尖晶石结构MFe2O4(M=Ca,Mg,Cu,Zn)纳米晶粉末的制备和磁性能
陈 鹏 姜林文*, 刘进军 杨姗姗 李江涛 陈红兵 何 骏 王 煜
(1宁波大学材料科学与化学工程学院,新型功能材料及其制备科学国家重点实验室培育基地,宁波 315211)
(2钢铁研究总院功能材料研究所,北京 100081)
0 Introduction
Recently,magnetic spinel-type ferrites have been investigated extensively for their excellent properties and broad applications[1-10].Spinel-type ferrites with a formula of MFe2O4and a structure of cubic unit cell can be found in a broad range of applications,such as transformer cores,high density storage,biomedical fields like hyperthermia of cancer treatment,targeted drug delivery[11-13].As is known to all,calcium ferrite(CaFe2O4)magnetic nanoparticles have the features of monodispersity,stability,superparamagnetism,and biocompatibility[14-15].Besides,CaFe2O4is biocompatible and eco-friendly owing to the presence of Ca2+instead of heavy metals[16].Magnesium ferrite(MgFe2O4)is a magnetic bi-oxide ceramic material,which possesses the excellent chemical stability and partial inverse spinel structured(Mg1-δFeδ)[MgδFe2-δ]O4,where δstands for the degree of inversion of cation in the structure[17-18].MgFe2O4can be used in the fields of treating tumors using a thermal coagulation technique[19],catalysis[20],gas sensors[21],as well as the fuel cell cathode for molten electrolytes due to the enhancement of the stability and performance[22].Copper ferrite(CuFe2O4)is one of the most important spinel-type ferrites,which has the structural phase transformation and the reduction of the crystal symmetry known as Jahn-Teller distortion[23].In addition,the magnetic properties of CuFe2O4are strongly determined by the Cu ions,especially in the nano-scale size when the magnetic domain size is smaller than the grain boundaries[24]. Zinc ferrite(ZnFe2O4)also has the partially inverted spinel structure of(Zn1-δFeδ)[ZnδFe2-δ]O4like MgFe2O4,and it also possesses rather good magnetic and electrical properties[25]. Furthermore, ZnFe2O4is a potential material to be widely in the applications of gas sensor,semiconducting photocatalysis,magnetic resonance imaging,drug-delivery,light-driven water-splitting,ferrofluid,magnetic data storage,etc[26-29].
At present,CaFe2O4have been investigated by some researchers.Sulaiman et al.reported that CaFe2O4nanoparticles synthesized by co-precipitation method have much larger saturation magnetization than those fabricated by auto-combustion method[30].Manohar et al.studied the room-temperature magnetic properties of CaFe2O4nanocrystals prepared by solvothermal reflux method[31].However,Zhang et al.have synthesized CaFe2O4nanocrystal by solution combustion,which could be used as a magnetically separable photocatalyst[32]. Da Dalt et al.studied magnetic and Mssbauer behavior of the nanostructured MgFe2O4spinel ferrite obtained at low temperature[33].Inaddition,Druc et al.invstigated magnetic and dielectric properties for MgFe2O4ferrite at room temperature[34].However,there are very few reports about the studies of zero-field-cooled(ZFC)and fieldcooled (FC)magnetic properties of CaFe2O4and MgFe2O4ferrites.
In this study,MFe2O4(M=Ca,Mg,Cu and Zn)nanocrystalline powders were synthesized by autocombustion method using a novel amino-based gel.ZFC and FC magnetic properties of CaFe2O4and MgFe2O4nanocrystalline were specially investigated in this work.The results showed that CaFe2O4exhibits a magnetic phase transition of antiferromagnetic state to paramagnetic state below 75 K,which would help us to better understand the mechanism of low-temperature magnetic phase transition.In addition,MgFe2O4nanocrystalline sample has a superparamagnetic behavior,making the ferrite promising for magnetic recording application.
1 Experimental
1.1 Materials
Ca(NO3)2·4H2O(98.5%),Mg(NO3)2·6H2O(98.5%),Cu(NO3)2·3H2O (98.5%),Zn(NO3)2·6H2O(98.5%),Fe(NO3)3·9H2O(98.5%),and alanine(C3H7NO2,AR)were all purchased from the Sinopharm Chemical Reagent Corporation(Shanghai China).
1.2 Synthesis of MFe2O4 nanocrystalline powders
A series of spinel-type ferrites of MFe2O4(M=Ca,Mg,Cu and Zn)were synthesized by sol-gel autocombustion method.The flowchart presenting the method of preparation is detailedly exhibited in Fig.1.Calcium nitrate[Ca(NO3)2·4H2O],magnesium nitrate[Mg(NO3)2·6H2O],copper nitrate[Cu(NO3)2·3H2O],zinc nitrate[Zn(NO3)2·6H2O],and ferric nitrate[Fe(NO3)3·9H2O]were used as the precursors,while alanine(C3H7NO2)was acted as the combustion agent for the auto-combustion process.M(NO3)2(M=Ca,Mg,Cu and Zn)and Fe(NO3)3·9H2O with an accurate stoichiometric ratio of nM∶nFe=1∶2 were dissolved in the distilled water by stirring vigorously.The alanine was added into above solution,and the molar ratio of alanine to metal ions was set at 1∶1.Subsequently,the mixture was stirred and heated continuously until the mixture was almost evaporated dried and the sticky gel was formed.Then the sticky gel was transferred into an open alumina crucible,and the gel was burned by an auto-propagating manner.In this typical synthesis process, magnetic nanocrystalline powders were obtained successfully.Finally,all of as-obtained nanocrystalline powders were annealed at different temperatures for 2 h in a muffle furnace.
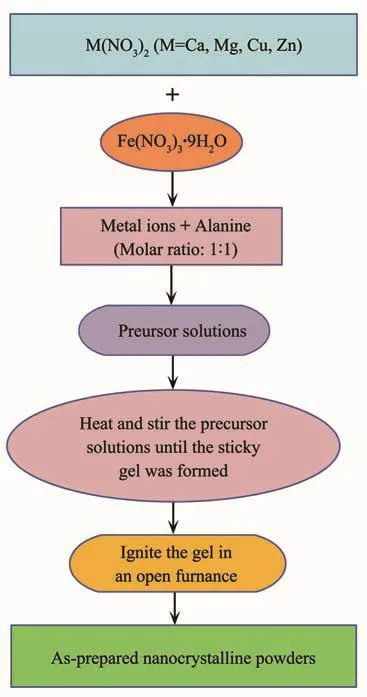
Fig.1 Flowchart of preparing MFe2O4(M=Ca,Mg,Cu and Zn)nanocrystalline powders by sol-gel autocombustion method
1.3 Sample characterization
The phase identification of MFe2O4nanocrystalline powders was confirmed using a Bruker D8 diffractometer with Cu Kα radiation (λ=0.154 06 nm,U=40 kV,I=40 mA)at a scan rate of 8°·min-1from 10°to 80°(2θ).The morphologies of the samples were characterized by a scanning electron microscope with 20 kV operating voltage,which was equipped an energy dispersive X-ray spectroscopy (EDS)analyzer(SEM,SU-70,Hitachi,Japan).The magnetic properties of the samples were investigated by a vibrating sample magnetometer(VSM Lakeshore-7410,America).
2 Results and discussion
2.1 X-ray analysis
The phase structures of the as-prepared MFe2O4(M=Ca,Mg,Cu and Zn)nanocrystalline powders annealed at 900℃were investigated by X-ray diffraction(XRD),as shown in Fig.2(a).The XRD patterns of four as-prepared nanocrystalline powders are all well in accordance with their standard cards,suggesting that the pure phase of nanocrystalline powders had been obtained successfully.However,there are residual organic compounds in the as-burnt nanocrystalline powders.Therefore,the as-burnt samples should be annealed.Fig.2(b)exhibits the XRD patterns of CaFe2O4powders annealed at the temperature of 600,700,800,900,1000,1 100 and 1 200℃,respectively.In Fig.2(b),the diffraction peaks of as-burnt CaFe2O4nanocrystalline exactly corresponded to the PDF#65-1333 until the annealed temperature is 700℃,suggesting that residual organic compounds in as-burnt CaFe2O4powders can be removed when the temperature is above 700℃.In addition,according to our previous work[35],the residual organic compounds in as-burnt GdFeO3powders prepared by sol-gel auto-combustion method can be removed by annealing at 800℃.Therefore,in order to ensure the removal of organic matter from the as-burnt powders,all of the samples for characterization were annealed at temperature of 900℃.

Fig.2 XRD patterns of four spinel-type ferrite nanocrystalline powders annealed at 900℃(a)and as-prepared CaFe2O4 powders annealed at different temperatures(b)
2.2 Microstructure analysis
Typical SEM micrographs of four kinds of asprepared nanocrystalline powders annealed at 900℃are well performed in Fig.3(a~h),respectively.In Fig.3(a,b),it can be seen that as-obtained CaFe2O4nanocrystalline powders were spongy and porous agglomerating with many pores,which were induced by the instantaneously-released gases during the autocombustion process.Fig.3(c,d) show the microscopic morphology of as-prepared MgFe2O4nanocrystalline powders,and it can be observed that the MgFe2O4powders presented sponge-like shape with lots of tiny bubbles.Fig.3(e,f)exhibit the micrograph of CuFe2O4nanocrystalline powders,and it can be seen that there were plenty of pores presented in the sample.In Fig.3(g),we can observe that ZnFe2O4powders were spongy and porous in morphology,and a high-magnification SEM image shown in Fig.3(h)clearly revealed that assynthesized ZnFe2O4nanocrystalline powders were spherical.Totally speaking,all of as-prepared powders have the porous and spongy morphology with plenty of pores.The pores could reduce the interactions between nanocrystalline powders,as well as be beneficial to magnetic materials.
2.3 EDS spectra analysis

Fig.3 SEM images of CaFe2O4(a,b),MgFe2O4(c,d),CuFe2O4(e,f)and ZnFe2O4(g,h)powders annealed at 900℃
As known to us all,magnetic properties of powders are sensitive to the constitutive components,such as Fe2O3,CaO,MgO,CuO,ZnO.The undesired matters may appear during the auto-combustion process owing to the drastic and uncontrollable reaction,which would seriously influence the results of intrinsic magnetic properties of the samples.In order to investigate the purity of as-synthesized powders,the EDS technique was used to measure the molar ratios of atoms in the samples.Fig.4(a~d)show the EDS spectra of as-obtained magnetic nanocrystalline powders:CaFe2O4,MgFe2O4,CuFe2O4and ZnFe2O4,respectively.It can be seen that the ratios of nM:nFe(M=Ca,Mg,Cu and Zn)are all close to the theoretical value of 1∶2.However,according to the statistical data in Table 1,we can know that the contents of O element of all the samples are obviously higher than the theoretical values.The much higher content of O element may be caused by the residual organic matters with oxygen element,which could enhance the content of detected oxygen[36].
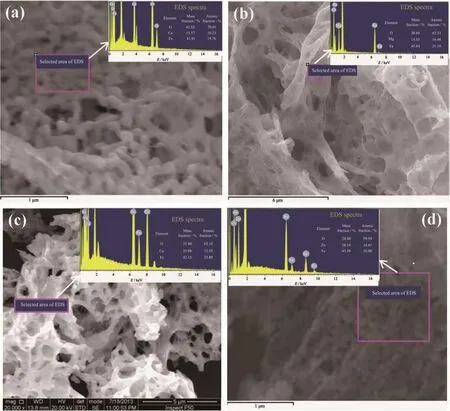
Fig.4 EDS spectra of as-synthesized nanocrystalline powders:(a)CaFe2O4;(b)MgFe2O4;(c)CuFe2O4;(d)ZnFe2O4
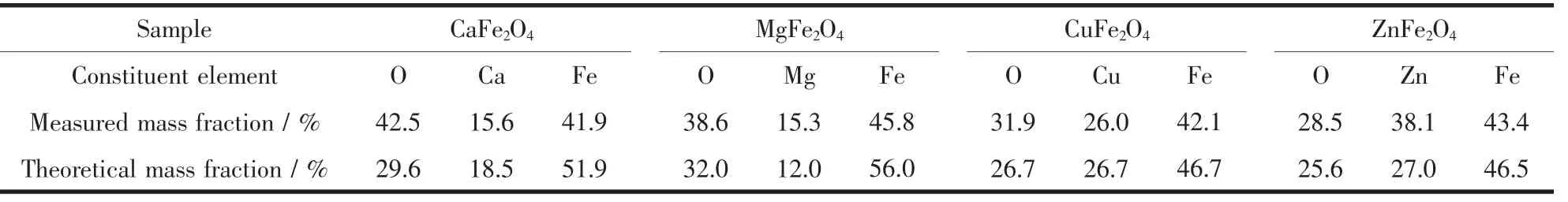
Table 1 Comparison of theoretical and measured values of element contents of as-prepared samples
2.4 Magnetic properties
Magnetic properties of the ferrite nanocrystalline powders are significant for their application in magnetic recording,magneto-optical recording,as well as other electronic devices.The hysteresis loops of asprepared MFe2O4(M=Ca,Mg,Cu and Zn)nanocrystalline powders measured using a vibrating sample magnetometer(VSM)at room temperature are exhibited in Fig.5(a~d).From Fig.5,it can be observed that all the samples presented a rather narrow hysteresis loop,suggesting a behavioral characteristic of soft magnetic features[34].The related hysteresis parameters of as-prepared samples are concretely described in the Table 2.From the hysteresis loops,it can be observed that CaFe2O4and ZnFe2O4powders have rather low saturation magnetization(Ms)and residual magnetization(Mr).Whereas,MgFe2O4and CuFe2O4powders have rather large Msand Mrvalues,which may be induced by their larger particle sizes.According to the references[37-39],the saturation magnetization(Ms)increases with the increase in particle size.In Fig.4(a~d),it can be observed that CuFe2O4powders have the largest coercive force(Hc)value in the four kinds of nanocrystalline powders.As we all know,Hcis different from Msand Mr,since particle size is not a unique factor determining Hc.Hcis determined by some complex factors,such as microstructure,particle/grain size,residual strain[40-41].Besides,Table 2 also exhibited the values of loop squareness ratios,Mr/Ms.In Table 2,it can be found that all of the calculated Mr/Msvalues were less than 0.5,indicating the particles could interact by magnetostatic interactions[42].According to the given values,it is quite possible for these materials to be used in the field of cores and coils with low inductance[43].
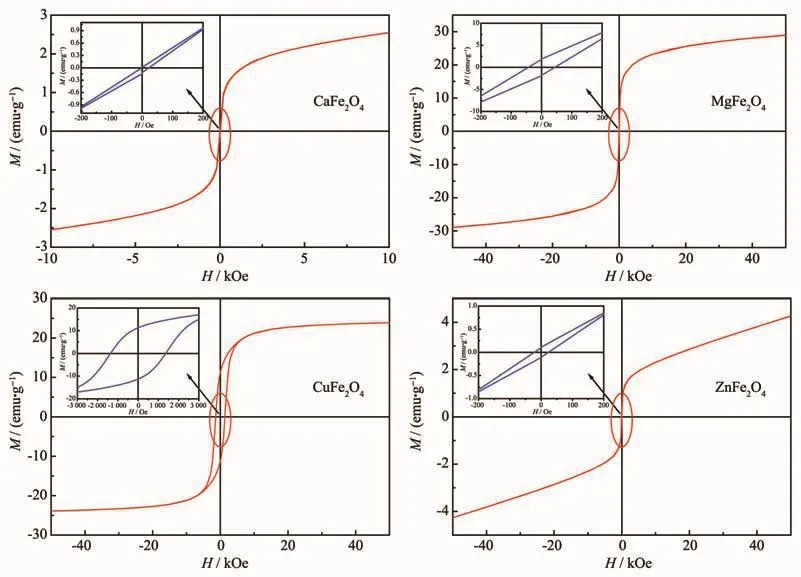
Fig.5 Hysteresis loops of CaFe2O4(a),MgFe2O4(b),CuFe2O4(c)and ZnFe2O4(d)powders
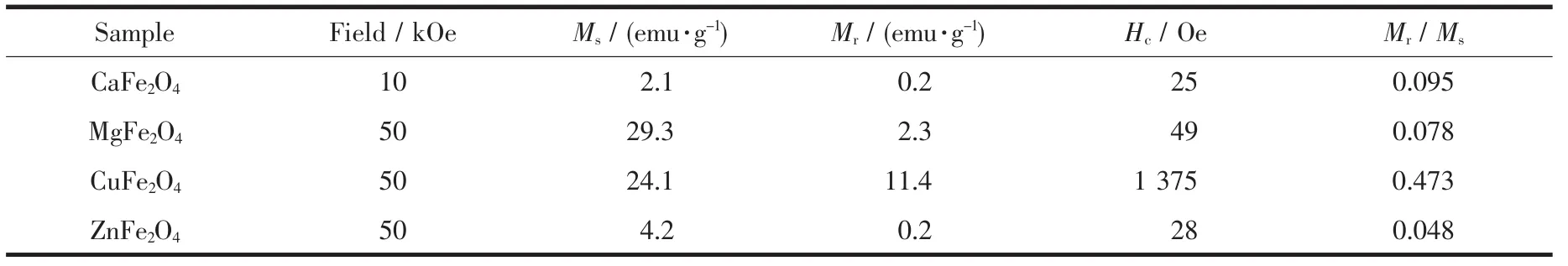
Table 2 Hysteresis parameters of as-prepared samples at the room temperature
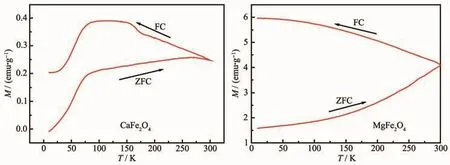
Fig.6 Temperature dependence of the magnetization in ZFC and FC for CaFe2O4(a)and MgFe2O4(b)powders
To investigate the detailed magnetic behaviors,CaFe2O4and MgFe2O4as two typical ferrites were employed to determine ZFC and FC properties.Fig.6(a,b)display the ZFC and FC magnetization curves for the two nanocrystalline samples of CaFe2O4and MgFe2O4,respectively.In Fig.6(a),the ZFC process of CaFe2O4had a sharp increase from 0 (5 K)to 0.20 emu·g-1(75 K),and a slow increase from 0.20 emu·g-1(75 K)to 0.24 emu·g-1(300 K).In the ZFC process,temperature plays a crucial role in determing the magnetization due to the absence of applied field.In this case,the high temperature can provide the related thermal disturbance,and cause the oriented rotation of magnetic domain,so the magnetization presents an obvious increase with the temperature increasing.When the sample was cooled from 300 K to 5 K(FC),the magnetization of CaFe2O4increased from 0.24 emu·g-1(300 K)to 0.38 emu·g-1(75 K),while decreased from 0.38 emu·g-1(75 K)to 0.24 emu·g-1(5 K).In contrast,in the FC process,the magnetization increased with the temperature decreasing in the condition of adding an applied field.It might be due to that the thermal disturbance has the random orientation,which can weaken the effect of applied field on the magnetization of sample.As in the reference[44],the magnetization of CaFe2O4is initially constant as the temperature is decreased.Further lowering the temperature,magnetization increases sharply,reaches a maximum value and then decreases almost linearly down to 30 K.This behavior is typical of an antiferromagnetic material exhibiting a transition to paramagnetic state.Therefore,it can be observed a similar behavior in Fig.6(a)that the magnetization presented an inconsistent variation tendency below 75 K,which may be due to the magnetic phase transition of CaFe2O4sample.For the sample of MgFe2O4,it can be seen from Fig.6(b)that the ZFC magnetization monotonically increased from 1.61 emu·g-1(5 K)to 4.18 emu·g-1(300 K).Similar with the CaFe2O4sample,temperature plays a crucial role in determining the magnetization during the ZFC process.For the FC process,magnetization monotonically increased with the decrease of temperature from 4.18 emu·g-1(300 K)to 5.95 emu·g-1(5 K).Similar with the CaFe2O4sample,the decreased magnetization might be due to the thermal disturbance that weaken the effect of applied field on the magnetization of sample.In addition,the separated ZFC and FC magnetization curves in Fig.6(b)indicate that the MgFe2O4sample has a superparamagnetic behavior.
3 Conclusions
Auto-combustion method was applied in the preparation of spinel-type ferrites nanocrystalline powders MFe2O4(M=Ca,Mg,Cu and Zn)in this work.The high purity of synthesized MFe2O4powders were certified by the fact that the ratios of nM∶nFewas close to the theoretical value of 1∶2 measured via an energy dispersive X-ray spectroscopy.The measured results obtained from the magnetic hysteresis loops indicated that all of the four samples have the moderate magnetism and the behavioral characteristic of soft magnetic features.CaFe2O4presents an inconsistent variation tendency below 75 K,which is caused by a magnetic phase transition of antiferromagnetic state to paramagnetic state below 75 K.The separated ZFC and FC magnetization curves indicate that the MgFe2O4sample has a superparamagnetic behavior.
Acknowledgements:This work is supported by Natural Science Foundation of China(Grant No.51701098),and Natural Science Foundation of Ningbo municipality (Grant No.2017A610100).This work is also sponsored by the Opening Project of State Key laboratory of Crystal Material in Shandong University (Grant No.KF1706),and K.C.Wong Magna Foundation in Ningbo University.
Conflict of Interest:The authors declare that they have no conflict of interest.

