Mixed vitamin C and zinc diet supplements co-administered with artemether drug improved haematological profile and survival of mice infected with Plasmodium berghei
Felici N. Ekeh, Nkiru E. Ekechukwu,b, Chimdinm F. Chukwum,Ifenyi Oscr N. Aguzie,∗, Chinenye M. Ohnu, Chike Ebido, Stnley N. Oluh
a Department of Zoology and Environmental Biology, University of Nigeria, Nsukka, Enugu State, Nigeria
b Center for Parasitology and Entomology, School of Life Sciences, Huxley Building, Keele University, ST5 5BG Keele, UK
Keywords:
A B S T R A C T
1. Introduction
Malaria is a preventable and curable disease, yet it remains a devastating tropical disease, with high infection and mortality statistics. Malaria, a life threatening disease is caused by a parasitic infection of the red blood cells by Plasmodium parasites transmitted through a bite of the female Anopheles mosquitoes. This disease is prevalent in tropical and subtropical regions and is mostly associated with poverty. Clinical symptoms of malaria include headache,fever, chills and vomiting which are usually mild but if not treated immediately could lead to delirium, metabolic acidosis, cerebral malaria and multi-organ system failure [1]. World Health Organization (WHO) estimates that each year, more than 200 million people are infected with malaria worldwide [2]. There were 214 million cases of malaria worldwide in 2015; 90% of which occurred in sub-Saharan Africa. Out of these, 438,000 resulted in death globally of which 78% were children under the age of five [3].
Children under the age of 5 (U5) in malaria-endemic areas are at risk of protein-energy malnutrition and deficiency in micronutrients such as iron and zinc [4]. Micronutrients are vitamins and minerals needed by man in small quantities for good health. These include vitamin C (ascorbic acid), iron, magnesium, calcium, etc.[5]. Iron is involved in numerous biological processes. It is the most important transition metal in all living organisms [6]. Vitamin C is a cofactor in many enzymatic reactions, including several collagen synthesis reactions that, when dysfunctional, cause the most severe symptoms of scurvy. In animals, these reactions are especially important in wound-healing and in preventing bleeding from capillaries [7]. Zinc is required for normal immune function [8]and reduces the incidence of diarrhoea and pneumonia [9]. Zinc is essential for a variety of lymphocyte functions implicated inresistance to malaria, including production of IgG, interferon-γ and tumor necrosis factor-α, and microbicidal activity of macrophages[8,10]. Zinc is the second most abundantly distributed trace element in the body after iron. It is an essential micronutrient for human metabolism that catalyzes more than 100 enzymes, facilitates protein folding, and helps regulate gene expression [11].

Table 1 Feed ingredient and quantities used in diet formulation.
Iron and zinc deficiencies is common in malaria endemic areas[12,13]. Children and pregnant women are particularly vulnerable[14]. Deficiency in iron results in anaemia. Anaemia affects 43%of children under 5 years and 38% of pregnant women globally[15]. Anaemia during pregnancy increases the risk of maternal and perinatal mortality and low birth weight. Maternal and neonatal deaths are a major cause of mortality, together causing between 2.5 million and 3.4 million deaths worldwide [15]. Direct iron supplementation during malaria may have negative effects [16]. Reports on the impact of direct iron supplement during plasmodium infection have been conflicting. Sazawal et al. [17] reported increased risk for severe illness from direct iron supplement during malaria.Direct iron supplement during malaria has also been reported to have no effect on malaria outcome [18]. This prompted Rahfiludin and Ginandjar [19] to hypothesize that iron adsorption from food instead of direct iron supplement is preferable during malaria infection. If this assumption is correct, then stimulating increased adsorption of iron from food could help ameliorate iron deficiency during malaria. Vitamin C is known to increase ability of the body to adsorb iron from food. Rahfiludin and Ginandjar [19] studied the effects of combined supplement of vitamin C and zinc on malaria patients. They observed that 45 days of vitamin C – zinc supplement resulted in positive haematological outcomes. Haemoglobin and haematocrit concentrations was significantly improved by zinc– vitamin C supplements [19]. Zinc only supplement have been reported not to have effect on morbidity from falciparum malaria among children, although it reduced morbidity associated with diarrhoea [20]. A combination of vitamin A and zinc supplementation have been reported to lower incidence of malaria by 27%in children [21]. These reports appear to suggest that the positive effects of zinc and vitamin supplements is due to their combined administration. Thus, this study evaluated the effect of different combinations of vitamin C and zinc on haematological parameters,P. berghei parasitaemia and mortality of infected mice.
We assumed that combined supplement of vitamin C and zinc given together with antimalarial drugs such as artemether could stimulate faster recovery from malaria induced anaemia. This assumption is premised on findings that zinc-vitamins supplements improved haematological outcomes during malaria [19],and that the supplement probably provides protection by reducing malaria morbidity [21]. Artemether administered during malaria is believed to contain factors that may facilitate recovery from anaemia [22]. Though it is uncertain whether the improvement in anaemia condition may be entirely due to clearance of malaria parasitaemia and the nutritional status of patients. In Nigeria malarial drugs are severally administered along with multivitamins or direct iron supplements in the belief that it facilitates quick recovery and blood synthesis especially in the face of malnutrition. The duration of supplement intake tends to terminate with the duration of treatment, though sometimes it extends well beyond. Thus,it becomes necessary to understand the impact of zinc-vitamin supplement co-administered with antimalarial drugs. Here haematological outcomes and survival rate after three days standard course of artemether treatment co-administered with vitamin Czinc supplement was evaluated, and the effects observed for three weeks. The supplements was administered as formulated diet. Plasmodium berghei-infected laboratory mouse model was used.
2. Materials and methods
2.1. Management of Albino Mice
Sixty-three albino male mice weighing 19 – 25 g were used for the study. They were housed in stainless steel cages with wire screen top. The animals were approximately 6 weeks old. They were allowed to acclimatize for 1 week in the laboratory environment under atmospheric temperature and humidity, and were maintained on commercial feeds and tap water ad libitum. Good hygiene was maintained by constant cleaning and removal of faeces and spilled feeds from the cages daily.
2.2. Ethical considerations
The University Research Policy on Research Involving Animal Subjects was adhered to in the use and care for the experimental animals [23].
2.3. Preparation of inoculum of Plasmodium berghei
Donor mouse with a rising Plasmodium berghei parasitemia of 20% – 30% confirmed by thin and thick blood film microscopy was used. 1 ml of parasitized blood was collected from the donor mouse and diluted in 15 ml normal saline at pH 7.2 so that each 0.2 ml contained approximately 107 infected red cells [24,25]. Each animal received inoculum of about 10 million parasites per kilogram body weight, which is expected to produce a steadily rising infection in mice.
2.4. Processing and preservation of dietary ingredients
The dietary ingredients required for the research were corn,soybean, fish meal, Bambara nut chaff, vitamin C and Zinc. Three kilograms of yellow corn and 2 kg of soybean were purchased from a feed mill, grand and stored. Two kg of fish meal was purchased from feed mill and stored in an airtight tin. Two kg of Bambara nut and 5 g each of vitamin C and zinc (as zinc sulphate) were purchased.
2.5. Diet formulation
Weight of ingredients used and procedure for diet formulation is shown in Table 1. Group A was fed diet without vitamin C and zinc while groups B, C, D, E, F and G were fed diets with vitamin C and zinc in different rations. The ingredients were weighed out using a sensitive beam balance. 500 g each of the formulated diet was homogeneously mixed with water to produce dough. The mixed diet was then transferred into heat resistant polythene and boiled for two hours. According to Eyo [26], such heat treatment was necessary for the gelatinization of starch, reduction of cyanide activity and activation of nutrients. Each diet was then pelletized using an improvised sieve. The resulting pellets were dried and stored. Vitamin C and zinc inclusion in the formulated diet were combined in ratios that adds up to 150 mg where 50:50 is equivalent to 75 mg of each supplement, 30:70 is 45 mg to 105 mg, and 40:60 is 60 mg to 90 mg. Before inclusion of zinc and vitamin, proximate composition of the cornmeal, soya bean, fishmeal and Bambara nut chaff formulated diets was 6.21 ± 0.21% moisture, 7.15 ± 0.07% crude fat,17.02 ± 3.35% crude protein, 3.93 ± 0.02% total ash, 2.38 ± 0.04%crude fibre and 63.31 ± 2.13% carbohydrate. The energy level was approximately 354.60 kcal/100 g.
2.6. Preparation of standard drug (Artemether)
Artemether injection was used. Each vial of injection contained 80 mg/ml of artemether. Average body weight of the mice was taken and used to calculate the dosage.2.6.1. Dosage calculation
According to the Organization of Economic Corporation and Development’s (OECD) guidelines, dosage of drug (mg) should be constituted in an appropriate volume not usually exceeding 10 ml/kg (1 ml/100 g) body weight of experimental animals (mice and rats) for non-aqueous solvent in oral route of administration[27]. Mice collected were between 17 to 22 g, hence the dosage of drug according to standard should not exceed 0.2 ml.
Dosage (mg) = [Average weight of mice (g) × Dose (mg)] / 1000 g
2.7. Experimental design
The experimental set up comprised seven (7) groups labeled A– G each replicated thrice and three mice per replicate. The seven groups were as follows:
i Group A (No-supplement Control). Infected and treated with standard drug only;
ii Group B. Infected and treated with standard drug + vitamin C and zinc (50:50) supplemented diet;
iii Group C. Infected and treated with standard drug + vitamin C and zinc (30:70) supplemented diet;
iv Group D. Infected and treated with standard drug + vitamin C and zinc (60:40) supplemented diet;
v Group E. Infected and treated with standard drug + vitamin C and zinc (70:30) supplemented diet;
vi Group F. Infected and treated with standard drug + vitamin C and zinc (40:60) supplemented diet; and
vii Group G (No-infection Control). Not infected but administered vitamin C and zinc (50:50) supplemented diet.
After acclimatization, the mice were inoculated with P. berghei.Three days post-inoculation and establishment of infection, they were treated with the standard drug and administered supplemented diets depending on the group. Artemether was given intra-peritoneal once daily for three consecutive days. Feeding with supplemented diet commenced and ended with the three days treatment. Body weight of mice was measured before parasite inoculation, just before treatment with standard drug and weekly for three weeks post-treatment. Blood samples were collected for haematological tests and parasitaemia count weekly and at 2-day interval respectively. Haematological parameters were measured post-treatment while parasitaemia was determined pre- and posttreatment.
2.8. Calculation of the blood parasite infection load
Thin smears were prepared from mouse tail venous blood, fixed with methanol, and stained with 10% Giemsa for 10 min [28]. The slides were then examined with an oil immersion lens. A total of ten fields were counted. Parasitaemia was calculated using the equation:
Parasitaemia (%) = Infected RBCs/ total RBCs x 100
2.9. Determination of haematological parameters
Packed cell volume and white blood cell count was carried out as described by Aka et al. [29] while red blood cell count and haemoglobin concentration was carried out as described by Ochei and Kolhatkar [30]. The total white blood cell count was done using the improved Neubauer’s counting chamber. Mean cell volume(MCV), mean cell haemoglobin (MCH) and mean cell haemoglobin concentration (MCHC) were derived using the formulae:

2.10. Data analysis
Data was analysed using SPSS version 20.0 (IBM Corporation, Armonk, New York). Repeated Measures univariate ANOVA full factorial model using default Type III sum of square and polynomial level test of within-subjects contrasts and no contrast between-subjects effects, under the Generalized linear model(GLM) procedure was used to assess the responses of mice haematological parameters (PCV, RBC, HB, WBC, MCV, MCH and MCHC)to the supplement for the duration of exposure. Repeated Measure univariate ANOVA was also used to model response of mice body weight and P. berghei parasitaemia to supplement. The general design of the model was: intercept + experimental groups(between-subjects design), repeated for the duration of treatment (i.e. within-subjects design). Estimated marginal means of responses, estimates of effect sizes, least significance difference LSD(with no-supplement Control, i.e. Group A, as reference), and mean estimates from pairwise comparison are presented in the result section. Mortality of mice per group was calculated in percentage as proportion of total number of death per group divided by initial number of mice per group. Chi-square test was used to compare mortality between the groups. Level of significance was set at 95%probability decision boundary (p < 0.05 was considered significant).

Table 2 Haematological changes in mice infected with P. berghei, treated with standard drug and fed vitamin C and zinc supplemented diet
3. Results
3.1. Response of Plasmodium berghei-infected mice haematological parameters to treatment with Artemether under vitamin C - zinc supplement
Before infection with Plasmodium berghei, haematological status of all the mice was similar. PCV, HB, RBC, WBC and other haematological indices evaluated was not different between the groups before P. berghei infection. The response in some of the haematological parameters was indicative of the effect of the supplements.PCV and HB responded positively to the supplements. The response was significant for PCV (F = 3.973, P = 0.005, ηp2= 0.460) and HB(F = 3.977, P = 0.005). Compared to no-supplement control (Group A), marginal PCV in all groups fed supplemented diet was higher.But only Group B which received equal amount of vitamin C and zinc (50:50), and group G (the uninfected but supplemented control) had marginal mean PCV significantly higher than the nosupplement control (P < 0.05). HB in plasmodium infected groups,co-administered vitamin C - zinc and artemether was higher than in no-supplement control (Group A). However, HB in the uninfected but supplemented control (Group G) was highest (Table 2).RBC was not affected by combined vitamin C and zinc supplement. WBC did not appear to show a clear cut response to the supplement, though group E (70:30 vitamin C - zinc) WBC increased significantly compared to no-supplement control (Group A, P = 0.039).
Groups fed supplemented diets and the uninfected control had higher marginal mean MCV than the infected no-supplement control. However, only groups B (50:50), C (30:70 vitamin C - zinc) and G were significantly higher (p < 0.05). Marginal MCHC showed minimal difference between the groups (Fig.1). Overall, there was no significant between group difference in MCH (F = 1.729, P = 0.151).But MCH in groups C and G was significantly higher than in group A. MCH in group C was similarly significantly higher than in group E (P = 0.049; Fig.1).
In all infected and treated groups, PCV increased significantly on week 3 compared to week 1. At the end of week 1, PCV in all the groups, except group B (50:50 vitamin C to zinc), was less than PCV of the uninfected control (Group G) significantly (P < 0.05). The decline was greatest in group A, the no-supplement group. PCV in groups fed 50:50, 60:40, 70:30 and 40:60 vitamin C - zinc supplemented diet and the no-supplement control was similar to the no-infection control (group G) on week 2, but remained significantly lower in group C (30:70 vitamin C - zinc) (Table 3). On week 3, PCV in all groups attained the same level.
Week 1 RBC in all the groups were similar except group G which was significantly higher. At the end of week 2 RBC in all the groups had attained the same level as group G. The supplementation had no effect on RBC (Table 3). Week 1 HB in all the infected groups were significantly less than the uninfected control (P < 0.05). They all attained similar level on week 2 and remained so. However, HB appeared to have increased slightly more in the groups infected,treated and fed supplemented diet than in the no-supplement control at the end of week 3 (Table 3). WBC in all the infected and treated groups were similar from weeks 1 to 3. In group E (70:30 vitamin C - zinc) week 1 WBC was significantly higher than those of groups D (60:40 vitamin C - zinc), F (40:60 vitamin C - zinc) and G(no-infection control) (P < 0.05); but from week 2 they all attained the same level of WBC.

Fig.1. Response of mean cell volume (MCV, fl), mean cell haemoglobin concentration (MCHC, g/dl) and mean cell haemoglobin (MCH, pg) to vitamin C and zinc supplement.Values as mean ± Standard error. Bars with different alphabet label were significantly different (p < 0.05). Key (Treatment): A. infected and treated with standard drug only;B. infected and treated with standard drug + vitamin C and zinc (50:50); C. infected and treated with standard drug + vitamin C and zinc (30:70); D. infected and treated with standard drug + vitamin C and zinc (60:40); E. infected and treated with standard drug + vitamin C and zinc (70:30); F. infected and treated with standard drug + vitamin C and zinc (40:60). G. Not infected but administered vitamin C and zinc (50:50).

Table 3 Haematological changes from weeks 1 to 3 in mice infected with P. berghei, treated with standard drug and fed vitamin C and zinc supplemented diet.
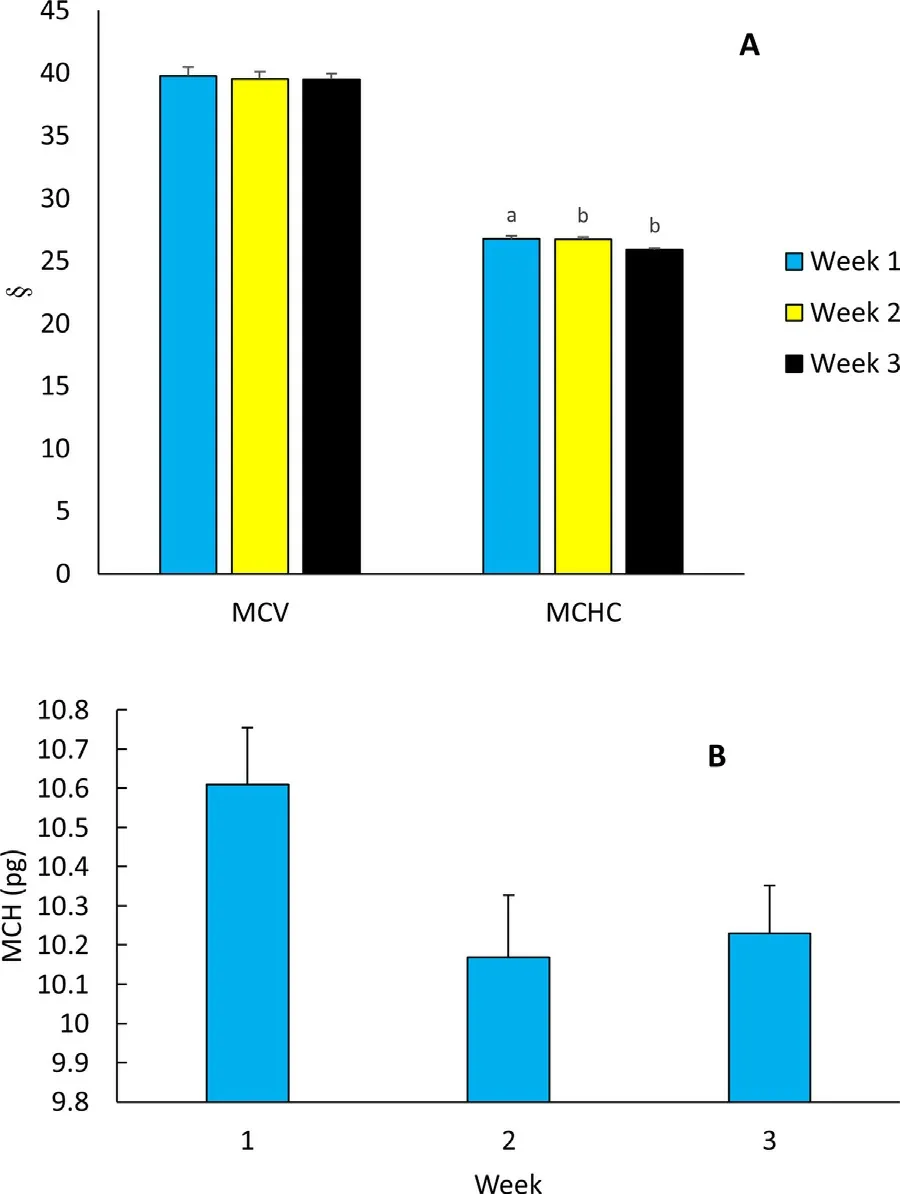
Fig.2. Duration of supplementation associated changes in haematological derivatives of mice infected with P. berghei, treated with standard drug and fed vitamin C and zinc supplemented diet. (A) MCV (fl) and MCHC (g/dl); (B) MCH (pg). Bars with different alphabet label were significantly different (p < 0.05).
The effect of the vitamin C – zinc co-administered treatment on the haematological parameters PCV, RBC, HB and WBC was dependent on duration of observation post-withdrawal of the treatment.The interaction of treatment and duration of observation postwithdrawal of treatment was significant for these haematological parameters (p < 0.05).
Marginal mean MCV was similar for weeks 1 – 3 (Fig.2A). MCHC on week 1 was significantly higher than weeks 2 and 3 values(F = 14.975, p = 0.001, Fig.2A). MCH also changed significant for the duration of the study (F = 4.288, p = 0.048), weeks 2 and 3 MCH were low compared to week 1 (Fig.2B).

Fig.3. Plasmodium berghei Parasitaemia pre- and post-treatment with supplemented standard drug. Key (Treatment): A. infected and treated with standard drug only; B. infected and treated with standard drug + vitamin C and zinc (50:50); C.infected and treated with standard drug + vitamin C and zinc (30:70); D. infected and treated with standard drug + vitamin C and zinc (60:40); E. infected and treated with standard drug + vitamin C and zinc (70:30); F. infected and treated with standard drug + vitamin C and zinc (40:60). G. Not infected but administered vitamin C and zinc (50:50). Bf – before.
3.2. Response of Plasmodium berghei parasitaemia to treatment with Artemether under vitamin C and zinc supplement
Before treatment with standard drug, P. berghei parasitaemia was approximately 9% in all the groups. After 3 days treatment with artemether standard drug, parasitaemia dropped to approximately 2% on days 3, 5, 7, and 9 (Fig.3). It dropped further to below 1%on day 11. Compared to the no-supplement control (group A), the vitamin C - zinc supplement did not affect P. berghei parasitaemia.However, group C (30:70 vitamin C to zinc) had the least parasitaemia compared to the no-supplement control (Fig.4).
3.3. Mortality of Plasmodium berghei-infected mice on treatment with Artemether under vitamin C - zinc supplement
Mortality of mice was recorded in some groups for the three weeks duration (Fig.5). The highest percentage mortality of 22.2%occurred in group A (no-supplement control); this was higher than 11.1% mortality in each of the groups B, E and F. One death was recorded in these supplement groups compared to two out of nine in the no-supplement control. No mortality was observed in groups C, D and G. But the difference in mortality between the groups was not significant (χ2= 5.214, df = 6, p = 0.5167).
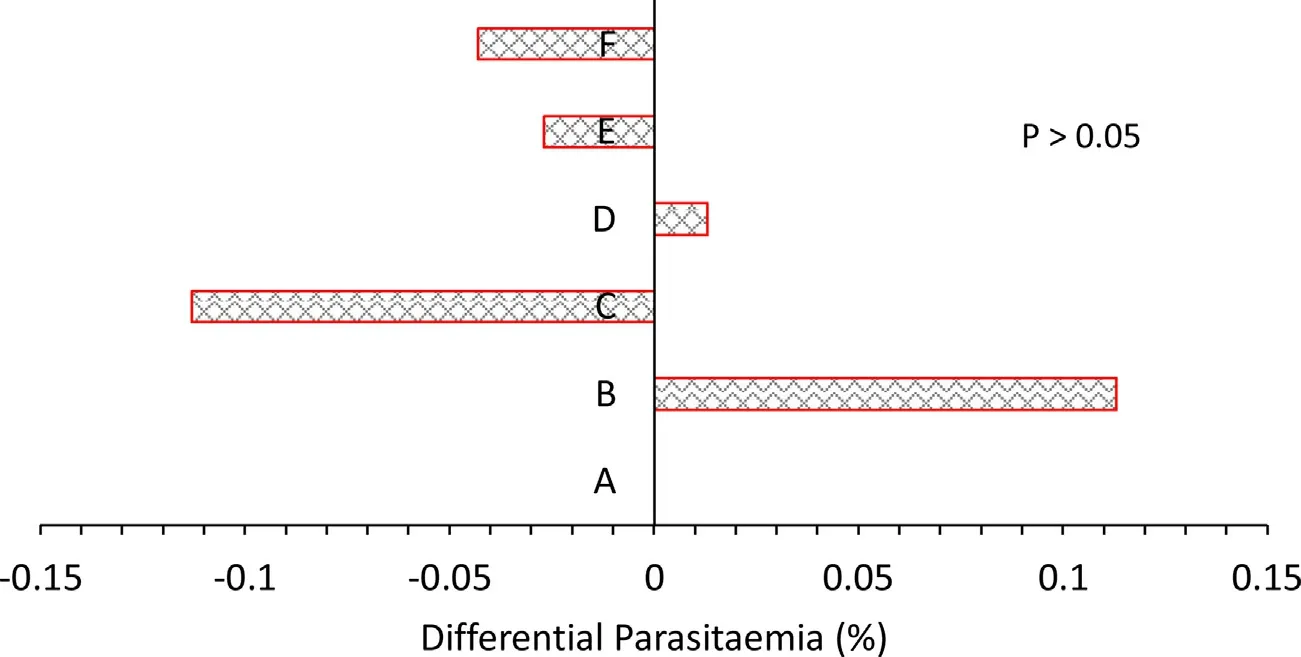
Fig.4. Differential marginal mean P. berghei parasitaemia in relation to group A (no-supplement control). Key (Treatment): A. infected and treated with standard drug only;B. infected and treated with standard drug + vitamin C and zinc (50:50); C. infected and treated with standard drug + vitamin C and zinc (30:70); D. infected and treated with standard drug + vitamin C and zinc (60:40); E. infected and treated with standard drug + vitamin C and zinc (70:30); F. infected and treated with standard drug + vitamin C and zinc (40:60). G. Not infected but administered vitamin C and zinc (50:50).

Fig.5. Mortality of mice throughout the course of the study. Key (Treatment): A. infected and treated with standard drug only; B. infected and treated with standard drug + vitamin C and zinc (50:50); C. infected and treated with standard drug + vitamin C and zinc (30:70); D. infected and treated with standard drug + vitamin C and zinc (60:40); E. infected and treated with standard drug + vitamin C and zinc (70:30); F. infected and treated with standard drug + vitamin C and zinc (40:60). G. Not infected but administered vitamin C and zinc (50:50).
3.4. Response of Plasmodium berghei-infected mice body weight to treatment with Artemether under vitamin C - zinc Supplement
Pre-inoculation with P. berghei, body weight of the mice were similar (p > 0.05). Marginal mean weights of mice in all groups except group G were similar at the end of three weeks. Marginal mean weight of mice in group G (no-infection control) was significantly higher compared to other groups (p < 0.0001). Marginal mean weight of all the groups infected with P. berghei treated with standard drug and provided vitamin C - zinc supplemented diets were not different from weight of mice treated with standard drug only (Fig.6 I). Weight of the mice only returned to pre-infection level three weeks post-infection and treatment (Fig.6 II). The increase in weight was significant (F = 45.404, p < 0.0001). There was no significant interaction between treatment and duration of observation in response of weight. Marginal mean weight one week after treatment was similar to pre-treatment weight. But from week 2 post-treatment, the rise was significant (p < 0.05; Fig.6 II).
4. Discussion
The study assessed the impact of combined vitamin C and zinc supplementation co-administered with artemether on haematological parameter, P. berghei parasitaemia, and mortality of infected mice. Vitamin C - zinc supplemented diet in addition to 3 days daily artemether treatment did not improve weight gain compared to 3-day sole treatment with artemether standard drug. PCV and HB was improved by the three days treatment course. RBC and WBC showed no supplement–dependent change for the general course of treatment. P. berghei parasitaemia was not affected by the coadministered vitamin C –zinc supplements. Mortality of mice was lower in all the groups fed the supplemented diets compared to the no-supplement control. Therefore, the supplement promoted survival of the mice, although the impact was not significant statistically. Overall, the 30:70 vitamin C - zinc (Group C) supplement performed better for all parameters evaluated except for weight gain.
Generally, malaria is associated with reduction in PCV, HB and RBC. This results from destruction of infected RBC and depletion of haemoglobin [31]. This contributes to the morbidity associated with the disease. WBC drops in response to demands of immune maintenance [32]. Malaria morbidity is also known to cause weight loss. Similar scenarios were noticed in this study. But these are not the main thrust of these study, and thus will not be discussed further.

Fig.6. Marginal mean body weight of mice in the experimental groups for before infection, before treatment and post-treatment. (I) Comparison of betweensubjects effects; (II) comparison of within-subject effects. Values as mean ± Standard error. Bars with different alphabet label were significantly different (p < 0.05). Key(Treatment): A. infected and treated with standard drug only; B. infected and treated with standard drug + vitamin C and zinc (50:50); C. infected and treated with standard drug + vitamin C and zinc (30:70); D. infected and treated with standard drug + vitamin C and zinc (60:40); E. infected and treated with standard drug + vitamin C and zinc (70:30); F. infected and treated with standard drug + vitamin C and zinc (40:60). G. Not infected but administered vitamin C and zinc (50:50).
There was generally, rise in PCV post-treatment in all infected groups. Marginal mean rise was higher in the supplemented groups compared to their no-supplement infected counterpart (Group A).The marginal increase was especially obvious at the 50:50 vitamin C - zinc supplementation. A similar significant increase in PCV from vitamin C - zinc supplement in Plasmodium vivax infected patient has been reported. Rahfiludin and Ginandjar [19] observed significant rise in PCV after a 45 d daily supplementation with 40 mg vitamin C and 10 mg zinc in patients infected with P. vivax. They also reported a significant increase in haemoglobin concentration(HB) resulting from the supplementation. Although in the present study, there was increase in HB as a result of the vitamin C - zinc supplement compared to the group that did not receive supplement, the difference was not significant (p < 0.05). Vitamin C and zinc both influence haemoglobin synthesis indirectly [33]. Vitamin C increases the absorption of iron from dietary sources [19].It may also improve red cell production by mobilizing stored iron[19,34]. Zinc influences heme synthesis through its effects on δaminolevulinic acid dehydratase (ALAD), an enzymes relevant in the synthetic pathway [35]. Therefore, vitamin C - zinc combined supplement may serve as alternative to direct iron supplement.
Vitamin C - zinc supplement did not affect P. berghei parasitaemia in this study. In a previous study, zinc only supplement had no effect on malaria morbidity among children in rural West Africa[36]. Zinc supplement did not reduce the median time to reduction of fever in preschool children [20]. However, Darling et al. [37]noticed a reduction in histopathology-positive placental malaria among pregnant women who received zinc supplement from first trimester of pregnancy compared to those who did not. From the same study, vitamin A did not have any additional protection [37].There are no reports available on the impact of combination of zinc and vitamin C on Plasmodium parasitaemia. But micronutrients are known to enhance immune response [38,39], and zinc is involved in the regulation of innate and adaptive immune responses [39].Though a study designed to evaluate changes in interferon-γ and interleukin 10 concentration as indicators of immuno-protective effect of a combined zinc and vitamin C supplement reported no immune enhancing activity [19]. Findings from this study supports the view that zinc - vitamin C supplement during malaria treatment did not affect clearance of parasitaemia. One weakness, however,is the inability to tell what would have happened if the mice were not treated with artemether drug, but administered vitamin C -zinc supplements. However, based on our study design, the supplements had no effect on B. berghei parasitaemia.
Mortality was highest in the groups that did not received the vitamin C – zinc supplement. This may suggest additional protection resulting from the supplements. Though the difference in mortality was not significant statistically. The lack of significant effect was probably due to the small sample sizes (nine mice per group) and low mortality (two maximum). But the observation is important and requires further study. The supplements may work by means not explored in this study to improve conditions of the infected and treated mice. Zinc for example is critical for sustaining proper immune response [39]. The micronutrients may have also worked by reducing oxidative stress from malaria and maintaining erythrocyte membrane integrity [40].
Conclusively, combination of the micronutrients vitamin C - zinc with standard antimalarial drugs has potential therapeutic benefits in the management of malaria. They may be of benefit as therapeutic options when compounded with existing antimalarial formulations. Vitamin C - zinc are compatible with the antimalarial artemether in the control of Plasmodium berghei in albino mice. The 30:70 vitamin C - zinc was the most suitable combination.
Conflict of interest
The authors declare no conflict of interest.
External funding
The study did not receive external funding.
- 食品科学与人类健康(英文)的其它文章
- Risk assessment of chemical substances of safety concern generated in processed meats
- Seaweed nutraceuticals and their therapeutic role in disease prevention
- Occurrence, properties and biological significance of pyroglutamyl peptides derived from different food sources
- Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants
- Antioxidant peptides encrypted in flaxseed proteome: An in silico assessment
- Hyperinsulinemia, cancer and maqui berry: The promise of nutritional supplementation

