曝气灌溉条件下土壤N2O排放特征及影响因子分析
雷宏军,刘 欢,臧 明,潘红卫,陈德立
曝气灌溉条件下土壤N2O排放特征及影响因子分析
雷宏军1*,刘 欢1,臧 明1,潘红卫1,陈德立2
(1.华北水利水电大学水利学院,河南 郑州 450046;2.墨尔本大学粮食与土地资源学院,澳大利亚 维多利亚 3010)
为了明确曝气灌溉下土壤N2O排放特征及主要影响因子,实验设置了2个灌水量(70%和90%田间持水量)和2个增氧水平(5,40mg/L),采用静态箱法和qPCR技术对土壤N2O通量及土壤关键功能基因进行测定,研究不同灌水量和增氧水平对土壤充水孔隙度、溶解氧、氧化还原电位(h)、矿质氮及氨氧化古菌(AOA)、氨氧化细菌(AOB)和反硝化基因(和)的影响.结果表明:培养过程中,各处理N2O排放通量均呈现先增加后降低的趋势,于灌溉后1d达到峰值;曝气量和灌水量的增加可显著增加土壤N2O的排放通量和排放峰值.灌溉造成土壤含水量增加的同时,降低了土壤溶解氧和h;曝气可提高土壤溶解氧和h,改善土壤通气性(<0.05),而对土壤充水孔隙度无显著影响.土壤充水孔隙度、h、NO3--N含量是曝气灌溉下驱动土壤N2O排放的主要理化因子.曝气显著增加了AOA的基因拷贝数, 且N2O排放与AOA的基因拷贝数呈显著正相关关系(<0.05).研究结果为进一步明确曝气灌溉对土壤N2O排放的影响机制和曝气灌溉模式下农田N2O排放管理提供支撑.
曝气灌溉;N2O排放;溶解氧;氧化还原电位;基因;拷贝数;影响因子
氧化亚氮(N2O)是全球气候变暖的主要元凶,其增温潜力是CO2的300倍左右[1],持续破坏大气臭氧层[2].农业土壤是全球N2O排放的主要来源,占人为N2O排放量的84%[3].
土壤N2O排放的影响因素是多种环境因子的相互叠加[4].土壤中CO2、CH4和N2O排放主要受到基因和细胞水平上的微生物途径驱动[5].影响N2O排放的微生物群可分为2类:参与硝化反应的氨氧化古菌(AOA)和氨氧化细菌(AOB);参与异养反硝化微生物的关键功能基因、、和等[6]. Han等[7]研究了不同灌水量下温室番茄地土壤N2O排放和AOA、AOB、反硝化细菌基因(亚硝酸还原酶/和NO还原酶)之间的关联关系, 结果表明不同灌溉方式仅影响AOA的拷贝数.土壤非生物因子不仅可通过调节微生物丰度和活性间接地影响N2O的排放,而且可通过影响气体扩散速率直接影响N2O的排放[8].Gödde等[9]研究表明土壤水分和温度可掩盖其他土壤因子对N2O排放的影响,且25℃的标准化土壤温度可有效降低土壤温度对N2O排放的掩盖效应.土壤的矿质氮浓度亦会影响土壤N2O的产生和排放.Wang等[10]研究了硝酸盐的添加对土壤N2O排放的影响,结果表明N2O排放量随着NO3--N添加浓度的增加而增加,且10cm土层的N2O浓度最高.
曝气灌溉(Aerated irrigation, AI)是通过滴灌或地下滴灌系统将微纳米气泡水输送至作物根区的新型高效节水灌溉技术[11-12],已广泛应用于设施农业[13-14].
曝气灌溉改变着土壤的水分分布和通气状况,可有效降低作物根区土壤的紧实度,维持良好的土壤充气孔隙度[15].曝气灌溉可提高根区土壤pH值,为微生物的生长和繁殖营造良好的化学环境,提高土壤酶活性[16].曝气灌溉可有效缓解灌溉造成的根区土壤缺氧胁迫,促进土壤中好氧微生物的活动和繁殖[17].李元等[18]研究了曝气灌溉对大棚甜瓜土壤细菌、真菌和放线菌数量的影响,结果表明土壤中细菌和放线菌的数量随着土壤加气量和加气频率的增加而显著增加.曝气灌溉改变着土壤的物理、化学和生物学因子,势必会影响土壤的硝化和反硝化过程,进而影响着土壤N2O的排放.目前关于曝气灌溉条件下土壤N2O排放量及其影响因子的研究较少.
为了明确曝气灌溉对土壤N2O排放的影响,实验中于设施菜地采集原状土进行室内恒温培养,通过对N2O排放通量及相关物理、化学和生物学因子的监测,拟明确曝气灌溉下土壤N2O排放特征,并分析N2O排放与土壤物理、化学和生物学因子之间的相关关系,辨识曝气灌溉下土壤N2O排放的主要影响因子.
1 材料和方法
1.1 实验概况
实验于郑州设施菜地采集原状土,土柱直径为30cm,高度为40cm,在华北水利水电大学农业高效用水实验室(34°47′23″N, 113°47′41″E)开展室内培养实验.供试土壤的砂粒(0.02~2mm)、粉粒(0.002~ 0.02mm)和黏粒(<0.002mm)质量分数分别为42.87%、35.26%和21.87%,为壤质黏土.土壤的田间持水量为36.64%,土壤容重1.20g/cm3, NO3-- N5.68mg/kg, NH4+-N3.36mg/kg,速效钾3.42mg/kg,速效磷9.98mg/kg,有机质21.54g/kg, pH值6.30.
1.2 实验设计
实验中采用微纳米气泡水制备技术[19]进行曝气, 设置了2个增氧水平(40,5mg/L,记为DA和DC)和2个灌水量(灌溉至90%和70%田间持水量,记为W1和W2),共4个处理,分别为DAW1、DAW2、DCW1和DCW2,每个处理4次重复.供试土壤初始体积含水率为22.17%,经预备实验计算,W1和W2分别为2.0, 1.0L.实验中取每个土柱上层10cm原状土进行拌肥,称量3.397g NH4NO3(相当于基肥用量180kg N/hm2)溶于100mL去离子水中,均匀喷洒于土壤表面,静置15min,拌匀后回填于土柱至原容重.实验中采气与采土土柱分开,于灌溉后的0,0.25,0.5,1,2,4,6,8d进行气样采集.实验中土壤温度控制为25℃.
实验中采用的滴头为压力补偿式滴头(NETAFIM,以色列奈特菲姆灌溉公司), 额定流量为2.2L/h,埋深为5cm.滴灌毛管从距土柱上沿向下2cm处打孔穿出,用胶密封.首部供水压力设置为0.10MPa.灌溉装置如图1所示.

图1 灌溉装置示意Fig.1 Schematic diagram of irrigation device
1.循环曝气装置; 2.常规滴灌供水装置; 3.储水箱; 4.闸阀1; 5.水泵1; 6.压力表1; 7.氧气罐; 8.减压阀; 9.水泵2; 10.文丘里; 11.承压水罐; 12.排气阀; 13.压力控制器; 14.溶解氧控制器; 15.水表; 16.压力表2; 17.闸阀2; 18.闸阀3; 19.压力补偿式滴头; 20.土柱; 21.供水毛管; 22.供水干管
1.3 样品采集及指标测定
采气静态箱为圆柱体,直径为30cm,高为10cm.于静态箱侧壁中部打孔,向内延伸装入平衡软管,用于平衡箱内压强,直径为4mm,长度为10cm.采气时,分别于盖上静态箱后的0,10,20,30min,用50mL带三通阀的注射器采集30mL箱内气体,注入12mL的真空气瓶中,2周内完成测量.
实验前对土柱侧壁进行压实、灌浆和凡士林浇筑,以防止灌溉过程中灌溉水沿侧壁渗漏.采气的同时监测土柱0~10cm的土壤含水率、溶解氧和氧化还原电位(h).采用光纤式溶氧测量仪连接溶解氧敏感探针(PyroScience GmbH,德国Aachen公司)测定土壤溶解氧含量;通过h电极(IQ150,美国SPECTRUM公司)监测土壤h;采用土壤水分速测仪监测(TRIME-T3/T3C,德国TRIME-FM公司) 0~10cm的土壤平均含水率.采气的同时进行破坏性取土,测定NO3--N和NH4+-N含量.土壤NO3--N和NH4+-N以2mol/L KCl为提取剂,利用紫外分光光度计测定;土壤速效钾的测定以乙酸铵为提取剂,采用火焰光度法测定;速效磷的测定以碳酸氢钠为提取剂,采用钼锑抗比色法测定;土壤有机质采用重铬酸钾容量法测定[20].
1.4 相关指标计算
1.4.1 N2O排放通量计算,利用气相色谱仪(GC- 2010PLUS,日本岛津有限公司)测定气体样品中的N2O浓度,N2O排放通量[21]的计算如式(1)所示.
=××273/(273+) ×(/0)×d/d(1)
式中:为标准气体的浓度,g/cm3,为1.96g/cm3;为土面距离静态箱顶部距离,m,为0.15m;为采集N2O时静态箱内的温度,℃;为采集N2O时静态箱内的压强,mm Hg;0为标准大气压,mm Hg;为测定的气体浓度,mg/L; d/d为mg/(m3·h);为N2O排放通量,mg/(m3·h).
1.4.2 N2O排放总量计算 N2O排放总量的计算如式(2)[7]所示.

式中:为N2O排放通量,mg/(m3·h);为第次测量的时间,h;(t+1-t)为2次测量之间的时间间隔,h.
1.4.3 充水孔隙度 土壤充水孔隙度(WFPS)是反映土壤水分状况的重要指标,其计算公式如式(3)所示.
WFPS=θ/(1-/2.65) (3)
式中:θ为土壤的体积含水率,%;为土壤容重,g/cm3.
1.5 DNA提取及qPCR分析
实验中于灌溉后1d采集0~10cm的土壤,混匀后保存于-80℃的冰箱中,用于DNA提取和qPCR分析.土壤样品中微生物DNA总量提取方法参照Chen等[22]提供的方法.利用核酸测定仪(Nanodrop ND- 1000UV-Vis分光光度计)测定DNA浓度.PCR引物序列信息列于表1.AOA和AOB实时定量PCR扩增条件均为:95℃预变性2min,95℃ 5s,55℃ 30s, 72℃ 10s,40个循环; 95℃ 15s,60℃ 15s,95℃ 15s.土壤反硝化基因和实时定量PCR扩增条件均为:95℃预变性30s,95℃ 5s,60℃ 30s,72℃10s, 40个循环; 95℃ 15s,60℃ 15s,95℃ 15s.

表1 PCR引物序列信息
1.6 数据分析
采用EXCEL 2013软件进行数据分析;采用SPSS 22统计软件进行显著性和相关性分析.显著性分析采用Fisher LSD方法,检验水平为0.05.
2 结果与分析
2.1 曝气灌溉对土壤N2O排放的影响
如图2所示,实验中各处理N2O排放通量均呈现先增加后降低的变化趋势,于灌溉后1d出现排放峰值,之后下降,于灌溉后4d趋于稳定,且呈现较低排放水平.在培养期内,各处理N2O排放通量的关系:DAW1>DCW1> DAW2>DCW2.

图2 曝气灌溉下土壤N2O排放特征
DA为曝气灌溉;DC为常规滴灌;W1为高湿度处理;W2为低湿度处理,下同
由表2可知,曝气可显著增加土壤N2O的排放通量、排放峰值和排放总量(<0.05).与常规滴灌相比,W1和W2水量下曝气处理的土壤N2O排放通量、排放峰值、排放总量分别增加了41.08%和58.70%、158.79%和167.30%、127.46%和97.27%.

表2 不同处理下土壤N2O排放通量、峰值及排放总量
注:同列不同小写字母表示差异性显著(<0.05).
灌水量的增加可显著增加N2O排放通量和排放峰值(<0.05).另外,灌水量的增加可显著提高曝气条件下的N2O排放总量(<0.05),而对常规滴灌的N2O排放总量无显著影响(>0.05).曝气条件下高湿度处理(W1)的N2O排放总量较低湿度处理(W2)增加了73.46%.
2.2 曝气灌溉下土壤N2O排放的影响因子
各处理的充水孔隙度随着培养时间的增加而逐渐降低(图3a).曝气处理的土壤溶解氧呈现“N”型变化,于灌水后0.5d降到最低,之后逐渐上升,于灌水后2d逐渐趋于稳定;常规滴灌的土壤溶解氧呈现先降低后增加的变化趋势,于灌溉后0.5d达到最低,之后逐渐上升,于灌溉后4d逐渐趋于稳定(图3b).培养过程中土壤h的变化与常规滴灌条件下土壤溶解氧的变化趋势一致,但于灌水后1d达到最低值(图3c).培养期内各处理土壤溶解氧含量和h的关系均为DAW2>DAW1>DCW2>DCW1.由于灌水和曝气的差异造成土壤氮素的迁移和转化.在培养过程中,土壤的NO3--N和NH4+-N含量均呈现逐渐降低的变化趋势(图3d和图3e).
由表3可知,曝气可显著提高土壤溶解氧和h(<0.05),改善土壤通气性, 而对土壤充水孔隙度无显著影响(>0.05).W1水量下,曝气条件的土壤溶解氧和h均值较对照分别提高了7.79%和4.72%;W2水量下,曝气条件的土壤溶解氧和h均值较对照分别提高了7.52%和4.00%.
灌水量的增加可显著增加充水孔隙度,降低土壤溶解氧含量(<0.05).曝气条件下,W1水量的充水孔隙度较W2增加了14.88%,而溶解氧降低了3.23%.常规滴灌下, W1水量的充水孔隙度较W2增加了14.33%,而溶解氧降低了3.47%.
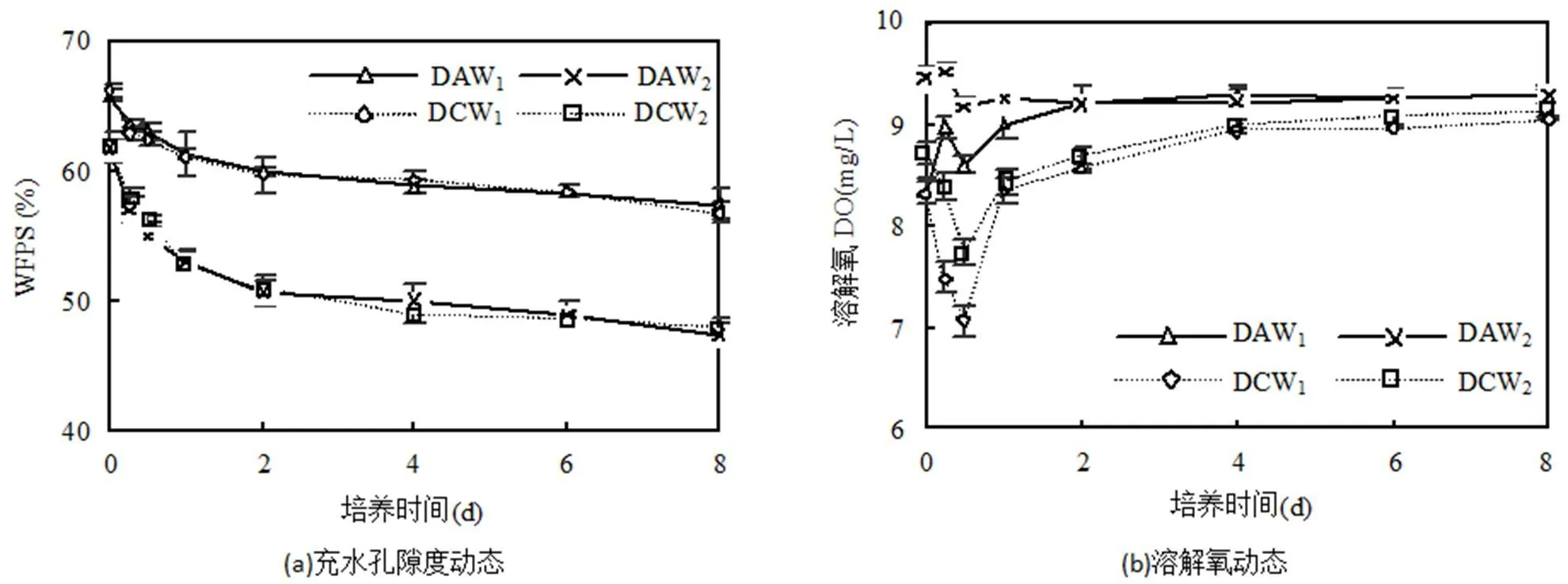
DO为土壤溶解氧

表3 影响土壤N2O排放的物理化学因子平均值
注:同列不同小写字母表示差异性显著(<0.05).
曝气可增加土壤NO3--N含量(<0.05),而对土壤NH4+-N含量无显著性影响(>0.05).低湿度处理的土壤NO3--N含量显著高于高湿度处理(<0.05).
由表4可知,曝气可显著增加高湿度处理下土壤拷贝数,减少低湿度处理下反硝化基因nosZ拷贝数(<0.05).灌水量的增加可增加古菌拷贝数(<0.05).曝气条件下,W1水量的拷贝数较W2增加了21.21%;常规滴灌条件下,W1水量的土壤古菌拷贝数较W2增加了20.95%.

表4 培养1d的土壤硝化与反硝化基因拷贝数
注:同列不同小写字母表示差异性显著(<0.05).
2.3 曝气灌溉下土壤N2O排放与各影响因子间的关系
由表5可知,高湿度处理下,土壤充水孔隙度、NO3--N含量和古菌拷贝数均与N2O排放呈显著的正相关关系(<0.05),而h与N2O排放呈极显著的负相关关系(<0.01).低湿度处理下,土壤充水孔隙度和古菌拷贝数与N2O排放之间分别呈极显著正相关关系(<0.01)和显著正相关关系(<0.05),而土壤h与N2O排放之间呈显著的负相关关系(<0.05).综上所述,实验中土壤充水孔隙度、h、NO3--N含量和AOA为曝气灌溉下N2O排放的主要影响因子.

表5 曝气灌溉下N2O排放与各影响因子间的相关系数
注:*表示在<0.05水平显著相关,**表示在<0.01水平显著相关.
3 讨论
土壤从干燥或适当干燥的环境变成湿润的环境会增加土壤碳氮的可利用性,造成土壤微生物量剧增[25].土壤微生物的迅速繁殖,促进土壤N2O的产生和排放[5].实验中灌溉后的土壤平均质量含水率达32.72%(初始平均质量含水率为22.17%),增加了土壤微生物数量,促进了土壤N2O的排放.灌溉会造成土壤含水量剧增,促进N2O排放峰值的出现[26],故灌溉后的N2O排放剧烈增加,并于灌水后1d出现N2O排放峰值,与Włodarczyk等[25]研究结果相似.有研究表明,当土壤WFPS在35%~60%时,N2O排放主要来源于硝化反应,当WFPS超过70%时,土壤N2O排放主要来源于反硝化反应[27-28].实验中高湿度处理的平均WFPS在60%以上,低湿度处理的平均WFPS达53%,故从土壤水分含量角度,高湿度处理下造成N2O排放可能以反硝化反应为主,而低湿度处理下以硝化反应为主.
土壤通气状况从2个方面影响着土壤N2O的排放:一方面是土壤的氧气含量影响着硝化和反硝化过程,从而制约了土壤N2O的产生数量;另一方面土壤孔隙为N2O排放至空气提供通道,通气性好坏决定了土壤N2O的排放数量[29].常规灌溉下由于水分的入渗驱替了土壤孔隙中的空气而造成土壤厌氧环境,导致土壤N2O产生主要来源于反硝化作用[30].曝气灌溉不仅为作物根系土壤提供充足的水分,还可有效弥补常规灌溉造成的土壤氧气逃逸[14],保持良好的气体交换通道.曝气激发了土壤好氧微生物的繁殖潜力,促进了土壤硝化作用,并提供了气体扩散的通道,增加了土壤N2O的排放.
土壤氧化还原状况和土壤水分含量密切相关,影响着土壤N2O的排放.Liu等[31]研究了水田淹水-落干过程中h对N2O排放的影响,结果表明稻田土壤中N2O排放与土壤h显著相关.侯会静等[32]研究表明土壤N2O排放通量的峰值出现在土壤h值为207.5~275.2mV,而h值低于120mV或者高于300mV时,土壤N2O排放通量处于较低水平.本研究中N2O排放峰值亦出现在该h范围,且在h值高于300mV时,N2O排放通量较低.有研究表明,当h在+300~ +400mV时,硝化作用是控制氮素转化反应的主要过程,反硝化作用相对较弱;当h介于+200mV和+300mV间时,反硝化作用出现且0mV时N2O排放量达到最高[33].研究中在灌溉后0.25~2d内土壤h均在+300mV以下,开始出现反硝化作用,而其他培养时间段内土壤h均在+300mV以上,以硝化反应为主.
土壤NO3--N易溶于水,且易随水分的入渗而迁移,而土壤NH4+-N不易在土壤中流失[34],故实验中低湿度处理的NO3--N含量显著高于高湿度处理,且随着培养时间的增加呈现降低的变化趋势.土壤NH4+-N是硝化微生物的作用底物,在各种氧化酶的作用下生成NO2--N和NO3--N[35].土壤NO3--N是反硝化作用的直接底物,缺氧条件下NO3--N被逐步还原为N2O和N2,底物供应充足可能直接促进反硝化作用强度[36].实验中曝气处理向土壤提供了充足的有效氧含量,在好氧微生物作用下,土壤NH4+-N逐步生成NO2--N和NO3--N,故土壤NH4+-N含量呈现降低的趋势,且高湿度处理和低湿度处理下N2O排放与NO3--N含量呈正相关关系,表明曝气条件下N2O排放以硝化反应为主.
微生物是驱动土壤氮元素生物地球化学循环的关键驱动力.灌溉不仅引起土壤含水量和Eh的变化,还可引起微生物群落结构发生变化[37].当土壤pH值在4.9~7.5时,有利于AOA基因拷贝数的增加和活性的表达,并且其拷贝数和表达活性随pH值的降低而升高[38].研究表明,AOA是水稻根际土壤的优势微生物,且AOA比AOB更易受土壤氧气含量的影响[39].实验中供试土样的pH值为6.30,为弱酸性土壤,有利于AOA的生存和繁殖.另外,土壤氧气含量的增加激发了AOA的繁殖潜力,故AOA的基因拷贝数显著高于AOB,且AOA基因拷贝数与N2O排放通量呈极显著正相关关系(<0.01),表明曝气灌溉条件下土壤以硝化反应为主,与Han等[7]研究结果相似.曝气灌溉对土壤硝化和反硝化过程N2O产生的来源及贡献分析对明确曝气滴灌条件下N2O排放机制有重要意义,有待进一步研究.
4 结论
4.1 实验中各处理N2O排放通量均呈现先增加后降低的趋势,于灌溉后1d达到峰值,于灌溉后的4d趋于稳定,且呈现较低排放水平.曝气可显著增加N2O的排放通量、排放峰值和排放总量.灌水量的增加可显著增加N2O的排放通量和排放峰值.
4.2 灌溉造成土壤含水量增加的同时,降低了土壤溶解氧和h.曝气可提高土壤溶解氧和h,改善土壤通气性(<0.05),而对土壤的充水孔隙度无显著影响(>0.05);低湿度处理的土壤NO3--N含量显著高于高湿度处理(<0.05).
4.3 通过相关性分析,土壤充水孔隙度、h和NO3--N含量为曝气灌溉下土壤N2O排放的主要理化因子.另外,AOA对曝气灌溉下土壤N2O排放起着重要的作用.
[1] Stocker T, Qin D, Plattner G, et al. IPCC, 2013: Climate Change 2013: The Physical Science Basis. Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [J]. Computational Geometry, 2013,18(2):95-123.
[2] Delgado J A, Mosier A R. Mitigation alternatives to decrease nitrous oxides emissions and urea-nitrogen loss and their effect on methane flux [J]. Journal of Environmental Quality, 1996,25(5):1105-1111.
[3] Smith P, Martino D, Cai Z, et al. Greenhouse gas mitigation in agriculture [J]. Philosophical Transactions Biological Sciences, 2008, 363(1492):789-813.
[4] Nishina K, Takenaka C, Ishizuka S. Relationship between N2O and NO emission potentials and soil properties in Japanese forest soils [J]. Soil science and plant nutrition, 2009,55(1):203-214.
[5] Singh B K, Bardgett R D, Smith P, et al. Microorganisms and climate change: terrestrial feedbacks and mitigation options [J]. Nature Reviews Microbiology, 2010,8(11):779.
[6] 贺纪正,张丽梅.土壤氮素转化的关键微生物过程及机制[J]. 微生物学通报, 2013,40(1):98-108. He J, Zhang L. Key processes and microbial mechanisms of soil nitrogen transformation [J]. Microbiology China, 2013,40(1):98-108.
[7] Han B, Ye X, Li W, et al. The effects of different irrigation regimes on nitrous oxide emissions and influencing factors in greenhouse tomato fields [J]. Journal of Soils & Sediments, 2017,17(10):1-12.
[8] Martins C S C, Macdonald C A, Anderson I C, et al. Feedback responses of soil greenhouse gas emissions to climate change are modulated by soil characteristics in dryland ecosystems [J]. Soil Biology & Biochemistry, 2016,100:21-32.
[9] Gödde M, Conrad R. Influence of soil properties on the turnover of nitric oxide and nitrous oxide by nitrification and denitrification at constant temperature and moisture [J]. Biology & Fertility of Soils, 2000,32(2):120-128.
[10] Wang L, Sheng R, Yang H, et al. Stimulatory effect of exogenous nitrate on soil denitrifiers and denitrifying activities in submerged paddy soil [J]. Geoderma, 2017,286:64-72.
[11] Bhattarai S P, Midmore D J, Su N. Sustainable irrigation to balance supply of soil water, oxygen, nutrients and agro-chemicals [M]. Biodiversity, biofuels, agroforestry and conservation agriculture. Springer, Dordrecht, 2010:253-286.
[12] 雷宏军,冯 凯,张振华,等.河南3种典型土壤曝气灌溉草莓生长与品质 [J]. 排灌机械工程学报, 2017,35(2):158-164. Lei H, Feng K, Zhang Z, et al. Growth and quality of potted strawberry under aerated drip irrigation in the three typical soils in Henan Province [J]. Journal of drainage and irrigation machinery engineering, 2017,35(2):158-164.
[13] Bhattarai S P, Midmore D J, Pendergast L. Yield, water-use efficiencies and root distribution of soybean, chickpea and pumpkin under different subsurface drip irrigation depths and oxygation treatments in vertisols [J]. Irrigation Science, 2008,26(5):439.
[14] Ben-Noah I, Friedman S P. Aeration of clayey soils by injecting air through subsurface drippers: Lysimetric and field experiments [J]. Agricultural Water Management, 2016,176:222-233.
[15] 雷宏军,胡世国,潘红卫,等.土壤通气性与加氧灌溉研究进展 [J]. 土壤学报, 2017,54(2):297-308. Lei H, Hu S, Pan H, et al. Advancement in research on soil aeration and oxygation [J]. Acta Pedologica Sinica, 2017,54(2):297-308.
[16] 赵丰云,杨 湘,董明明,等.加气灌溉改善干旱区葡萄根际土壤化学特性及细菌群落结构 [J]. 农业工程学报, 2017,33(22):119-126. Zhao F, Yang X, Dong M, et al. Aeration irrigation improving grape rhizosphere soil chemical properties and bacterial community structure in arid area [J]. Transaction of the Chinese Society of Agricultural Engineering, 2017,33(22):119-126.
[17] Pendergast L, Bhattarai S P, Midmore D J. Benefits of oxygation of subsurface drip-irrigation water for cotton in a Vertosol [J]. Crop & Pasture Science, 2013,64(11/12):1171-1181.
[18] 李 元,牛文全,张明智,等.加气灌溉对大棚甜瓜土壤酶活性与微生物数量的影响 [J]. 农业机械学报, 2015,46(8):121-129. Li Y, Niu W, Zhang M, et al. Effects of aeration on rhizosphere soil enzyme activities and soil microbes for muskmelon in plastic greenhouse [J]. Transactions of the Chinese Society for Agricultural Machinery, 2015,46(8):121-129.
[19] 雷宏军,臧 明,张振华,等.循环曝气压力与活性剂浓度对滴灌带水气传输的影响 [J]. 农业工程学报, 2014,30(22):63-69. Lei H, Zang M, Zhang Z, et al. Impact of working pressure and surfactant concentration on air-water transmission in drip irrigation tape under cycle aeration [J]. Transactions of the Chinese Society of Agricultural Engineering, 2014,30(22):63-69.
[20] 鲁如坤.土壤农业化学分析方法 [M]. 北京:中国农业科技出版社, 2000:106-193. Lu R. Soil argrochemistry analysis protocoes [M]. Beijing: China Agriculture Science Press, 2000:106-193.
[21] 陈 慧,侯会静,蔡焕杰,等.加气灌溉温室番茄地土壤N2O排放特征 [J]. 农业工程学报, 2016,(3):111-117. Chen H, Hou H, Cai H, et al. Soil N2O emission characteristics of greenhouse tomato fields under aerated irrigation [J]. Transactions of the Chinese Society of Agricultural Engineering, 2016,(3):111-117.
[22] Chen Z, Luo X, Hu R, et al. Impact of long-term fertilization on the composition of denitrifier communities based on nitrite reductase analyses in a paddy soil [J]. Microbial ecology, 2010,60(4):850-861.
[23] Sahan E, Muyzer G. Diversity and spatio-temporal distribution of ammonia-oxidizing Archaea and Bacteria in sediments of the Westerschelde estuary [J]. Fems Microbiology Ecology, 2008,64(2): 175–186.
[24] Rotthauwe J H, Witzel K P, Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations [J]. Applied & Environmental Microbiology, 1997,63(12):4704.
[25] Włodarczyk T, Szarlip P, Kozieł W, et al. Effect of long storage and soil type on the actual denitrification and denitrification capacity to N2O formation [J]. International Agrophysics, 2014,28(3):371-381.
[26] Dobbie K E, Smith K A. Nitrous oxide emission factors for agricultural soils in Great Britain: the impact of soil water‐filled pore space and other controlling variables [J]. Global Change Biology, 2003, 9(2):204-218.
[27] Peng S, Hou H, Xu J, et al. Nitrous oxide emissions from paddy fields under different water managements in southeast China [J]. Paddy & Water Environment, 2011,9(4):403-411.
[28] Case S D C, Mcnamara N P, Reay D S, et al. The effect of biochar addition on N2O and CO2, emissions from a sandy loam soil – the role of soil aeration [J]. Soil Biology & Biochemistry, 2012,51(3):125-134.
[29] Ludwig J, Meixner F X, Vogel B, et al. Soil-air exchange of nitric oxide: An overview of processes, environmental factors, and modeling studies [J]. Biogeochemistry, 2001,52(3):225-257.
[30] 郑 欠,丁军军,李玉中,等.土壤含水量对硝化和反硝化过程N2O排放及同位素特征值的影响 [J]. 中国农业科学, 2017,50(24):4747- 4758. Zheng Q, Ding J, Li Y, et al. The Effects of soil water content on n2o emissions and isotopic signature of nitrification and denitrification [J]. Scientia Agricultura Sinica, 2017,50(24):4747-4758.
[31] Liu J, Hou H, Sheng R, et al. Denitrifying communities differentially respond to flooding drying cycles in paddy soils [J]. Applied Soil Ecology, 2012,62(62):155-162.
[32] 侯会静,杨士红,徐俊增,等.水稻控制灌溉对稻田N2O排放的影响机理研究 [J]. 中国科学:技术科学, 2015,45(4):443-448. Hou H, Yang S, Xu J, et al. Influence mechanism of controlled irrigation on N2O emissions from paddy fields [J]. Sci Sin Tech, 2015, 45(4):443-448.
[33] Kralova M, Masscheleyn P H, Lindau C W, et al. Production of dinitrogen and nitrous oxide in soil suspensions as affected by redox potential [J]. Water, Air, and Soil Pollution, 1992,61:37-45.
[34] 刘秋丽.不同灌水量下土壤水氮运移及氨挥发特性研究[J]. 人民黄河, 2015,37(12):146-148. Liu Q. Soil water and nitrogen transport and ammonia volatilization characteristics research under different irrigation water [J]. Yellow River, 2015,37(12):146-148.
[35] Braker G, Conrad R. Diversity, structure, and size of N2O-producing microbial communities in soils—what matters for their functioning? [J] Advances in Applied Microbiology, 2011,75:33-70.
[36] Hunt P G, Matheny T A, Stone K C. Denitrification in a coastal plain riparian zone contiguous to a heavily loaded swine wastewater spray field [J]. Journal of Environmental Quality, 2004,33(6):2367–2374.
[37] Bapiri A, Bååth E, Rousk J. Drying-rewetting cycles affect fungal and bacterial growth differently in an arable soil [J]. Microbial Ecology, 2010,60(2):419-428.
[38] 侯海军,秦红灵,陈春兰,等.土壤氮循环微生物过程的分子生态学研究进展 [J]. 农业现代化研究, 2014,35(5):588-594. Hou H, Qin H, Chen C, et al. Research progress of the molecular ecology on microbiological processes in soil nitrogen cycling [J]. Research of Agricultural Modernization, 2014,35(5):588-594.
[39] Chen X P, Zhu Y G, Xia Y, et al. Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? [J]. Environmental Microbiology, 2010,10(8):1978-1987.
致谢:本研究得到“中国科学院亚热带农业生态研究所”和“中国农业科学院农田灌溉研究所”支持,在此表示感谢.
Characteristics and influencing factors of N2O emission from incubated soil under aerated irrigation.
LEI Hong-jun1*, LIU Huan1, ZANG Ming1, PAN Hong-wei1, CHEN De-li2
(1.School of Water Conservancy, North China University of Water Conservancy and Electric Power, Zhengzhou 450046, China;2.Faculty of Land and Food Resources, University of Melbourne, Victoria 3010, Australia)., 2019,39(5):2115~2122
To clarify the characteristics of soil N2O emission and identify the main factors under aerated irrigation (AI), the soil culture experiments were conducted at 2 irrigation levels with upper soil moisture limit as 70% and 90% of field capacity and 2 dissolved oxygen (DO) levels at 5 and 40mg/L. Soil N2O emission fluxes were monitored using static chamber-gas chromatography method and the copy number of nitrification and denitrification gene were determined using the real-time quantitative polymerase chain reaction (qPCR) technique. In addition, the main factors on soil N2O fluxes were analyzed, including soil water filling porosity (WFPS), DO, redox potential (h), mineral nitrogen content (NO3--N and NH4+-N), as well as the abundance of soil ammonia-oxidizing bacterial (AOB) and ammonia-oxidizing archaea (AOA) and denitrifier genes (and). Results showed that soil N2O flux increased from the beginning, peaked at 1d after irrigation, dropped in the following 3days, and then stabilized. An increase of aeration and irrigation amount resulted in the increase of average values and peak values of soil N2O fluxes. Irrigation caused an increase of WFPS, while a decrease of soil DO andh. Aeration treatments increased soil DO concentration andh (<0.05), improved soil aeration. However, aeration treatments showed no significant impact on WFPS. The WFPS,h and NO3--N content were the main physical, chemical influencing factors driving soil N2O emission under AI. The AI significantly affected AOA copy numbers. In addition, soil N2O fluxes were significantly correlated with AOA copy number (<0.05). The results could provide scientific support for the influential mechanism of AI on soil N2O and the farmland N2O emission management.
aerated irrigation;N2O emission;dissolved oxygen;redox potential;gene;copy numbers;influencing factor
X511,S275
A
1000-6923(2019)05-2115-08
雷宏军(1975-),男,湖北大冶人,博士,博士生导师,主要从事节水灌溉理论与技术研究.发表论文100篇.
2018-10-19
国家自然科学基金资助项目(U1504512,51779093);河南省科技创新人才项目(174100510021);华北水利水电大学研究生创新课题(YK2017-02);中原科技创新领军人才项目(194200510008)
*责任作者, 教授, hj_lei2002@163.com

