cord and poly(lactic-co-glycolic acid) nanoparticles, which was then encapsulated with vascular endothelial growth factor. This poly(lactic-co-glycolic acid)/nanoparticle scaffold promoted angiogenesis and vascular remodeling in a rat spinal cord hemisection model. Composite scaffolds using natural and synthetic materials are a promising research direction for SCI repair in the future. To achieve this goal will require closer cooperation of materials synthesis experts and neurobiologists.
Clinical Application of Biomaterial Scaffolds
Although there are many regenerative biomaterials applied in SCI research, few of them have been translated from bench to clinic. Collagen scaffolds, such as Neuragen®,NeuraWrapTM, NeuroMatrixTM, and NeuroFlexTM, have been approved for clinical use as nerve conduits (Straley et al.,2009). Furthermore, several synthetic material-based products have been approved by the Food and Drug Administration for commercial use (Cai et al., 2010). For example,NeuroTube®, consisting of polyglycolic acid, is an approved scaffold as a nerve conduit. SalutunnelTMand NeurolacTMare made of polyvinyl alcohol and poly(D, L-lactide-co-ε-caprolactone), respectively. Polyvinyl alcohol is also one component of SaluBridgeTM. Some researchers in particular have made great contributions to the development of various scaffolds for SCI repair. Jianwu Dai and colleagues have focused on developing a collagen scaffold for SCI recovery for almost 10 years and they have made recent clinical research progress in this field; they developed a collagen scaffold and transplanted it into the injured spinal cord of patients to investigate its safety and effectiveness (Xiao et al., 2016, 2018;Zhao et al., 2017). During 1-year follow-up, they observed neither early adverse events, such as headache, fever, infection, allergic reaction, shock, or perioperative complications, nor late adverse events, such as cancer or aggravation of neurological status in recipients. In some patients, they observed measures of therapeutic effectiveness, including enhanced finger activity, expansion of sensory level, enhanced trunk stability, and recovery of sense function in the bowel and bladder. One form of chitosan scaffold also has been developed from basic research to clinical research (Amr et al., 2014). In the study, a complication of postoperative seroma formed due to chitosan disintegration. Hematoma complication was observed and led to delayed wound healing. However, recovery was not influenced by seroma formation. Complications reported by other researchers include transient post-operative hypotonicity and meningitis (Dobkin et al., 2006). In addition, the Neuro Spinal Scaffold made of poly(lactic-co-glycolic acid) and poly(L-lysine) has been applied in a clinical research study (Theodore et al., 2016). No safety issues due to the scaffold implantation or procedural complications appeared after 6 months. These studies demonstrate the possibility of these materials for use in clinics.Although we still face huge challenges in tissue engineering,more biomaterial scaffolds as an alternative approach for SCI will be translated for clinical use in the future.
Conclusion and Perspective
We have discussed various biomaterial scaffolds that have been used to treat SCI. The physicochemical and biological properties of these biomaterials and the structure of the scaffolds play an important role in therapy. Hydrogel scaffolds can work well with surrounding spinal tissue because of their similar mechanical properties to spinal cord tissue,and can be implanted into the body through a simple injection. Natural regenerative biomaterials are more suitable for hydrogel scaffolds than solid scaffolds because of their mechanical properties (Itosaka et al., 2010; Alhosseini et al.,2012). Synthetic biomaterials are generally used for fabricating channel-type scaffolds, because they are easy to form into various shapes and structures (Wong et al., 2008).
The biological activity of reparative scaffolds can be improved by seeding with various cells, such as MSCs and NSCs. To enhance the cell adhesive property and promote cell growth, cellular adhesion molecules, such as integrin or cadherin, can be incorporated into the scaffolds. Growth factors, such as basic fibroblast growth factor, brain-derived neurotrophic factor, nerve growth factor and glial cell line-derived neurotrophic factor, are also being integrated into scaffolds to improve biological performance. A combinatorial approach using cells, growth factors and regenerative biomaterials should be applied to attain ideal scaffolds for spinal cord regeneration.
Although some progress has been made in SCI therapy by tissue engineering techniques, there are still many problems to be resolved. It is still a challenge to build an ideal regenerative microenvironment at the lesion site, because of the dynamic pathology of SCI. There are still no uniform international evaluation criteria for the safety of scaffold materials and the effectiveness of treatment. Additionally, the detailed mechanism of SCI treatment by tissue engineering technology has not been fully studied. Each material has its own advantages and disadvantages. It is currently unknown which is most beneficial combination of biomolecules and the best time for transplantation. Moreover, the location and degree of SCI also need to be considered. The microstructure of scaffolds, and controllable distribution of living cells and bioactive molecules need to be further studied.
Future research should focus on developing and applying novel regenerative biomaterials to maximize the potential of drugs and cells. The advances in mechanical engineering,mathematical science, and computer science may together play important roles in screening the best operating parameters of biomaterials. A combinatorial strategy can maximize functional recovery and provide a robust effect that can be translated into the clinic. Furthermore, the combination of neuroprotection and neural inhibition may achieve the desired therapeutic effect. Generally, all characteristics of spinal cord tissue can be modeled and used as a guide to modify biomaterials. We hope that the combination of several strategies can provide a powerful effect for clinical research and application.
Author contributions:All authors participated in the writing and evaluation of the manuscript, and approved the final version of the manuscript.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81571213 (to BW), No. 81800583 (to YYX); the 13thSix Talent Peaks Project (C type) of Jiangsu Province of China (to BW); the Medical Science and Technique Development Foundation of Nanjing of China, No. QRX17006 (to BW); and the Medical Science and Innovation Platform of Nanjing of China, No. ZDX16005(to BW). The funding sources had no role in manuscript conception and design, data analysis or interpretation, manuscript writing or deciding to submit this paper for publication.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Josue Ordaz, University School of Medicine, USA;Stephanie K. Seidlits, University of California Los Angeles, USA.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Improvement of ataxia in a patient with cerebellar infarction by recovery of injured cortico-ponto-cerebellar tract and dentato-rubro-thalamic tract: a diffusion tensor tractography study
- Tandem pore TWIK-related potassium channels and neuroprotection
- Dendritic shrinkage after injury: a cellular killer or a necessity for axonal regeneration?
- Regenerative biomarkers for Duchenne muscular dystrophy
- Exploring the efficacy of natural products in alleviating Alzheimer's disease
- Role of macrophages in peripheral nerve injury and repair

