Electroacupuncture at ST36 modulates gastric motility via vagovagal and sympathetic reflexes in rats
Meng-Jiang Lu, Zhi Yu, Yan He, Yin Yin, Bin Xu
Abstract BACKGROUND Electroacupuncture (EA) at ST36 can significantly improve gastrointestinal symptoms, especially in promoting gastrointestinal motility. The automatic nervous system plays a main role in EA, but few studies exist on how vagovagal and sympathetic reflexes affect EA to regulate gastrointestinal motility.AIM To study the role of vagovagal and sympathetic reflexes in EA at ST36, as well as the associated receptor subtypes that are involved.METHODS Gastric motility was measured with a manometric balloon placed in the gastric antrum area in anesthetized animals. The peripheral nervous discharge was measured using a platinum electrode hooking the vagus or greater splanchnic nerve, and the central nervous discharge was measured with a glass microelectrode in the dorsal motor nucleus of the vagus (DMV). The effects and mechanisms of EA at ST36 were explored in male Sprague-Dawley rats which were divided in to a control group, vagotomy group, sympathectomy group, and microinjection group [including an artificial cerebrospinal fluid group, glutamate(L-Glu) group, and γ-aminobutyric acid (GABA) group] and in genetically modified male mice [β1β2 receptor-knockout (β1β2-/-) mice, M2M3 receptorknockout (M2M3-/-) mice, and wild-type control mice].RESULTS EA at ST36 promoted gastric motility during 30-120 s. During EA, both vagus and sympathetic nerve discharges increased, with a much higher frequency of vagus nerve discharge than sympathetic discharge. The gastric motility mediated by EA at ST36 was interdicted by vagotomy. However, gastric motility mediated by EA at ST36 was increased during 0-120 s by sympathectomy, which eliminated the delay effect of EA during 0-30 s, but it was lower than the control group during 30-120 s. Using gene knockout mice and their wild-type controls to explore the receptor mechanisms, we found that EA at ST36 decreased gastric motility in M2/3-/- mice, and promoted gastric motility in β1/2-/- mice. Extracellular recordings showed that EA at ST36 increased spikes of the DMV. Microinjection of L-Glu into the DMV increased gastric motility, while EA at ST36 decreased gastric motility during 0-60 s, and promoted gastric motility during 60-120 s.Injection of GABA reduced or increased gastric motility, and reduced the promoting gastric motility effect of EA at ST36.CONCLUSION These data suggest that EA at ST36 modulates gastric motility via vagovagal and sympathetic reflexes mediated through M2/3 and β1/2 receptors, respectively.Sympathetic nerve activity mediated through β1/2 receptors is associated with an early delay in modulation of gastric motility by EA at ST36.
Key words: Gastric motility; Electroacupuncture; Vagovagal reflex; Sympathetic nerve;Rats
INTRODUCTION
Extrinsic neural inputs originating in the central nervous system (CNS) provide modulation of gastric motility, especially in the upper gastrointestinal tract. In particular, brainstem vagovagal parasympathetic neurocircuits have the most prominent role in the CNS-mediated control of upper gastrointestinal tract motility[1].Vagovagal neurocircuits comprise the nucleus tractus solitarius (NTS), the dorsal motor nucleus of the vagus (DMV), and the nucleus ambiguous. The DMV is the nuclei of origin of vagal motor fibers. The efferent fibers from the DMV form synaptic contacts with postganglionic neurons located in the target organ which modulate gastric motility.
Gastric dysmotility is a common symptom of gastrointestinal diseases such as functional dyspepsia and diabetic gastroparesis. Although cisapride and domperidone can promote gastric motility in patients with insufficient gastric motility, adverse cardiac reactions are often reported in the clinic[2-5]. Electroacupuncture (EA) is widely used in clinical practice due to its safety, high efficacy,and low toxicity. Studies have shown that EA at ST36 can significantly improve gastrointestinal symptoms, especially in promoting gastrointestinal motility[6-9].However, the mechanism underlying its efficacy remain exploring. Most studies have shown that EA at ST36 is closely related to vagus nerve activity, especially the dorsal vagal complex of the neural pathway[10-13].
In this study, we measured intragastric pressure to observe the effect of EA at ST36 on gastric motility at different time intervals. The role of the peripheral autonomic nervous system in EA was determined using the vagus nerve and splanchnic nerve severance model, as well as by detecting peripheral autonomic nerve discharge. M2/3 and β1/2 receptor knockout mouse models were further used to identify autonomic receptor subtypes specifically involved in the regulation of gastric motility. Finally,we studied the role of brainstem neurocircuits during EA at ST36 by detecting DMV neuron discharge and the effect of microinjection of γ-aminobutyric acid (GABA) and glutamate (L-Glu) to the DMV. Using these approaches, the role of vagovagal and sympathetic reflexes in regulating gastric motility by EA at ST36 was determined.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats weighing 250-300 g (Model Animal Research Center of Nanjing Medical University, China) were divided into a control group, sham surgery group, vagotomy group, sympathectomy group, and microinjection group (including an artificial cerebrospinal fluid group, GABA group, and L-Glu group). M2/3-/-mice(M2/3-/-, D2; 129-Chrm2tmlChrm3tml, D0407, Kumamoto University, Japan),β1/2-/-mice (β1/2-/-; Adrb1tmlBkkAdrb2tmlBkk/J, J003810, donated by the Jackson Laboratory, United States), and their wild-type mice (male, 20-25 g; Model Animal Research Center of Nanjing Medical University, China) were applied. Gene knockout in mice was verified by PCR.
All animals were housed under controlled environmental conditions (22 °C, 40%-60% relative humidity, 12/12 h light/dark cycle) and were given free access to water and food. All animals were allowed 1 week of feeding adaptation. All experimental manipulations were undertaken in accordance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals, published by the National Science Council, China.
Drugs
Animals were anaesthetized with urethane (U2500; Sigma, St. Louis, MO, United States). L-Glu (G1251-100G; Sigma), GABA (A2129-10G; Sigma), artificial cerebrospinal fluid (R22153; Yuan Ye Biological Co., Ltd., Shanghai, China), and Pontamine sky blue (24410; Sigma) were administered via microinjection prior to surgery. Penicillin (2011; Shandong Shengwang Pharmaceutical Co., Ltd., Shandong,China) was administered after surgery. The concentrations and doses of the drugs were as follows: urethane (20%; 0.8 g/kg for rats and 5 mL/kg for mice), L-Glu (0.1 mol/L, 0.2 μL), GABA (0.1 mol/L, 0.2 μL), artificial cerebrospinal fluid (0.2 μL),penicillin (0.2 mL/d of 800 IU penicillin in 5 mL saline per rat, intramuscular), and Pontamine sky blue (1% dissolved in 0.5 mol/L sodium acetate).
Assessment of gastric motility
Experimental animals were fasted for 12 h and were free to drink. A small skin incision (length: 5-8 mm in rats, 2-3 mm in mice) was made below the xiphoid process, then a small balloon made of flexible rubber (about 2 mm in diameter for rats and 1 mm in diameter for mice) was inserted into the duodenum and placed in the antrum of the stomach. The small balloon was connected to a polyethylene tube filled with 0.05-0.1 mL of warm water. The pressure in the balloon was further analyzed using a transducer (YP201; Chengdu Instrument Factory) and a physiological signal collection system (RM6240; Chengdu Instrument Factory). Baseline intragastric pressure was maintained at 0.1 Kpa, and the experimental animals were maintained at a temperature of 37 ± 0.5 °C with an electric heating pad.
Surgery
For vagotomy, rats were fasted for 24 h before surgery and anesthetized with urethane. The skin was prepared with iodophor disinfection. A small skin incision was introduced along the midline of the abdomen, and the stomach and subphrenic surface of the animal were exposed. The distal and proximal ventral gastric vagus branches were separated and cut. After surgery, penicillin was injected for 3 d to prevent infection. The rats were fed liquid food within 3 d after surgery. After 5 d, the rats were used in the experiment. For sympathectomy, preoperative preparation was the same as for vagotomy. Between the left iliac horn and the lumbar muscle, the left greater splanchnic nerve was separated and cut. Postoperative treatment was the same as that for vagotomy.
Parasympathetic and sympathetic nerve discharge
Vagus nerve discharge:Rats were anesthetized with urethane, a small skin incision was made in the midline of the abdomen, and the left vagus nerve was separated as for vagotomy. The nerve was hooked with the positive electrode, and the surrounding tissue was hooked with the reference electrode.
Sympathetic nerve discharge:Rats were anesthetized with urethane, a small skin incision was made in the midline of the abdomen, and the left greater splanchnic nerve was separated as for sympathectomy. The nerve was hooked with the positive electrode, and the surrounding tissue was hooked with the reference electrode. Nerve discharge was recorded using a preamplifier (NL100, CED, United Kindom) and a Micro1401-3 bioelectric module (NL125NL126, CED, United Kindom) connected to a biosignal acquisition and analysis system (Microl 1401-3, CED, United Kindom). The signal filtering was 10-1000 Hz, sampling frequency was 20000Hz, and amplification was 1000 times. The data were recorded with Spike2 software.
Localization, microinjection, and nerve discharge of the dorsal motor nucleus of the vagus nerve
Rats were anesthetized with 20% urethane (8 mL/kg) and placed in a prone position on a brain stereotaxic instrument maintained at a temperature of 37 °C using an electric heating pad. The head was fixed with an ear rod and rat head clip. After removing the cranial fur, a longitudinal incision was made in the middle of the head,the subcutaneous tissue was separated and the skull was clearly exposed, and the anterior fontanelle and posterior fontanelle were adjusted to the same horizontal line.The vagus dorsal motor nucleus was determined according to the brain localization map of Paxinos and Watson for rats (coordinate: AP 13.2 mm, RL 0.5 mm, H 8 mm)[14].
For microinjection of the rostral part DMV, a small hole (about 2 mm diameter) was drilled into the skull using an electric bone drill. A customized injection cannula (a stainless steel catheter with an outer diameter of 0.7 mm, inner diameter of 0.4 mm,and length of 10 mm) was inserted through the hole, and zinc phosphate cement was used to seal the hole. After surgery, penicillin was injected for 3 d to prevent infection.The rats were fed liquid food within 3 d after surgery. After 5 d, the rats were used in the experiment. Thereafter, the rats received artificial cerebrospinal fluid (0.1 mol/L,0.2 μL), L-Glu (0.1 mol/L, 0.2 μL), and GABA (0.1 mol/L, 0.2 μL) via the microinjection catheter. The rats were divided into three groups depending on the drug they received.
To measure nerve discharge of the DMV, a glass microelectrode (0.5 mol/L sodium acetate electrolyte filled with 1% Pontamine sky blue) was passed through a microinjection thruster to reach the DMV nerve (coordinate: AP 13.2 mm, RL 0.5 mm,H 8 mm), and its extracellular nerve discharge was recorded. When spontaneous neuronal discharge appeared, neuronal discharge before EA (1 min), during EA (2 min), and after EA (1 min) was recorded. After completion of the recording, the next intervention was performed after the baseline returned to the pre-EA level.
The tissue recording site was localized after each recording of nerve discharge. A digital DC stabilized power stimulator was used to pass reverse direct current (10 μA,20 min) to the microelectrode, and Pontamine sky blue was passed through the microelectrophoresis mode to the tip of the electrode.
Dissected brains were soaked in 4% paraformaldehyde solution. Thick sections (40-60 μm) of brain tissue were used to observe the location of the Pontamine sky blue marker under a light microscope. The location of the dye marker was compared with the position of the vagus nerve dorsal motor nucleus based on Paxinos and Watson rat brain location to determine the accuracy of vagus dorsal motor nucleus recording localization.
EA stimulation
ST36 (Zusanli) is located in the posterolateral aspect of the knee joint, about 5 mm below the capitulum fibulae; the stimulation method was EA stimulation, and the needle was connected to Han's EA instrument (LH402A; Beijing Huawei Technologies Co., Ltd.). The stimulating intensity was 2 mA, frequency was 2/15 Hz, and stimulation time was 2 min.
Statistical analysis
Discharge data were collected using the amplifier and biosignal acquisition device,and recorded and analyzed using the Spike2 software. The recorded intragastric pressure data were analyzed with a physiological signal collection system. The change in discharge frequency or percentage change in gastric motility was compared between during and before EA. An absolute value of change in discharge frequency ≥20% was regarded as an effective excitation or inhibition effect, and an absolute value of gastric motility change ≥ 5% was regarded as effective excitation or inhibition. The following effect formula (1) was used to indicate the change in percentage:
Percentage change = [ (DreEA-PreEA)/PreEA] × 100% (1)
Data were analyzed with SPSS 23.0 software and GraphPad Prism 6.0. All data are expressed as the mean ± standard deviation. Any two groups were compared using independent sample t-tests, and one-way analysis of variance was used for more than two groups. P < 0.05 indicated statistical significance.
RESULTS
Effect of EA at ST36 on gastric movement and peripheral autonomic discharge
In order to understand the effect of EA at ST36 on gastric motility, we monitored changes in intragastric pressure during EA for 120 s. We observed that there was no significant change in intragastric pressure at 0-30 s during EA (P > 0.05), and the intragastric pressure was significantly increased from 30-60 s (P < 0.05, Figure 1A).Simultaneously, we examined discharge of the vagus nerve and the greater visceral nerve during EA. The discharge frequency during EA was significantly increased compared to the pre-EA frequency, and the vagus nerve discharge frequency was significantly higher than that of the greater visceral nerve (P < 0.05, Figure 1C). These findings suggest that the vagus nerve and the greater visceral nerve are both affected by EA at ST36, with the vagus nerve affected to a greater degree.
Effect of EA at ST36 on gastric motility in different nerve transection groups
In order to further understand the role of the vagus nerve and the greater visceral nerve in gastric movement related to EA at ST36, we observed changes in intragastric pressure in different neurotomy groups. Compared with the normal control group,the intragastric pressure during EA in the vagus nerve transection group was significantly reduced. During EA at 0-30 s, the intragastric pressure of the greater visceral nerve transection group was significantly increased (P < 0.05, Figure 2). These results suggest that the promotion of gastric motility caused by EA at ST36 is related to vagus nerve activation, and the delayed aspect of this effect (no significant change in intragastric pressure at 0-30 s) depends mainly on activation of the greater visceral nerve.
Effect of EA at ST36 on gastric motility in M2/3-/- and β1/2-/- mice
To further study the effects of different receptor subtypes of the vagus nerve and the greater visceral nerve on gastric movement induced by EA at ST36, we measured associated changes in intragastric pressure in different receptor knockout mouse models. Compared with wild-type B6 mice, EA at ST36 inhibited gastric movement in M2/3-/-mice. At 0-30 s, the intragastric pressure of β1/2-/-mice was significantly increased by EA at ST36 (P < 0.05, Figure 3). These results suggest that the effect of EA at ST36 on gastric motility depends on the vagus nerve M2/3 receptor. The delayed function of this effect is mainly dependent on activation of the greater visceral nerve β1/2 receptor.
Effect of EA at ST36 on the discharge of neurons in the dorsal motor nucleus of the vagus nerve
In order to further understand the mechanism of the vagus nerve circuit in EA at ST36, we measured neuronal discharge in the DMV nerve during EA at ST36. Seventy percent of the 47 neurons recorded showed excitatory responses to EA at ST36, and the frequency of neuronal discharge during EA was significantly higher than that before EA (P < 0.05, Figure 4).
Effect of microinjection of L-Glu and GABA into the DMV on gastric motility
Studies have shown that L-Glu and GABA play an important role in the brainstem vagus nerve circuit. In order to clarify the effect of L-Glu and GABA on gastric motility regulation in the DMV, we microinjected L-Glu and GABA into the DMV to detect changes in intragastric pressure between 60 s before injection and 60 s after injection. Microinjection of L-Glu significantly increased intragastric pressure in the DMV compared with the artificial cerebrospinal fluid control group, while microinjection of GABA produced both excitatory and inhibitory effects (P < 0.05,Figure 5).
Effect of EA at ST36 on gastric movement after microinjection of L-Glu and GABA in the DMV
In order to clarify transmitter regulation during EA at ST36 in the brainstem vagus nerve circuit, we measured the effect of EA at ST36 on gastric motility after microinjection of L-Glu and GABA into the DMV. Compared with the control group,the effect of EA at ST36 was significantly reduced after injection of GABA. During EA 0-60 s, the gastric motility was inhibited after injection of L-Glu (P < 0.05, Figure 6).These results suggest that both GABA and L-Glu are involved in the brainstem vagus nerve circuit of EA at ST36.
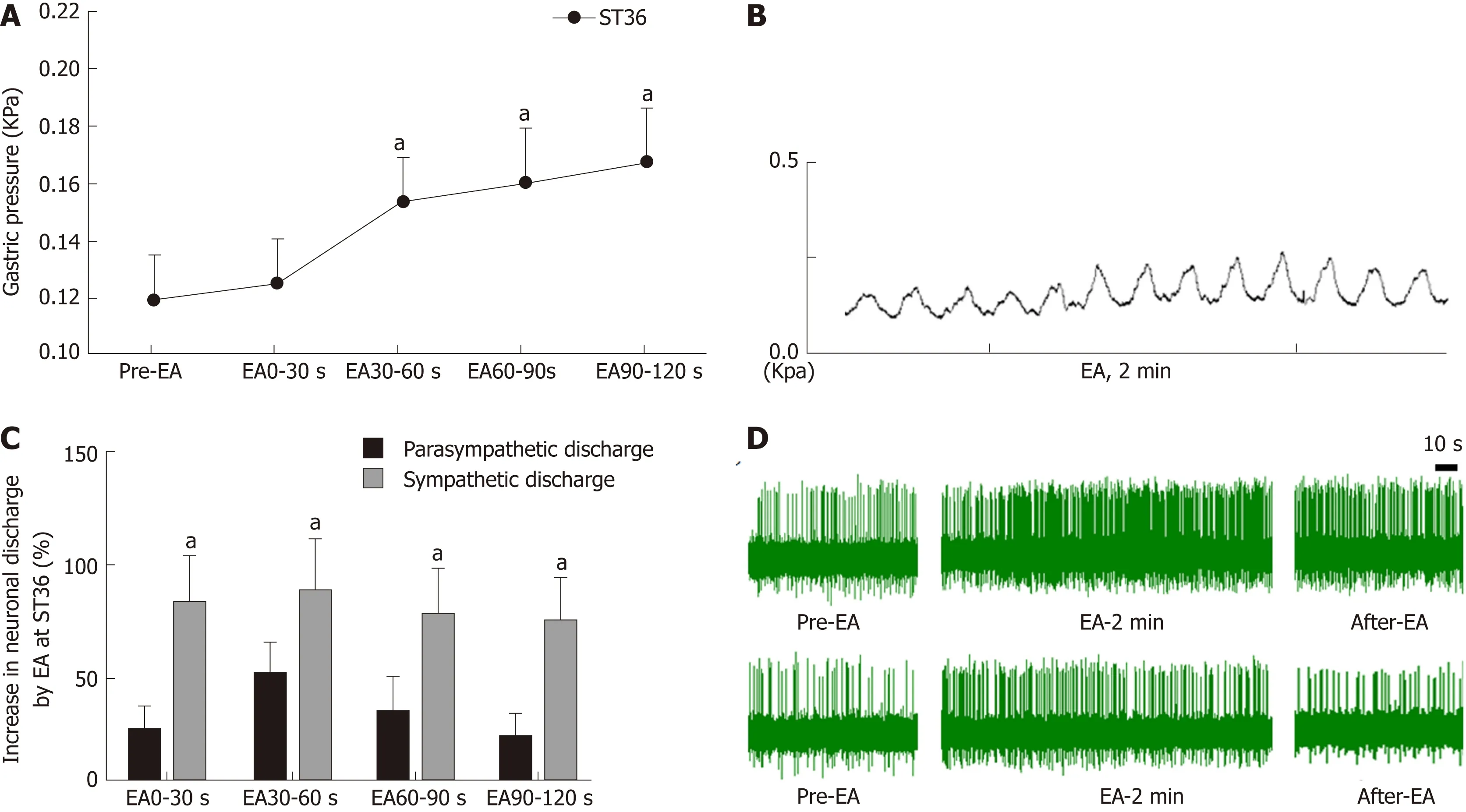
Figure 1 Effect of electroacupuncture at ST36 on gastric motility and vagus and sympathetic discharge.
DISCUSSION
Along with the rapid pace of life and changes in dietary habits, the incidence of gastrointestinal dysfunction is increasing, with abdominal distension, abdominal pain, nausea, and vomiting being the main symptoms. Many patients also experience these symptoms due to gastric dysmotility. Acupuncture therapy is widely used to treat gastrointestinal diseases because of its low side effect profile and good curative effect, and ST36 is the most frequently used acupoint for the treatment of gastrointestinal dysfunction. Previous studies have shown that acupuncture at ST36 regulates gastrointestinal motility mainly via the autonomic nervous system[15-17].However, there is no clear study on how the vagus nerve and sympathetic nerves participate in regulation, and what role the brainstem neural circuit plays.
Current research has found that acupuncture of the lower limbs can cause excitability of the vagus nerve, thereby increasing gastric motility[18,19]. However, the vagus nerve is not exclusively involved in regulation of gastric motility during acupuncture at ST36. Our study found that the time period during which EA at ST36 started to take effect was 30 s after stimulation onset, and that gastric movement did not change significantly during the first 0-30 s (Figure 1). We also examined vagus nerve and greater splanchnic nerve activity during EA. Neuronal discharge of the vagus nerve and the splanchnic nerve was significantly increased compared with the time period before EA (Figure 2), while the frequency of vagus nerve discharge was significantly higher than that of the greater splanchnic nerve (Figure 3). These data suggest that the vagus nerve and sympathetic nerve are involved in EA regulation,and that the vagus nerve may play a major role in increasing gastric motility.
To further clarify the autonomic nervous system mechanisms involved in gastric motility regulation, we observed changes in gastric motility after transecting the vagus nerve and greater splanchnic nerve. We found that after the vagus nerve was transected, the gastric motility-promoting effect of EA at ST36 was essentially abolished. When the greater splanchnic nerve was transected, the delayed effect of EA at ST36 disappeared (Figure 2). Therefore, EA at ST36 can increase gastric movement via the vagus nerve, and this effect is delayed due to involvement of sympathetic nerves.
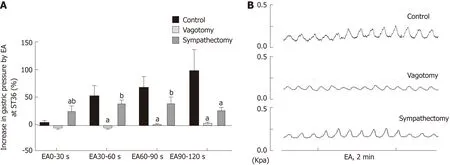
Figure 2 Effect of electroacupuncture at ST36 on gastric motility in different surgical model groups.
The vagus nerve and sympathetic nervous system regulate gastric motility together[20]. The vagus nerve neural circuit involves the brainstem vagus nerve neural circuit and the vagus nerve efferent fibers, which are transmitted via posterior membrane receptors of the gastric wall, with different receptors having different regulatory roles[21]. Studies have shown that when the vagus nerve releases the excitatory neurotransmitter acetylcholine, it mainly acts through two major receptor systems of the postsynaptic membrane, the nicotinic receptor (N) and muscarinic receptor (M), to promote gastric motility[22]. When the vagus nerve releases inhibitory neuronal NANC transmitter, it inhibits gastric motility. Five subtypes of M receptors exist[23], with M2M3 being the primary receptors distributed in the gastrointestinal smooth muscle[24]. Sympathetic nerves release norepinephrine and adrenaline through the branch of the spinal cord that innervates the gastric segment via the communicating branch, anterior ganglia, and the posterior ganglia. These transmitters act on postsynaptic β and α2 receptors, thereby inhibiting smooth muscle contraction and decreasing gastric motility.
As stated, EA regulates gastrointestinal motility via the vagus and sympathetic nerves. Studies have shown that injection of the M receptor blocker atropine can inhibit the gastric motility-promoting effect of EA at LI11 (Quchi)[25], and the injection of the beta blocker propranolol can abrogate the inhibition of jejunal motility[26].However, due to a poor specificity of the blockers, the specific receptor subtype(s)through which these effects are mediated is not clearly known. Our study found that compared with wild-type mice, the delayed effect of EA at ST36 disappeared in β1/2-/-mice, and gastric motility increased significantly during the 0-30 s period. EA at ST36 in M2/3-/-mice decreased gastric motility. These observations demonstrate that EA at ST36 acts through efferent vagus nerve activation of postsynaptic M2M3 receptors to promote gastric motility, while sympathetic activation of β1β2 receptors underlies the early delayed gastric motility response. Interestingly, EA at ST36 inhibited gastric motility in M2M3 receptor knockout mice.
Since the vagus nerve plays an important role in promoting gastric motility by EA at ST36, we focused on the effect of EA at ST36 on the vagus nerve neural circuit. We found that the brainstem vagus nerve neural circuit plays an important role in regulating gastric motility, including the solitary tract nucleus, the vagus nerve dorsal motor nucleus, and the nucleus ambiguus. The sensory afferent transmits the mechanical and chemical signals of the stomach to the solitary tract nucleus[27]through L-Glu; these signals are then transmitted to the DMV or the high-grade center via LGlu, GABA, and catecholamines, and the DMV transmits the signal through the efferent vagus nerve. The main neurotransmitters involved are L-Glu and GABA[28]. LGlu is a common excitatory neurotransmitter in the brain and its receptors include both ionotropic L-Glu receptors and metabotropic L-Glu receptors. Studies have found that microinjection of L-Glu into the DMV produces different effects. Sun et al[29]injected L-Glu into the right DMV and NTS to induce inhibition of gastric motility;Cruz et al[30]found that injecting L-Glu into the rostrum of the DMV promoted gastric motility, while injecting L-Glu into the tail of the DMV induced inhibition of gastric motility. These effects can be blocked by transecting the vagus nerve.
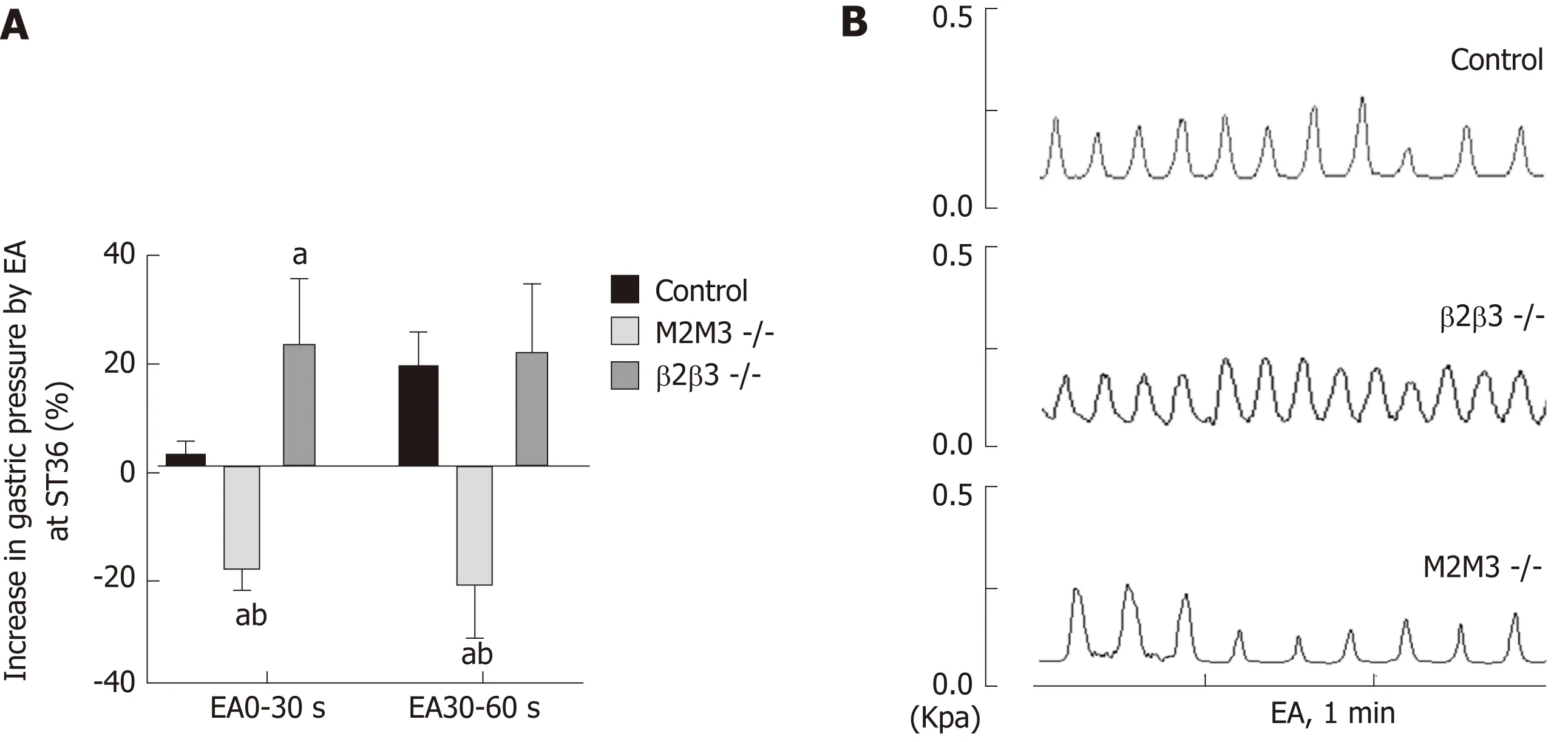
Figure 3 Effect of electroacupuncture at ST36 on gastric motility in mouse receptor knockout models.
GABA is the major inhibitory amino acid in the brain, with three major receptor types and multiple receptor subtypes. Browning et al[31]believe that glutamatergic neurons between the NTS and DMV do not affect the regulation of gastric motility.On the contrary, GABAergic neurons are important for the regulation of gastric motility; Pearson et al[32]found that the brainstem neural circuit that regulates the gastric antrum involves GABAergic transmission. Multiple studies have shown that GABAergic synaptic input in the solitary tract nucleus inhibits the efferent vagus nerve which regulates the upper digestive tract, thereby affecting gastric function[33].
Studies have shown that EA can affect the brainstem, as supported by imaging and c-fos immunopositive expression[34,35], and have also shown that the solitary nucleus is involved in acupuncture regulation of the gastrointestinal tract[36,37]. Our study found that injecting L-Glu into the rostrum of the DMV promotes gastric motility, while injecting GABA produces both inhibitory and stimulatory effects. To examine whether EA at ST36 involves the brainstem vagus nerve neuronal circuit, we examined the neuronal discharge of the DMV. Compared to baseline spontaneous discharge, EA at ST36 can significantly excite DMV neurons. To study the involvement of L-Glu and GABA in the EA process, we performed EA at ST36 along with the injection of L-Glu and GABA, respectively, into the DMV. Gastric motility decreased during the first 0-60 s after injecting L-Glu followed by EA; the motilitypromoting effect of EA showed a significant decline after the injection of GABA.These observations suggest that both L-Glu and GABA are involved in the brainstem nerve circuit of EA at ST36 that regulates gastric motility. GABA in the DMV antagonized the effect of EA at ST36.
In conclusion, our findings show that EA at ST36 mainly regulates gastric movement through the DMV vagovagal reflex circuit by L-Glu and GABA. While M2M3 receptors play a major role in mediating the vagus nerve efferent effect on gastric motility, the involvement of the sympathetic nervous system and β1β2 receptors may be the cause of delayed initiation of gastric motility. The vagussympathetic circuit is involved in the neural circuit that regulates gastric movement by EA at ST36.
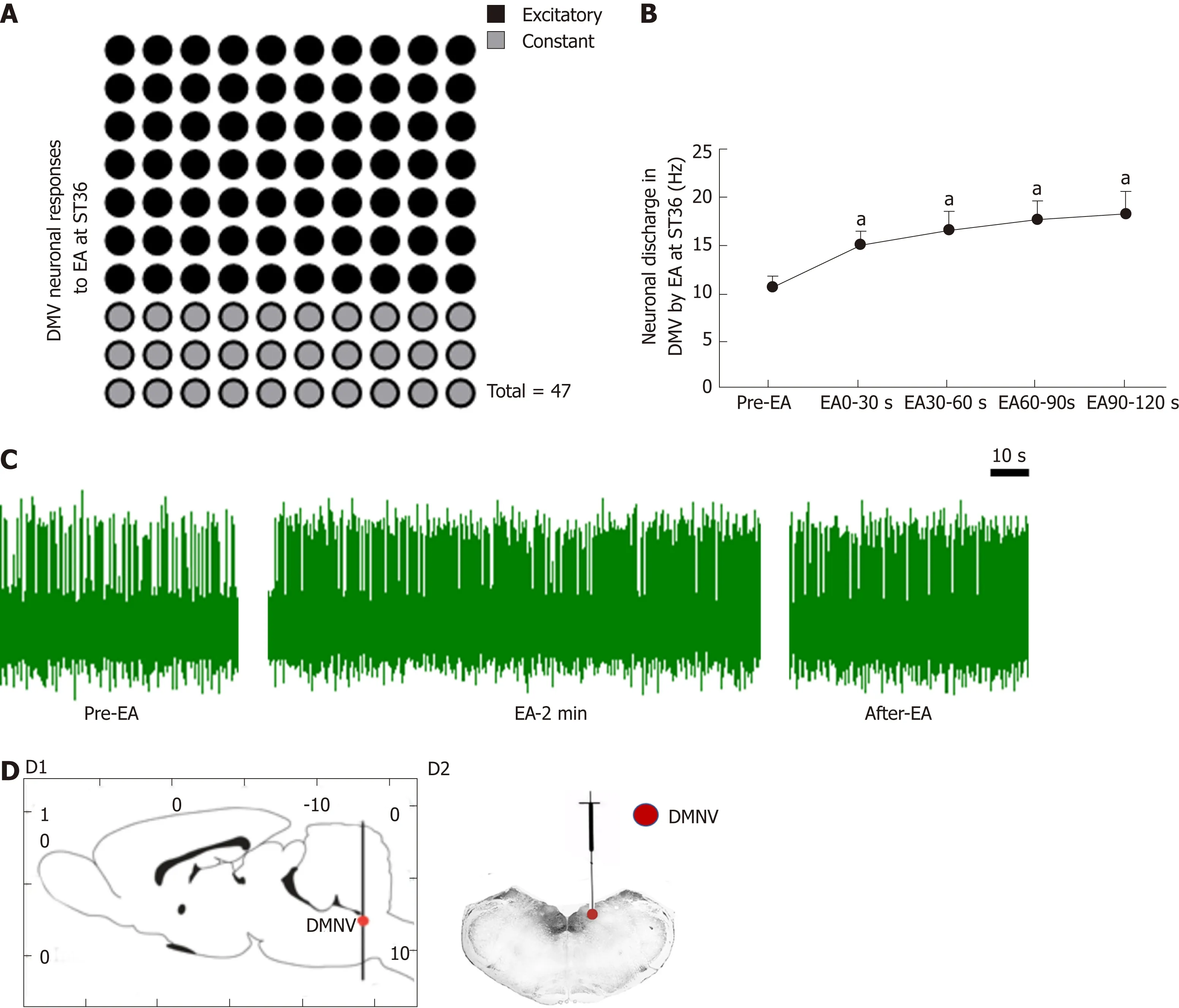
Figure 4 Effect of electroacupuncture at ST36 on neurons in the dorsal motor nucleus of the vagus nerve.
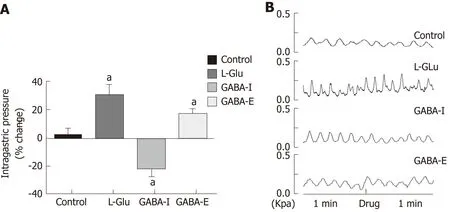
Figure 5 Gastric motility changes after dorsal motor nucleus of the vagus nerve microinjection of different amino acids.

Figure 6 Effect of electroacupuncture at ST36 on gastric motility after microinjection of different amino acids.
ARTICLE HIGHLIGHTS
Research background
Electroacupuncture (EA) at ST36 can significantly improve gastrointestinal symptoms, especially in promoting gastrointestinal motility. The automatic nervous system plays a main role in EA at ST36, but few studies exist on how vagovagal and sympathetic reflexes affect EA in regulating gastrointestinal motility.
Research motivation
This study aimed to investigate the mechanism of the automatic nervous system in promoting gastrointestinal motility by EA at ST36. The results obtained may be used to explain the mechanism of EA in regulating gastrointestinal motility. Furthermore, it may provide a reference to neurostimulation therapy.
Research objectives
The objective of this study was to determine the role of vagovagal and sympathetic reflexes in EA at ST36, as well as the associated receptor subtypes that are involved. The results obtained may be used to explain the mechanism of EA in regulating gastrointestinal motility.Furthermore, it may provide a reference to neurostimulation therapy.
Research methods
Gastric motility was measured with a manometric balloon in anesthetized animals. The peripheral nervous activity was measured with a platinum electrode hooking the vagus or greater splanchnic nerve, and the central nervous activity was measured with a glass microelectrode in the DMV. The effects and mechanisms of EA at ST36 were explored in male Sprague-Dawley rats which were divided into a control group, vagotomy group,sympathectomy group, and microinjection group [including an artificial cerebrospinal fluid group, glutamate (L-Glu) group, and γ-aminobutyric acid (GABA) group] and in genetically modified male mice [β1β2 receptor-knockout (β1β2-/-) mice, M2M3 receptor-knockout (M2M3-/-)mice, and wild-type control mice].
Research results
EA at ST36 promoted gastric motility during 30-120 s. During EA, the vagus nerve activity was much higher than sympathetic activity. The gastric motility mediated by EA at ST36 was interdicted by vagotomy. However, the delay effect of EA during 0-30 s was eliminated by sympathectomy. EA at ST36 decreased gastric motility in M2/3-/- mice and promoted gastric motility in β1/2-/- mice. Extracellular recordings showed that EA at ST36 increased spikes of the dorsal motor nucleus of the vagus (DMV). Microinjection of L-Glu into the DMV increased gastric motility, while EA at ST36 decreased gastric motility during 0-60s, and promoted gastric motility during 60-120 s. Injection of GABA reduced or increased gastric motility, and reduced the gastric motility-promoting effect of EA at ST36.
Research conclusions
EA at ST36 modulates gastric motility via vagovagal and sympathetic reflexes mediated through M2/3 and β1/2 receptors, respectively. Sympathetic nerve activity mediated through β1/2 receptors is associated with an early delay in the modulation of gastric motility by EA at ST36.GABA and L-Glu in the DMV are involved in regulating gastric motility by EA at ST36.
Research perspectives
The results prove that both vagal and sympathetic reflexes are involved in regulating gastric motility by EA at ST36. Future studies should investigate specific transcutaneous stimulation which can regulate gastric motility accurately.
 World Journal of Gastroenterology2019年19期
World Journal of Gastroenterology2019年19期
- World Journal of Gastroenterology的其它文章
- Cyst fluid glucose: An alternative to carcinoembryonic antigen for pancreatic mucinous cysts
- Mechanisms of hepatocellular carcinoma progression
- Congenital peritoneal encapsulation: A review and novel classification system
- Autoimmune hepatitis and IgG4-related disease
- Role of D2 gastrectomy in gastric cancer with clinical para-aortic lymph node metastasis
- Risk factors for progression to acute-on-chronic liver failure during severe acute exacerbation of chronic hepatitis Β virus infection
