Cell structure of microcellular combustible object foamed by supercritical carbon dioxide
Ya-jun Ding, San-jiu Ying, Zhong-liang Xiao, Xu Wu
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
Keywords:Cell structure Microcellular combustible object Solubility Foaming temperature Foaming time
A B S T R A C T In order to solve the issue that the combustible objects for cased telescoped ammunition (CTA) didn't burn completely during the combustion process, the microcellular combustible objects were foamed with numerous cells in the micron order to improve the combustion performance by the supercritical carbon dioxide (SC-CO2) foaming technology. As the cell structure determined the combustion properties of microcellular combustible objects, the solubility of SC-CO2 dissolved into the combustible objects was obtained from the gravimetric method,and scanning electron microscope(SEM)was applied to characterize the cell structure under various process conditions of solubility,foaming temperature and foaming time. SEM images indicate that the cell diameter of microcellular combustible objects is in the level of 1 μm and the cell density is about 1011 cell·cm-3. The microcellular combustible objects fabricated by the SC-CO2 foaming technology are smooth and uniform, and the high specific surface area of cell structure can lead to the significant combustion performance of microcellular combustible object for CTA in the future.
1. Introduction
In the traditional ammunition, the warheads are located in front of artillery shells, and the gun propellants are filled in the artillery shells. The concept of cased telescoped ammunition(CTA) was put forward in 1954 by the American air force. The warheads of CTA is placed inside the cylinder of whole cartridge,and the short length of ammunition cuts down the storage space.The regular and simple shape of CTA simplifies the design of shell magazine. Meanwhile, CTA has the higher charge of gun propellant than the traditional ammunition, resulting in improving the launch power. CTA has the features of small volume, light weight, low cost, high muzzle speed and long range, and it has been drawn more and more attention in the field of weapons[1-3]. The combustible object technology is often applied as the caseless ammunition cartridge case for CTA. The combustible object not only has the function of metal case for CTA charge,but also acts as a part of energy to provide the launch power for ammunition. Moreover, the weight of combustible object is far lower than that of the metal case, enhancing the bomb load.
However, as the traditional combustible objects are the polymer based composite materials, they cannot burn completely in the shooting process of gun weapons. Many researchers explore the ways to improve the oxygen balance and the combustion process from the aspects of the formula system and the internal structure of combustible object. B¨ohnlein-Mauß and his coworkers [4,5] produces the foamed thermoset polyurethane bonded hexogen (RDX) propellants by the reaction injection moulding process, which presents good vulnerability performance and high burning rate. Ying [6-8] puts forward the supercritical carbon dioxide (SC-CO2) foaming technology to prepare the microcellular gun propellants,and the presence of cell structure enhances the specific surface area, which promotes the combustion property of gun propellants. Based on this principle,Yang [9,10] reports the mechanical and combustion properties of microcellular combustible objects with hexogen/poly(methyl methacrylate) (RDX/PMMA) and hexogen/cellulose acetate (RDX/CA) formula system. The microcellular combustible objects performs higher burning rate than the original samples, but the mechanical strength is not ideal for CTA.
In order to solve the current problems of combustion and mechanical properties, triethylene glycol dinitrate (TEGN) is used as an energetic binder in this work, and it can also absorb some amounts of CO2. The content of RDX (45%) and TEGN(45%) is as high as 90% to improve the oxygen balance of combustible objects. A small amount of PMMA is added to adjust the foaming property of combustible objects [11]. Methyl methacrylate (MMA), acted as the thermoplastic elastomer, is blended with the matrix to prepare the novel semiinterpenetrating network structure, which promotes the mechanical strength of samples.
The numerous cells in the microcellular combustible objects have crucial effects on the mass transfer process of the burning gas,and the distribution of cells determines the stress condition when the combustible objects are burning. Therefore, it is foundational and important to investigate the cell structure of the microcellular combustible objects to improve the combustion and mechanical properties in the future.In the aspect of the formation mechanism of cell structure,the classic nucleation theory is applied to expound cell nucleation as following [12,13]where N0is the cell nucleation rate,C0is the fluid concentration,T is temperature, ΔG*homis the free energy, f0is the frequency factor and k is the Boltzmann constant.

According to Eq. (1), the fluid concentration and temperature are the main factors affecting the cell nucleation rate. Besides, the time of cell growth decides the cell distribution. Therefore, the solubility of SC-CO2, foaming temperature and foaming time are three determining factors influencing the cell structure. In this work, the solubility of SC-CO2dissolved in combustible objects with high energetic component (90%) is measured by the gravimetric method and Fickian diffusion law. The cell structure of the microcellular combustible object is characterized by scanning electron microscope (SEM), and the microcellular combustible objects for CTA is fabricated by the SC-CO2foaming technology and foaming mould.
2. Experimental
2.1. Materials
TEGN and RDX (10 μm) were provided by Luzhou North Chemical Industries Co.,LTD.PMMA(280 nm)and MMA were purchased from Nanjing Zhanyi Co., LTD. Ethanol (analytically pure, AR) and ethyl acetate (AR) were purchased from Sinopharm Chemical Reagent Co., LTD. Industrial CO2(purity≥99.9%) was supplied by Nanjing Wenda Special Gas Co., LTD.
2.2. Sample preparation
PMMA powder (2%), MMA (8%), TEGN (45%) and RDX (45%)were preliminarily kneaded in the mixed solvents of ethanol and ethyl acetate for 30 min to prepare the dough by a kneading machine (Jiangsu Guomao Reducer Group Co., LTD), while the bath temperature of the kneading machine was around 25°C.The dough was manufactured into sheets through the hydraulic machine and molding mould and the length,width and thickness of the samples were 12 mm, 12 mm and 2 mm, respectively.Samples were dried in the oven for 24 h at 60°C to drive out the mixed solvents. Meanwhile, the density of combustible object was 1.4 g·cm-3measured by the method of volumetric flask.
2.3. Foaming process
The microcellular combustible objects were foamed with SC-CO2by the typical intermittent warming-up supercritical process as Fig. 1. Firstly, the samples were placed in a high pressure vessel,and SC-CO2was injected into the vessel at the set saturation temperature (Ts) and saturation pressure (Ps) for some hours(saturation time, ts). Then the samples were removed from the vessel into a water or methylsilicone oil bath at a fixed temperature(foaming temperature, Tf) for several seconds (foaming time, tf).Thirdly,the samples were quenched into cold water later to prevent the growth of cells. Finally, the microcellular combustible objects were dried at 60°C in a water-jacketed oven for a week to wipe out the water.
On the other hand, the solubility property of SC-CO2in the combustible objects were investigated as our previous research[14].The samples were immediately taken off from the vessel to an analytical scale, and the data were recorded every 10 s.
2.4. Characterization
QUANTA FEG 250 scanning electron microscope (SEM, FEI LTD)was applied to observe and record the cell structure of the microcellular combustible objects. The foamed samples were immediately fractured after being immersed in the liquid nitrogen for about 10 min,and then the fractured surfaces were sputtered with gold. The cell structure was recorded with SEM at an acceleration voltage of 10 kV. The mean cell diameter and cell density of microcellular combustible objects were analyzed by Image-Pro Plus[15].
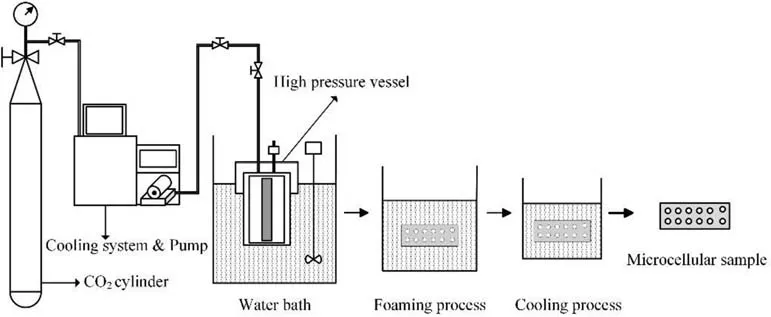
Fig.1. Flow chart of two-step foaming process.
3. Solubility property of SC-CO2 in combustible objects
As the sorption and desorption process of SC-CO2is complex,Fickian diffusion law is the easy and feasible method to solve the solubility property of SC-CO2in combustible objects[16].And our previous works have indicated that the gravimetric procedure and Fickian diffusion law are suitable to describe the mass transfer process of SC-CO2in gun propellants.
3.1. Effect of saturation time on solubility
Based on the Fickian diffusion law, the desorption curve of CO2in combustible objects is shown as Fig. 2 at 40°C and 20 MPa for 24 h when the desorption time is short. The figure displays the linear relationship between the square root of desorption time and the solubility of SC-CO2.By extrapolating the main linear-fit curve,the solubility can be obtained when the square root of desorption time is zero. And the linear-fit equation is calculated as following(R2=0.99)

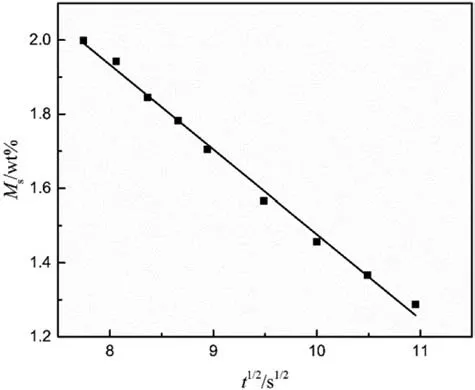
Fig. 2. Desorption curve of CO2 in combustible objects.
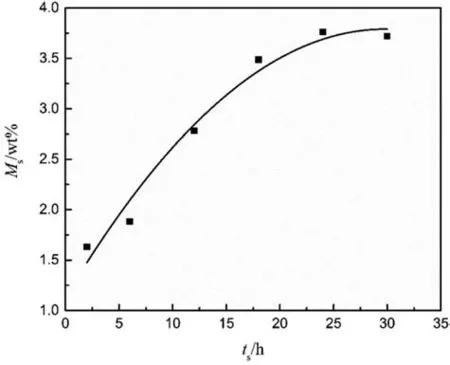
Fig. 3. Solubility curve of CO2 in combustible objects for various saturation time.
The equation means that the solubility of SC-CO2in combustible objects is 3.76 wt% under this condition. In the matrix of the combustible objects,SC-CO2can just be dissolved into PMMA and TEGN,while RDX doesn't absorb SC-CO2in the saturation process.The interactions between SC-CO2and polymers determine the solubility of SC-CO2dissolved into the polymers, and the Lewis acid-base theory is suitable to explain this phenomenon. As the carbon atom is the electron acceptor,SC-CO2acts as the Lewis acid,and the electron donor of carbonyl group makes PMMA as the Lewis base.The strong binding force produced from the Lewis acidbase action leads to the high solubility of SC-CO2in PMMA.Meanwhile, there is a literature reported that the solubility of SC-CO2in PMMA is approximately 18.17 wt%at 40°C and 10.5 MPa[17].The content of PMMA and TEGN is 2%and 45%in the formula of this work, respectively, so PMMA plays the crucial role in the solubility of SC-CO2.
The sorption and desorption process of SC-CO2dissolved into combustible objects is a diffusion process related to the saturation time. Based on the analysis above, the solubility for different saturation time is shown as Fig.3 at 40°C and 20 MPa.In the initial period of the diffusion process, the solubility increases with the increasing saturation time, and it trends to reach an equilibrium status (3.76 wt%) when the saturation time is more than 24 h. The concentration gradient of CO2is the main cause for this phenomenon.When the saturation time is less,the concentration of CO2in combustible objects is far less than that in the supercritical fluid surroundings,and it makes the fast sorption process of SC-CO2.But when the saturation time is more than 24 h,the sorption process of SC-CO2is equal to the desorption process, which leads to the dynamic equilibrium of solubility.
3.2. Effect of saturation temperature on solubility
The equilibrium process of solubility is connection with the thermodynamic and heat transfer,so the saturation temperature is an important factor to the solubility. Table 1 is the solubility of SC-CO2at various saturation temperatures for 24 h when the saturation pressure is 20 MPa. It exhibits that the solubility of SC-CO2constantly reduces while the saturation temperature increases.The solubility is 2.06 wt%when the saturation temperature is 60°C, and the solubility is 3.76 wt% when the saturation temperature is 40°C,which means that the decrement is 45.21%.In the solubility process, the saturation temperature has effects on many properties of the combustible object/SC-CO2system, such as the viscosity, structural strength and diffusion rate.
The increase of saturation temperature causes two competing mechanisms to the solubility of SC-CO2. The higher saturation temperature improves the free volume of combustible object and the kinetic motion of SC-CO2, which promotes the amount of SC-CO2dissolved into the matrix.However,the attraction between combustible object and SC-CO2is weakened by the higher saturation temperature, and it is not in favor of the dissolution of SC-CO2. Meanwhile, according to Arrhenius equation, the saturated vapor pressure rises with the higher temperature, which results in the escape tendency of SC-CO2. In the sorption and desorption process of SC-CO2, the primary factor decides the direction of solubility.As the latter factor plays the governing role in this case, the amount of SC-CO2reduces with the increasingsaturation temperature.

Table 1Solubility of CO2 in combustible objects at various saturation temperatures.

Table 2Solubility of CO2 in combustible objects at various saturation pressures.
3.3. Effect of saturation pressure on solubility
Saturation pressure is also an effective factor to the solubility.Table 2 is the solubility of SC-CO2at various saturation pressures while the saturation temperature is 40°C and the saturation time is 24 h. In the injection period, more CO2is injected into the high pressure vessel to obtain higher saturation pressure, and it makes the concentration gradient of CO2higher. Moreover, the potential energy of CO2is strengthened by the increasing pressure. These effects cause the growth trend of solubility. However, the increment of solubility is not remarkable when the saturation pressure increases from 10 MPa to 30 MPa in the supercritical region, so 20 MPa is selected as the suitable saturation pressure for the saturation process.
4. Cell structure of microcellular combustible object
4.1. Effect of solubility on cell structure
Due to the investigation of solubility above,the saturation time makes the most remarkable influence on the solubility of SC-CO2in combustible objects,so the saturation time is changed to reveal the impact of SC-CO2amount on the cell structure of microcellular combustible objects. Fig. 4 is the SEM images of cell structure for different saturation time when the saturation temperature,saturation pressure, foaming temperature and foaming time are 40°C, 20 MPa, 100°C and 30 s, respectively, and Table 3 is the diameter and density of cells. Compared with the original sample,the microcellular combustible objects own numerous cells in the level of 1 μm,and the amount of cells is around 10×1010cell·cm-3,which demonstrates that the SC-CO2foaming technology is ideal for fabricating microcellular combustible objects.And the presence of PMMA guarantees the uniform distribution of cells structure,promoting the ballistic stability of CTA. Because the more amount of SC-CO2dissolved into the combustible objects enhances the quantity and power for cell nucleation,the SEM images and Table 3 indicate that both the diameter and density of cell increase with the increasing saturation time.The values of cell diameter and density can be applied to explanation the specific surface of the microcellular combustible objects in the two-dimensional structure.
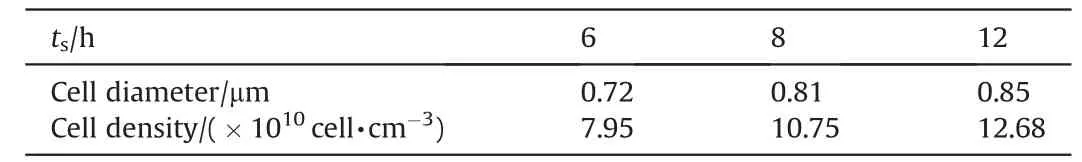
Table 3Cell diameter and density for different saturation time.
4.2. Effect of foaming temperature on cell structure
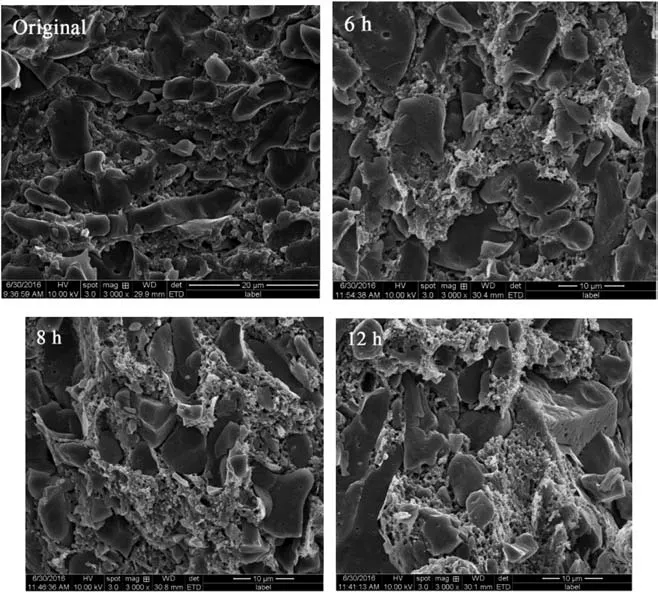
Fig. 4. SEM images of cell structure for different saturation time.
The foaming process is vital for the cell nucleation and growth,and the foaming temperature determines the nucleation power in intermittent warming-up supercritical process. Fig. 5 is the SEM images of cell structure at different foaming temperatures, and Table 4 is the diameter and density of cells. It shows that the cell density increases with the increasing foaming temperature when the foaming temperature is under 100°C, but the cell density trends to decrease when the foaming temperature is higher than 110°C. According to the classic nucleation theory, under the condition of the same amount of SC-CO2, the temperature difference between saturation and foaming temperature is the main factor for the driving force of cell nucleation, and the higher foaming temperature leads to the higher nucleation rate. Therefore, the higher foaming temperature contributes to increasing the diameter and density of cell. However, the foaming process is too fierce that it comes to cell coalescence and collapse when the foaming temperature is higher than 110°C,and the weak binding force between TEGN and RDX results in the exfoliation of RDX. What is worse,some amount of RDX separates from the matrix when the foaming temperature is 120°C.Hence,the foaming temperature needs to be controlled in a suitable range to improve the diameter and density of cell and maintain the enough strength of cell structure. In this work,the foaming temperature is located in the range from 90°C to 100°C.
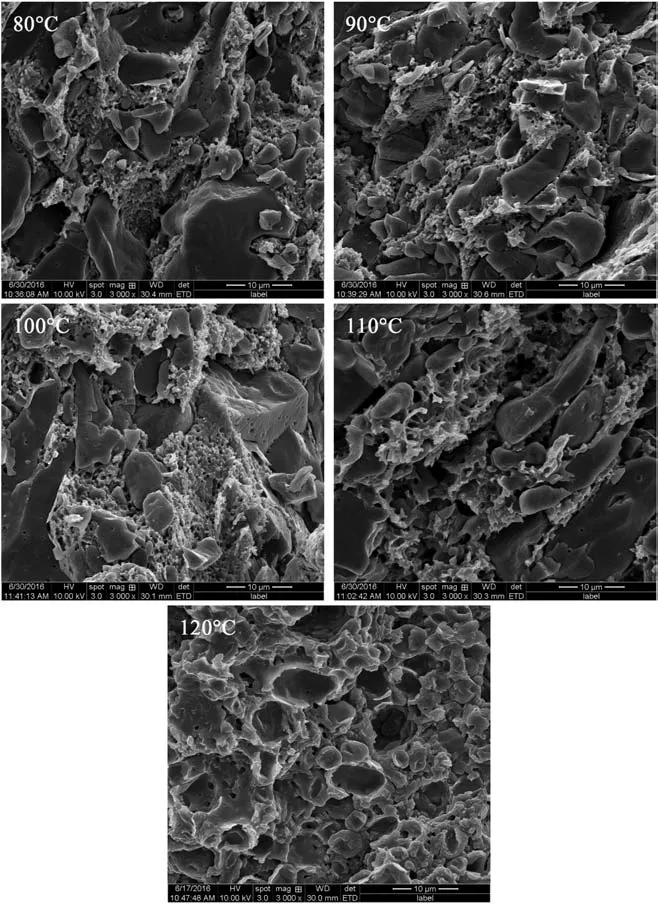
Fig. 5. SEM images of cell structure at different foaming temperatures.
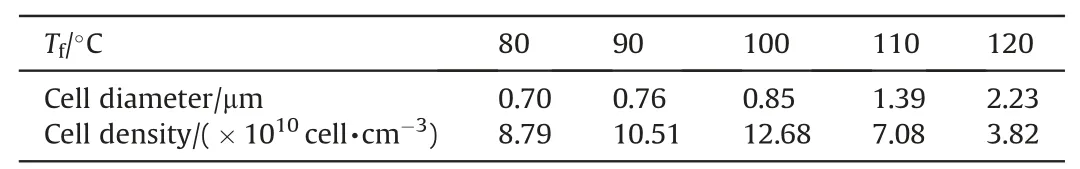
Table 4Cell diameter and density at different foaming temperatures.
4.3. Effect of foaming time on cell structure
The growth and shaping of cell are related to the foaming time in foaming process. Fig. 6 is the SEM images of cell structure for different foaming time when the foaming temperature is 100°C,and Table 5 is the diameter and density of cells.It is obvious that the diameter of cell rises with the increasing foaming time. Whereas,because of the immoderate growth of cell, it comes to the cell coalescence and collapse when the foaming time is 600 s. Table 5 reveals that the cell density decreases from 12.68×1010cell·cm-3to 5.93×1010cell·cm-3while the foaming time increases from 30 s to 600 s, and the decrement is 53.23%. In addition,the matrix is soaked in the boiling water for a long period,which leads to the weakness of the binding force between TEGN and RDX. In consequence,the foaming time is set near to 60 s.
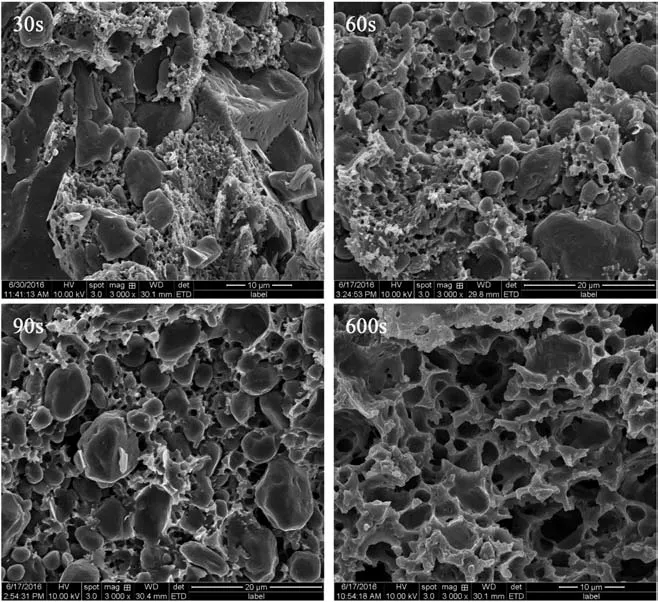
Fig. 6. SEM images of cell structure for different foaming time.
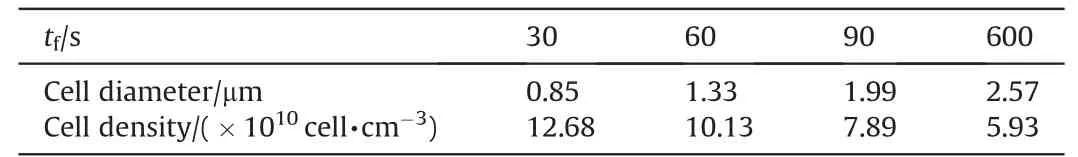
Table 5Cell diameter and density for different foaming time.
4.4. Fabrication of microcellular combustible object for CTA
According to the cell structure researched above, the microcellular combustible objects for CTA are fabricated by the SC-CO2foaming technology. The combustible objects are compressed by the hydraulic machine and molding mould,then they are foamed in the foaming mould. The samples of microcellular combustible objects for CTA are shown as Fig. 7, and Fig. 8 is the cell structure of microcellular combustible object.Because the cells are in the level of 1 μm and the foaming process is controlled in the foaming mould,the surface of microcellular combustible objects is smooth.The cells display the uniform distribution in the microcellular combustible object, and the high specific surface area can lead to the significant combustion performance for CTA.
5. Conclusions

Fig. 7. Samples of microcellular combustible objects for CTA.
The cell structure of microcellular combustible objects for CTA was characterized with the methods of SEM in this work.According to the classic nucleation theory, the effects of SC-CO2solubility,foaming temperature and foaming time on cell structure were investigated as three main factors. The results demonstrate that PMMA plays the crucial role in the solubility of SC-CO2, and the solubility is 3.76w%when the saturation temperature,pressure and time are 40°C,20 MPa and 24 h,respectively.SEM images indicate that the microcellular combustible objects own numerous cells in the level of 1 μm by the SC-CO2foaming technology, and the density of cell is in the level of 1011cell·cm-3. Both the diameter and density of cell increase with the increasing saturation time,resulting from the higher amount of SC-CO2. The foaming temperature located in the range from 90°C to 100°C is the ideal foaming temperature to improve the diameter and density of cell and maintain the enough strength of cell structure.Meanwhile,60 s is a suitable foaming time to prevent the weakness of the binding force between TEGN and RDX,which causes the separation of RDX from matrix. The microcellular combustible objects for CTA, fabricated by the SC-CO2foaming technology,are smooth and uniform,and the high specific surface area of cell structure can lead to the significant combustion performance for CTA.

Fig. 8. Cell structure of microcellular combustible object for CTA.
- Defence Technology的其它文章
- Body armour - New materials, new systems Ian G. Crouch*
- Special materials in pyrotechnics VII: Pyrotechnics used in thermal batteries☆
- Real-time calculation of fragment velocity for cylindrical warheads
- Heavy metal free primers: Polymorphism of gadolinium and titanium in the context of GSR glass phase Felice Nunziata
- Mitigation of EDFA transient effects in variable duty cycle pulsed signals
- Ballistic impact properties of woven bamboo- woven E-glassunsaturated polyester hybrid composites

