以HKUST-1为前驱体的Cu@Pt/C催化剂的结构及对甲醇的催化氧化性能研究
龙翔宇,雷霆,王坤婵,詹振翔
以HKUST-1为前驱体的Cu@Pt/C催化剂的结构及对甲醇的催化氧化性能研究
龙翔宇,雷霆,王坤婵,詹振翔
(中南大学 粉末冶金国家重点实验室,长沙 410083)
以硝酸铜和均苯三甲酸为原料,水热合成八面体结构的金属有机物框架(HKUST-1)前驱体,前驱体在Ar保护下煅烧发生热分解和碳热还原,得到Cu/C纳米颗粒,再与氯铂酸钾通过置换反应得到Cu@Pt/C催化剂。利用扫描电镜、X射线衍射仪以及X射线光电子能谱仪对Cu@Pt/C催化剂的形貌与结构进行表征,并测试其对甲醇溶液的电催化氧化性能。结果表明,Cu@Pt/C催化剂保留了前驱体HKUST-1的八面体结构,Pt包覆在Cu表面形成核壳结构。根据Cu@Pt/C催化剂在H2SO4溶液中的循环伏安曲线,计算出其电化学活性面积为74.3 m2/g,约为商业Pt/C的1.47倍,并且Cu@Pt/C催化电极在H2SO4与甲醇混合溶液中的循环伏安扫描曲线的正扫过程中的峰值电流密度(f)与负扫过程中的峰值电流密度(b)的比值(f/b)为2.18,远高于商业Pt/C电极,表现出优异的对甲醇的电催化氧化活性和抗CO中毒性能。
核壳结构;甲醇氧化;电催化;金属有机框架;置换反应
直接甲醇燃料电池(direct methanol fuel cell,DMFC)的功率密度大,转换效率高,在移动设备电池尤其是汽车动力电池领域具有广阔的发展前景,成为新能源领域的研究热点。然而目前直接甲醇燃料电池最常用的催化剂金属Pt因为过高的价格和稀缺性,限制了其商业应用[1]。同时,在催化甲醇氧化的过程中,甲醇分解产生的CO吸附在Pt的表面,阻碍反应进一步进行,严重影响Pt的催化性能和稳定性[2]。为了降低Pt的用量并提升其催化活性,一个有效的方法是将非贵金属元素作为合金元素,与Pt形成合金催化 剂[3−7]。在众多铂基双金属催化剂中,核壳结构的铂基催化剂由于具有更高的催化活性及稳定性而受到关注。置换法是一种制备核壳结构的铂基催化剂的简便方法,即以一种非贵金属作为核,通过置换反应在其表面包覆一层金属Pt[8]。铜是一种低成本的过渡金属元素,具有优良的导电性能及电催化性能[9],ADZIC等[10]首次采用Pt,Pd 或者 Ag置换单原子层Cu来制备核壳结构催化剂。KOKKIDINIS等[11]通过电化学沉积制备Cu或者Pd纳米颗粒,然后通过置换反应在其表面包覆一层Pt,得到具有核壳结构的铂基催化剂。SOTIROPOULOS等[12]以Cu/C粉末为前驱体,通过置换反应得到核壳结构的Cu-Pt催化剂,其催化甲醇氧化的性能优于商业20%Pt/C催化剂。El-Khatib等[13]制备了核壳结构的Cu@Pt催化剂并将其负载于XC-72R炭黑上,对甲醇具有良好的催化氧化活性,这是由于Cu的引入改变了Pt的电子结构[14−15]。现有的核壳结构Cu@Pt催化剂的大量文献报道,均采用炭黑、碳纳米管或普通碳粉作为载体,具有特殊几何结构的多孔碳材料载体的应用还不多见。金属有机物框架(HKUST-1)是一种新型的多孔材料,由金属离子与提供电子的有机配体构建而成,具有大的比表面积和特殊的几何构型,可以为反应提供更多的活性位点,提高催化剂的活性面积[16]。因此,本文以硝酸铜和均苯三甲酸为原料,通过水热法制得金属有机物框架化合物(HKUST-1),并将其作为碳源和铜源前驱体,在高温下煅烧发生热分解和碳热还原,再与氯铂酸钾溶液发生置换反应,制备以C为载体的Cu@Pt催化剂,并对Cu@Pt/C催化剂的结构及其对甲醇的电催化氧化性能进行测试,对于降低直接甲醇燃料电池的成本以及扩大其应用具有重要意义。
1 实验
1.1 Cu@Pt/C催化剂的制备
所用原料为:三水合硝酸铜(Cu(NO3)2∙3H2O),国药集团化学试剂有限公司,纯度≥99%;均苯三甲酸(H3BTC),国药集团化学试剂有限公司,纯度≥99%;聚乙烯吡咯烷酮(PVP),国药集团化学试剂有限公司,纯度≥99%;氯铂酸钾(K2PtCl6),阿拉丁试剂(上海)有限公司,纯度≥99%。
取1.305 g Cu(NO3)2·3H2O溶于18 mL蒸馏水中,取0.630 g H3BTC溶于18 mL乙醇溶液中。将这2种溶液混合均匀,再加入0.360 g PVP,超声搅拌30 min,然后倒入容积为50 mL的聚四氟乙烯反应釜中,置于烘箱内于120 ℃水热反应12 h,室温冷却后,用蒸馏水洗涤4次,最后于60 ℃下真空干燥,得到HKUST-1粉末,作为Cu/C的前驱体。将HKUST-1粉末置于管式烧结炉中,在600 ℃Ar气氛中保温2 h,发生热分解和碳热还原反应,得到Cu/C粉末。取少量Cu/C粉末,在磁力搅拌下加入到K2PtCl6与H2SO4的混合溶液中(混合液中K2PtCl6与H2SO4的浓度分别为4 mmol/L和0.5 mol/L),反应时间为30 min。反应结束后,将溶液离心并用蒸馏水洗涤、干燥,得到Cu@Pt/C粉末。
1.2 测试与表征
取3 mg Cu@Pt/C粉末加入到1 mL乙醇与水的混合溶液中(乙醇与水的体积比为1:4),再加入80 µL的Nafion溶液配制成浆料,超声分散30 min。取5 µL浆料滴于玻碳电极表面并干燥,即制得Cu@Pt/C工作电极。
用上海华辰CHI 660D电化学工作站测定Cu@ Pt/C电极的循环伏安曲线和计时电流曲线。采用三电极体系,分别用石墨电极和饱和甘汞电极(saturated calomel electrode, SCE)作为对电极和参比电极,以N2饱和的浓度为0.5 mol/L的 H2SO4溶液作为电解液,测定循环伏安曲线。以N2饱和的0.5 mol/L H2SO4+ 1 mol/L CH3OH溶液作为电解液,测定电极对甲醇的催化氧化活性。在进行电化学性能检测之前,将工作电极置于浓度为0.5 mol/L的 H2SO4溶液中,以100 mV/s的扫描速率循环伏安扫描25圈进行电极活化。另外,采用商业20%Pt/C进行对比试验。
2 结果与讨论
2.1 形貌与结构
图1所示为HKUST-1,Cu/C和Cu@Pt/C的SEM与TEM形貌。从图1(a)看出,Cu(NO3)2·3H2O与有机物H3BTC水热反应后,得到由铜离子和有机物配体构成的金属有机物框架化合物HKUST-1为八面体结构,且表面光滑,粒径均一,约为1 μm左右,与文献报道一致[17−19]。HKUST-1经过600 ℃高温处理后得到的Cu/C依旧保留前驱体的八面体结构,但其表面变得粗糙(如图1(b)所示),能谱分析结果表明其由C,Cu,O三种元素组成,这表明HKUST-1中的有机物在高温下分解,同时Cu2+离子被碳热还原为金属Cu。O元素的存在可能是由于Cu/C在空气中发生了部分氧化[19]。图1(c)所示为Cu/C在氯铂酸钾溶液中发生置换反应得到的Cu@Pt/C颗粒形貌。与Cu/C相比,Cu@Pt/C颗粒的形貌没有大的变化,Cu@Pt纳米颗粒均匀分散于八面体碳骨架表面。通过能谱分析得出C,Cu与Pt的原子分数分别为79.37%,12.54%和8.09%,这表明Pt与Cu颗粒表面的Cu发生了置换反应,形成Pt包覆在Cu表面的核壳结构。图1(d)和(e)所示为Cu@Pt/C颗粒的TEM形貌,可看出碳骨架保留完好,Cu@Pt纳米颗粒均匀分布,粒径约为2 nm。图1(f)为Cu@Pt/C颗粒的HR-TEM图,可得出其晶面间距为0.22 nm,介于Pt(111)晶面间距与Cu(111)晶面间距之间,这可能是由于Pt在Cu表面生长时导致Cu发生了晶格畸变[20]。
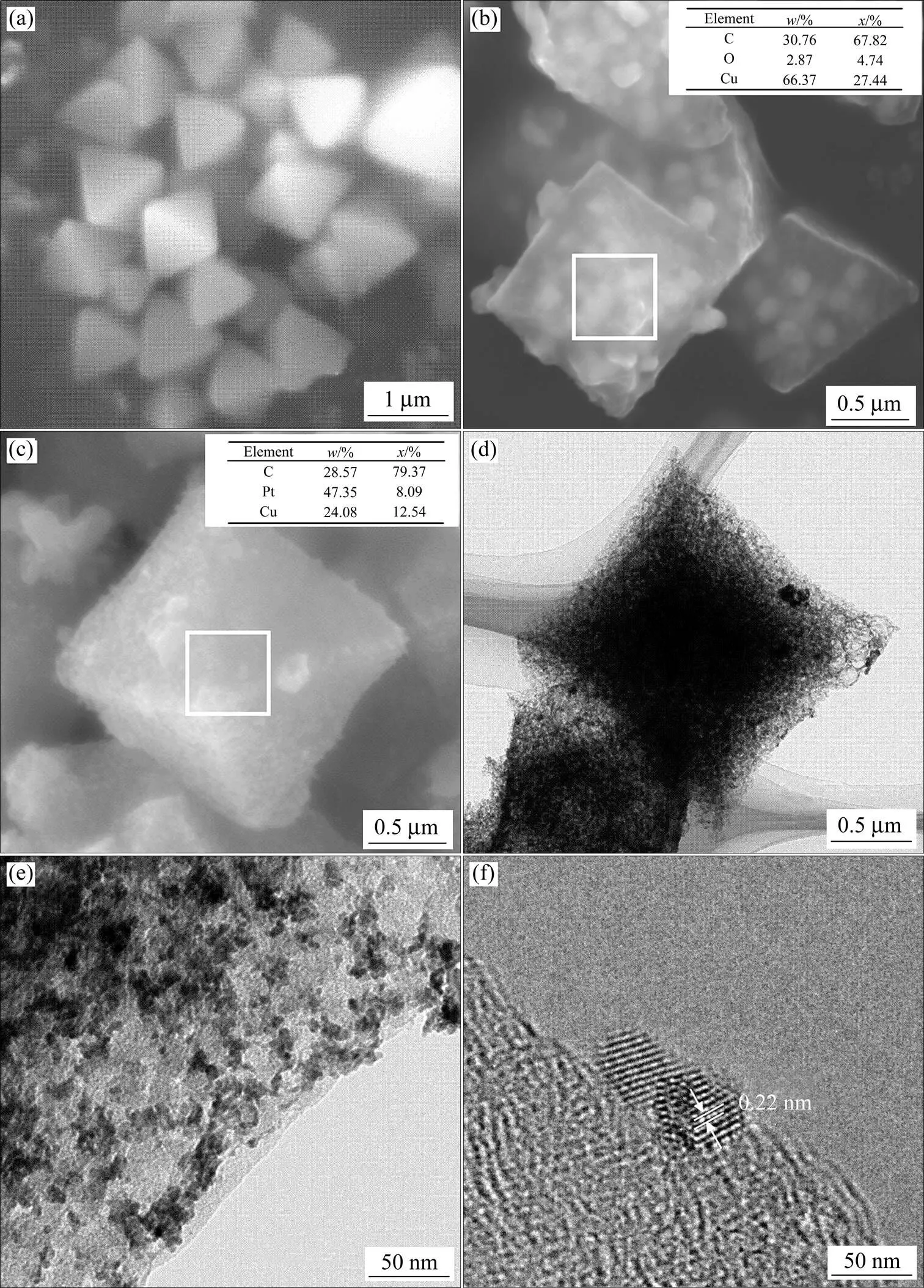
图1 HKUST-1, Cu/C和 Cu@Pt/C的组织结构与形貌
(a), (b), (c) Surface SEM images of HKUST-1, Cu/C and Cu@Pt/C, respectively; (d), (e), (f) TEM image and HRTEM images of Cu@Pt/C catalyst, respectively
图2所示为HKUST-1,Cu/C和Cu@Pt/C的XRD谱,图中的竖线为面心立方Pt的标准衍射峰。由图可见,HKUST-1的衍射峰与文献[19, 21]报道的完全一致。Cu/C在2为42.30°,50.40°和73.50°位置的衍射峰分别对应Cu的(111),(200)和(220)晶面[22]。Cu@Pt/C的衍射谱中只出现面心立方Pt的衍射峰,没有Cu的衍射峰,同时Pt的衍射峰向高角度偏移,根据文献报道[23−24],这是因为Pt将Cu包覆在内构成核壳结构所致。另外,与面心立方Pt的标准卡片(JCPDS#04-0802)相比,Cu@Pt/C衍射谱中Pt的衍射峰发生了宽化,结合TEM分析结果中Cu@Pt/C颗粒的晶面间距减小和谢乐方程[25]得知,这可能是由于Cu@Pt/C的晶粒尺寸减小造成的。
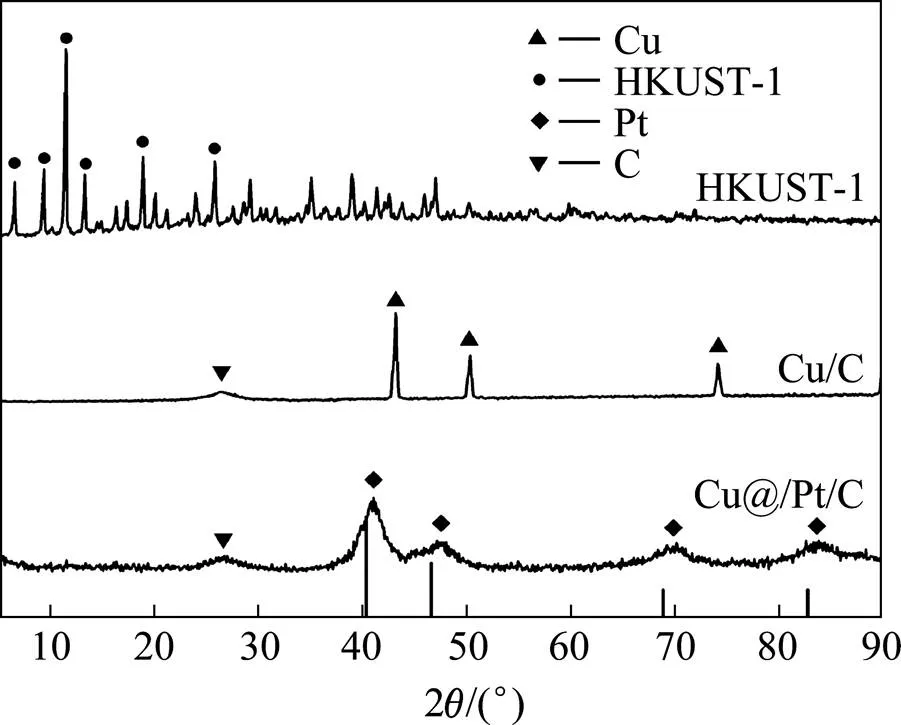
图2 HKUST-1, Cu/C和Cu@Pt/C的XRD谱
(The vertical lines represent the reference patterns of face-centered cubic structure of pure Pt)
图3(a)所示为Cu/C的XPS分析,可以看出在结合能为932.8 eV和952.7 eV处出现了Cu单质的光电子峰,而在933.9以及953.6 eV处出现CuO的光电子峰(938~945 eV和960~967 eV处为卫星峰)。图3(b)和(c)为Cu@Pt/C的Cu 2p 和Pt 4f的XPS谱,由图可知Cu@Pt/C中的Cu和Pt均为金属单质。值得注意的是,Cu的XPS图谱中不存在二价铜的光电子峰,这一方面是因为Cu/C粉末在空气中发生轻微氧化而生成少量铜的氧化物在氯铂酸钾酸性溶液中溶解,另一方面是由于形成核壳结构后,Pt对Cu核起到一定的保护作用,防止其氧化。因此,通过XRD和XPS的分析结果进一步推断,Cu@Pt/C催化剂具有以Cu为核,Pt为外壳的核壳结构。
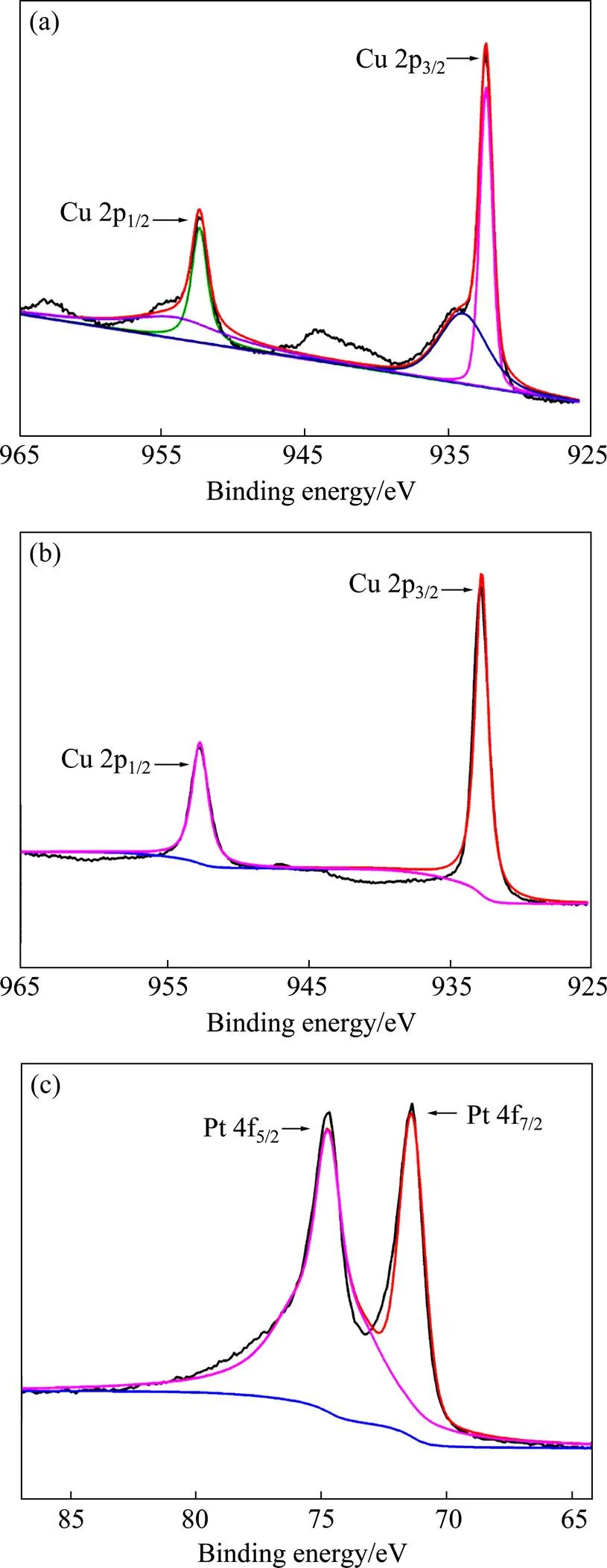
图3 Cu/C和Cu@Pt/C的XPS分析
2.2 催化活性
图4所示为Cu@Pt/C和商用Pt/C在N2饱和的浓度为0.5 mol/L的H2SO4溶液中的循环伏安曲线。从图中可见,Cu@Pt/C和Pt/C均在−0.2~0 V间出现了典型的氢吸附与脱附峰,这与多晶Pt的循环伏安曲线一致,表明Cu颗粒的表层确实被Pt原子置换,得到了具有核壳结构的Cu@Pt/C催化剂。在0.50~0.55 V左右形成的还原峰为之前正向扫描过程中Pt表面形成的氧化物发生了还原反应所致。
图5所示为Cu@Pt/C和Pt/C在CH3OH与 H2SO4的混合溶液中的循环伏安曲线。由图5可知,Cu@Pt/C和Pt/C的正扫过程中的峰值电流密度(f)均出现在0.65 V左右,负扫过程的峰值电流密度(b)出现在0.4~ 0.5 V左右。正扫描过程表示甲醇分解产生CO,峰电流密度(f)可表征催化剂对甲醇催化氧化的活性,f越大,表明催化剂催化甲醇氧化的活性越高;负扫描过程为吸附在催化剂表面的CO发生氧化,fb比值可表征催化剂抗CO中毒的能力[26−27],一般f/b的值越大,CO在催化剂表面的吸附越少,抗CO中毒性能越强。从图中可知,Cu@Pt/C和Pt/C正扫峰值电流密度分别为631.2 mA/mg和163.5 mA/mg,并且Cu@Pt/C的f/b值为2.18,远远超过商业Pt/C的0.74,说明Cu@Pt/C具有更高的催化活性及抗CO中毒性能[28]。
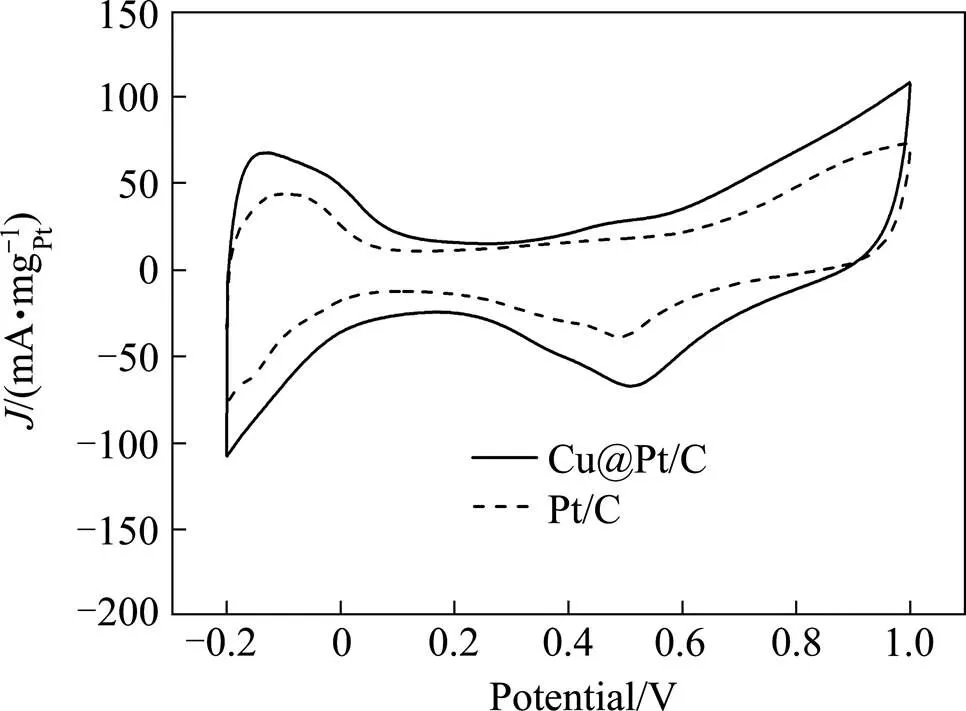
图4 Cu@Pt/C和Pt/C在0.5 mol/L浓度的 H2SO4溶液中的循环伏安曲线

图5 Pt/C和Cu@Pt/C在CH3OH+H2SO4混合溶液中的甲醇氧化曲线
电极的电化学活性面积(electrochemically active surface areas, ECSA)可用下式计算[29]:
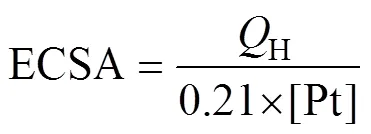
式中:H为在电极表面氢吸附的库伦电量或者氢脱附的库伦电量,通过对氢的吸附峰积分并扣除双电层得到;[Pt]为电极上铂的负载量,0.21表示在光滑的Pt电极表面氧化单分子层H2所需的电量。通过电感耦合等离子光谱分析(inductively coupled plasma spectro- metry, ICP)可得Cu@Pt/C和商业Pt/C的Pt负载量分别为1.0 g/m2和1.4 g/m2,通过式(1)计算出Cu@Pt/C和Pt/C的活性面积分别为74.3 m2/g和50.4 m2/g,Cu@Pt/C的活性面积大约是Pt/C的1.47倍。Cu@Pt/C具有很高的活性面积可能是由于其继承了Cu-有机物的八面体多孔骨架结构,能提供更多的活性位点,所以活性面积大,催化活性高。
甲醇的氧化过程通常包括甲醇分解产生CO,CO吸附于催化剂表面以及CO氧化这几个主要步骤。Cu@Pt/C催化剂在催化甲醇氧化过程中表现出相对于传统商业Pt/C更高的催化活性以及更优异的抗CO中毒性能,表明Cu的作用不容忽视。核壳结构的Cu@Pt/C催化剂中的Cu原子能改变Pt的d轨道电子状态,降低其费米能级,使得Pt与CO之间的相互作用降低,减少CO在Pt表面的吸附,从而提高催化剂的抗CO中毒性能[30−32]。另外,Cu可在较低电势下促使Pt表面形成OHads,CO被OHads氧化为CO2而脱离金属Pt表面,从而将Pt表面的催化活性位点再次释放出来用于甲醇氧化[33]。总之,电子效应、双功能机理以及大的活性面积是促成Cu@Pt/C催化剂具有更高的催化活性以及抗CO中毒性能的重要原因。
通过计时电流法来检测催化剂的稳定性。首先将电极在−200 mV电压下保持60 s,然后阶跃到600 mV下保持1 200 s,图6所示为Cu@Pt/C和Pt/C电极的电流密度随时间的变化曲线,可见二者的电流变化具有相同的趋势,在刚开始的一段时间内电流密度都急剧下降,随后趋于平缓,最终保持稳定。但与Pt/C相比,Cu@Pt/C的电流密度下降较慢,并且1 200 s时的电流密度72.9 mA/mg远远高于Pt/C的7.1 mA/mg。这表明Cu@Pt/C在催化甲醇氧化过程中具有更优异的稳定性。

图6 Pt/C和Cu@Pt/C在CH3OH+H2SO4混合溶液中的计时电流曲线
3 结论
1) 以硝酸铜和均苯三甲酸为原料,通过水热合成、碳热还原和置换反应可制备用于直接甲醇燃料电池的Cu@Pt/C催化剂。Cu@Pt/C为八面体结构,Pt包覆在Cu颗粒表面形成核壳结构。
2) Cu@Pt/C催化剂的活性面积大约是商用Pt/C的1.47倍,表现出更优异的对甲醇的电催化氧化活性和抗CO中毒性能以及更好的催化稳定性。
[1] IWASITA T. Electrocatalysis of methanol oxidation[J]. Electrochimica Acta, 2002, 47(22): 3663−3674.
[2] YANG L, YANG W, CAI Q. Well-Dispersed ptau nanoparticles loaded into anodic titania nanotubes: A high antipoison and stable catalyst system for methanol oxidation in alkaline media[J]. Journal of Physical Chemistry C, 2010, 111(44): 16613−16617.
[3] AMMAM M, EASTON E B. Oxygen reduction activity of binary PtMn/C, ternary PtMnX/C (X=Fe, Co, Ni, Cu, Mo and, Sn) and quaternary PtMnCuX/C (X=Fe, Co, Ni, and Sn) and PtMnMoX/C (X=Fe, Co, Ni, Cu and Sn) alloy catalysts[J]. Journal of Power Sources, 2013, 236(236): 311−320.
[4] MA X, LUO L, ZHU L, et al. Pt-Fe catalyst nanoparticles supported on single-wall carbon nanotubes: Direct synthesis and electrochemical performance for methanol oxidation[J]. Journal of Power Sources, 2013, 241(6): 274−280.
[5] MIN K J, YUAN Z, MCGINN P J. A comparative study of PtCo, PtCr, and PtCoCr catalysts for oxygen electro-reduction reaction[J]. Electrochimica Acta, 2010, 55(19): 5318−5325.
[6] WANG M, ZHANG W, WANG J, et al. Mesoporous hollow PtCu nanoparticles for electrocatalytic oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2013, 1(7): 2391−2394.
[7] CUI C H, LI H H, YU S H. Large scale restructuring of porous Pt-Ni nanoparticle tubes for methanol oxidation: A highly reactive, stable, and restorable fuel cell catalyst[J]. Chemical Science, 2011, 2(8): 1611−1614.
[8] YU Z, CHAO M, ZHU Y, et al. Hollow core supported Pt monolayer catalysts for oxygen reduction[J]. Catalysis Today, 2013, 202(1): 50−54.
[9] HUSSAIN N, GOGOI P, AZHAGANAND V K, et al. Correction: Green synthesis of stable Cu(0) nanoparticles onto reduced graphene oxide nanosheets: a reusable catalyst for the synthesis of symmetrical biaryls from arylboronic acids under base-free conditions[J]. Catalysis Science & Technology, 2015, 5(2): 1251−1260.
[10] BRANKOVIC S R, WANG J X, ADŽIĆ R R. Metal monolayer deposition by replacement of metal adlayers on electrode surfaces[J]. Surface Science, 2001, 474(1): L173−L179.
[11] BRUSSEL M V, KOKKINIDIS G, VANDENDAEL I, et al. High performance gold-supported platinum electrocatalyst for oxygen reduction[J]. Electrochemistry Communications, 2002, 4(10): 808−813.
[12] MINTSOULI, GEORGIEVA, ARMYANOV, et al. Pt-Cu electrocatalysts for methanol oxidation prepared by partial galvanic replacement of Cu/carbon powder precursors[J]. Applied Catalysis B Environmental, 2013, 136/137(21): 160− 167.
[13] EL-KHATIB K M, HAMEED R M A, AMIN R S, et al. Core–shell structured Cu@Pt nanoparticles as effective electro catalyst for ethanol oxidation in alkaline medium[J]. International Journal of Hydrogen Energy, 2017, 42(21): 14680−14696.
[14] PODLOVCHENKO B I, KRIVCHENKO V A, MAKSIMOV Y M, et al. Specific features of the formation of Pt(Cu) catalysts by galvanic displacement with carbon nanowalls used as support[J]. Electrochimica Acta, 2012, 76(8): 137−144.
[15] POOCHAI C. Highly active dealloyed Cu@Pt core-shell electro catalyst towards 2-propanol electro oxidation in acidic solution[J]. Applied Surface Science, 2017, 396(396): 1793− 1801.
[16] ROWSELL J L C, YAGHI O M. Metal–organic frameworks: a new class of porous materials[J]. Microporous & Mesoporous Materials, 2004, 73(1): 3−14.
[17] SCHLICHTE K, KRATZKE T, KASKEL S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu3(BTC)2[J]. Microporous & Mesoporous Materials, 2004, 73(1): 81−88.
[18] KUMAR R S, KUMAR S S, KULANDAINATHAN M A. Efficient electrosynthesis of highly active Cu3(BTC)2-MOF and its catalytic application to chemical reduction[J]. Microporous & Mesoporous Materials, 2013, 168(3): 57−64.
[19] ZHANG R, LIN H, BAO S, et al. Surface polarization enhancement: high catalytic performance of Cu/CuO/C nanocomposites derived from Cu-BTC for CO oxidation[J]. Journal of Materials Chemistry A, 2016, 4(21): 8412−8420.
[20] ZHU H, LI X, WANG F. Synthesis and characterization of Cu@Pt/C core-shell structured catalysts for proton exchange membrane fuel cell[J]. Fuel and Energy Abstracts, 2011, 36(15): 9151−9154.
[21] AMELOOT R, PANDEY L, VAN d A M, et al. Patterned film growth of metal-organic frameworks based on galvanic displacement[J]. Chemical Communications, 2010, 46(21): 3735−3737.
[22] CHENG W H. Reaction and XRD studies on Cu based methanol decomposition catalysts: Role of constituents and development of high-activity multicomponent catalysts[J]. Applied Catalysis A General, 1995, 130(1): 13−30.
[23] El-KHATIB K M, HAMEED R M A, AMIN R S, et al. Core–shell structured Cu@Pt nanoparticles as effective electrocatalyst for ethanol oxidation in alkaline medium[J]. International Journal of Hydrogen Energy, 2017, 42(21): 14680−14696.
[24] MINTSOULI I, GEORGIEVA J, ARMYANOV S, et al. Pt-Cu electrocatalysts for methanol oxidation prepared by partial galvanic replacement of Cu/carbon powder precursors[J]. Applied Catalysis B: Environmental, 2013, 136/137(Complete): 160−167.
[25] Star-like PtCu nanoparticles supported on graphene with superior activity for methanol electro-oxidation[J]. Electrochimica Acta, 2015, 177: S0013468615006489.
[26] LIU Z, Hong L. Electrochemical characterization of the electrooxidation of methanol, ethanol and formic acid on Pt/C and PtRu/C electrodes[J]. Journal of Applied Electrochemistry, 2007, 37(4): 505−510.
[27] BEDEN B, KADIRGAN F, LAMY C, et al. Oxidation of methanol on a platinum electrode in alkaline medium: Effect of metal ad-atoms on the electrocatalytic activity[J]. Journal of Electroanalytical Chemistry & Interfacial Electrochemistry, 1982, 142(1): 171−190.
[28] SHEN J, HU Y, CHEN L, et al. Pt-Co supported on single-walled carbon nanotubes as an anode catalyst for direct methanol fuel cells[J]. Electrochimica Acta, 2008, 53(24): 7276− 7280.
[29] TRASATTI S, PETRII O A. Real surface area measurements in electrochemistry[J]. Pure & Applied Chemistry, 1991, 63(5): 711−734.
[30] ZHANG J, VUKMIROVIC M B, SASAKI K, et al. Mixed-metal pt monolayer electrocatalysts for enhanced oxygen reduction kinetics[J]. Journal of the American Chemical Society, 2005, 127(36): 12480−12481.
[31] RUBAN A, HAMMER B, STOLTZE P, et al. Surface electronic structure and reactivity of transition and noble metals 1[J]. Journal of Molecular Catalysis A Chemical, 1997, 115(3): 421−429.
[32] KITCHIN J R, NØRSKOV J K, BARTEAU M A, et al. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3D transition metals[J]. Journal of Chemical Physics, 2004, 120(21): 10240−10246.
[33] TSIAKARAS P E. PtM/C (M=Sn, Ru, Pd, W) based anode direct ethanol–PEMFCs: Structural characteristics and cell performance[J]. Journal of Power Sources, 2007, 171(1): 107− 112.
Structure of Cu@Pt/C catalyst derived from HKUST-1 and its catalytic activity for methanol oxidation
LONG Xiangyu, LEI Ting, WANG Kunchan, ZHAN Zhenxiang
(State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China)
Copper nitrate and trimesic acid were used as raw materials to fabricate octahedral HKUST-1 by hydrothermal method. HKUST-1 was calcined in Ar protective atmosphere and Cu/C nanoparticles were derived through carbothermal reduction reaction. The Cu@Pt/C catalyst was obtained by soaking Cu/C in potassium chloroplatinic acid solution by galvanic displacement. The morphology and microstructure of Cu@Pt/C catalyst as well as its electrocatalytic activity towards methanol were further characterized by SEM, XRD, XPS and cyclic voltammetry (CV). The results show that the as-prepared Cu@Pt/C catalyst retains special octahedral structure of HKUST-1 and has a core-shell structure formed by Pt coating on the surface of Cu. The electrochemically active surface areas (ECSA) measured by cyclic voltammetry curves in H2SO4solution is 74.3 m2/g, about 1.47 times as much as that of commercial Pt/C. The cyclic voltammetry curves in H2SO4+CH3OH solution shows the ratio of positive sweep peak current density to reverse sweep peak current densityf/bis 2.18, which is much higher than that of commercial Pt/C. Thus Cu@Pt/C catalyst has better electrocatalytic activity to methanol oxidation and better CO tolerance.
core-shell structure; methanol oxidation; electrocatalysis; metal-organic framework; galvanic replacement
TB333
A
1673-0224(2019)03-289-07
国家自然科学基金资助项目(21673297)
2018−12−18;
2019−01−08
雷霆,教授,博士。电话:15974242599;E-mail: tlei@csu.edu.cn
(编辑 汤金芝)

