Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants
Adel F. Ahmed, Fatma A.K. Attia, Zhenhua Liu, Changqin Li, Jinfeng Wei,∗,Wenyi Kang,∗
a National R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng 475004, China
b Medicinal and Aromatic Plants Researches Department, Horticulture Research Institute, Agricultural Research Center, Egypt
c Joint International Research Laboratory of Food & Medicine Resource Function, Henan Province, Kaifeng 475004, China
d Department of Ornamental, Medicinal and Aromatic Plants, Faculty of Agriculture, Assiut University, Egypt
Keywords:
A B S T R A C T
1. Introduction
Antioxidants have been widely used as food additives to avoid the degradation of the foods. Also, antioxidants have an important role in preventing a variety of lifestyle-related diseases and aging because these are closely related to the active oxygen and lipid peroxidation [1]. Current research of free radicals confirmed that food rich in antioxidants play an essential role in reducing the risk of incidence of cardiovascular diseases as well as other chronic diseases and certain types of cancer [2,3]. A large number of plant species have already been tested for potential antioxidant activity [4,5].Ocimum basilicum L., named commonly as sweet basil, is a popular culinary herb belonging to the Lamiaceae family is originally native to India and other Asian regions. Nowadays, it is cultivated all over the world [6].
Traditionally, the basil leaves are used in folk medicine as a remedy for a large number of diseases, including cancer, convulsion, diarrhea, epilepsy, gout, nausea, sore throat, toothaches,and bronchitis [7–9]. It is also a source of essential oil containing biologically-active constituents which possess antioxidant and antimicrobial properties [6–11].
The chemical composition of basil oil has been the subject of several studies. Basil oils have been classified into four chemotypes according to their chemical composition and geographical source. The European type, cultivated in Europe, USA, and Africa,is characterized by linalool and methyl chavicol as the major oil constituents. The Reunion type, located in the Comoros and Seychelles Islands, Africa, and Reunion Island, is characterized by a high concentration of methyl chavicol. Tropical type originated from India, Pakistan, Guatemala, Haiti, and Africa is rich in methyl cinnamate. Another basil chemotype, with eugenol as the main component, is common in North Africa, Russia, Eastern Europe, and parts of Asia [6,12–14]. In addition to these, other basil oils have also been reported which contained various quantities of linalool,camphor, methyl chavicol, methyl cinnamate, and eugenol [15].
Several studies have investigated the chemical composition and antioxidant activity of the essential oil of basil from different origins. However, there are few reports on the antioxidant properties of basil extracts. Therefore, the aim of this study was to evaluate the chemical composition and antioxidant activity of the essential oils and extracts of sweet basil obtained from three locations of Egypt.
2. Materials and methods
2.1. Plants material
Sweet basil samples (leaves and stems) were collected from 3 locations of Egypt, Assiut, Minia and BeniSuef governorates. The samples were collected during summer 2018 and identified by Dr. Atef A. Kader Department of Medicinal and Aromatic Plants Researches, Horticulture Research Institute, Agricultural Research Center. The current study was conducted during 2018 and 2019 years at National R & D Center for Edible Fungus Processing Technology, Henan University, kaifeng, Henan, China.
2.2. Essential oil isolation
Dried basil samples (100 g) were crushed and subjected to hydrodistillation with sterile water (1 L) for 3 h using a Clevengertype apparatus. The obtained essential oil was dried over anhydrous sodium sulphate, and stored at 4°C for further use.
2.3. Gas chromatography/mass spectrometry analysis of oils
GC–MS analysis was carried out by an Agilent 6890 N gas chromatograph equipped with a capillary column DB-5 ms (30 m × 250 μm × 0.25 μm, Agilent Technologies, USA and coupled with a 5975 B mass selective detector spectrometer from the same company. The front inlet was kept at 250°C in split mode. The temperature program was as below: the initial column temperature was 60°C, held for 2 min, and then programmed to 120°C at a rate of 6°C per minute and held for 2 min; finally programmed to 230°C at a rate of 4°C per minute, held at 5 min. Flow rate of split injection was 1.0 mL per minute. As a carrier gas helium at 1.0 mL per minute was used. The MS detector was used in the EI mode with an ionization voltage of 80 eV. The ion source temperature was at 230°C.The transfer line was at 280°C. The spectra were collected over the mass rang (m/z) 30-1000. Retention indices were calculated by the retention times of C6-C26n-alkanes that were injected at the same chromatographic conditions. The volatile constituents were identified by comparison of their relative retention indices and their mass spectra with Nist 08.L library of essential oil constituents.
2.4. Preparation of basil samples extracts
Dried samples of basil were crushed and extracted by 70%ethanol under 50°C three times for 9 h totally. The extracts werefiltered under Buchner funnel and concentrated under reduced pressure at 40°C by a rotary evaporator. The extracts kept at – 40°C overnight then dried in a freeze dryer then collected and stored at– 4°C until used for further analyses.
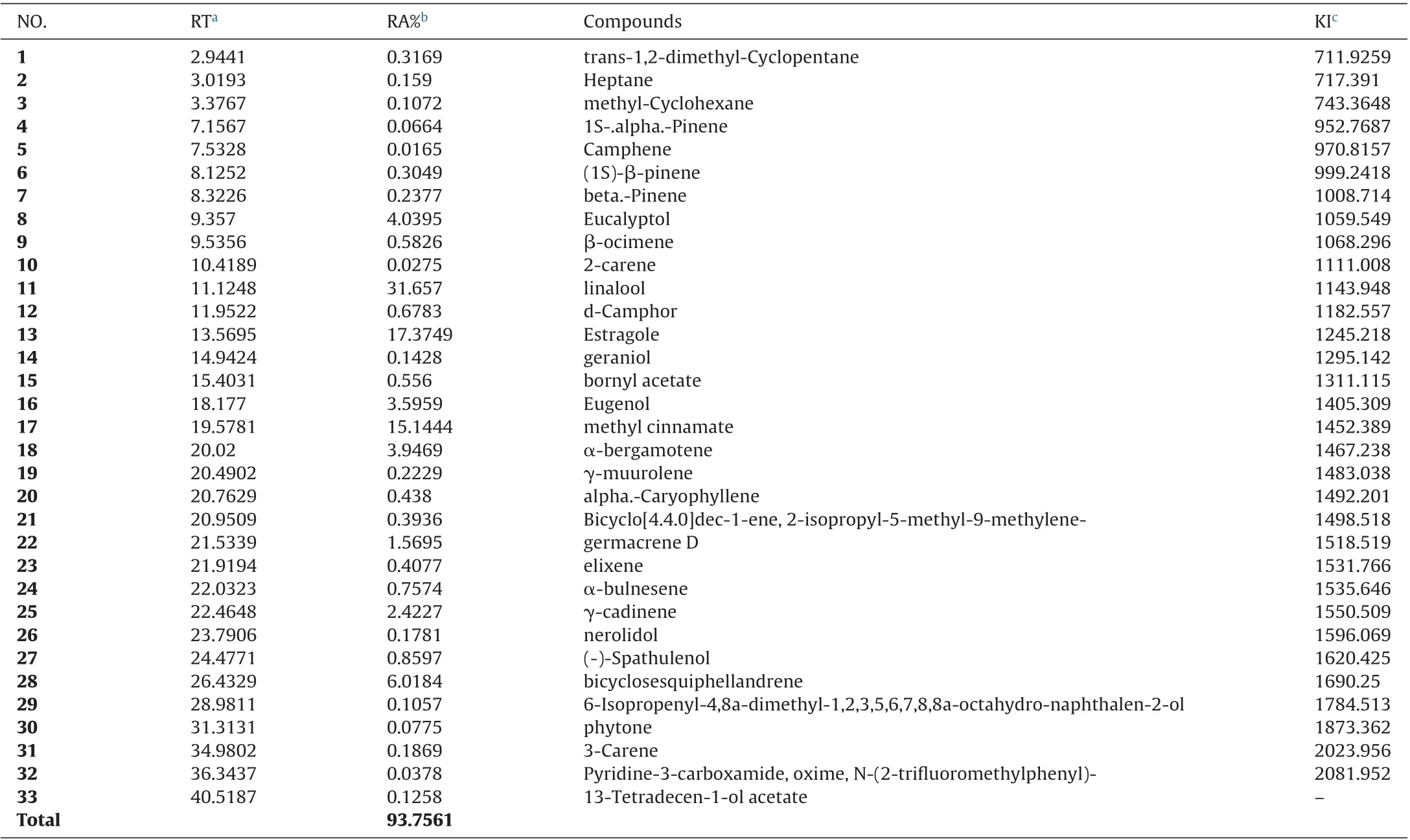
Table 1 Assiut basil essential oil composition (%), obtained by GC–MS.
2.5. DPPH radical scavenging assay
The method of Kang et al. [16] was used to assay the DPPH radical scavenging activity. The stable free radical DPPH was dissolved in methanol to give a 200 μmol/L solution; 10 μL of the essential oil and extract samples in methanol (or methanol itself as blank control) was added to 175 μL of the methanol DPPH solution. For each test compound, different concentrations were tested (20, 10,5, 2.5 and 1.25 mg/mL for extracts. 100, 50, 25, 12.5 and 6.25 mg/mL for oils). After further mixing, the decrease in absorbance was measured at 515 nm after 20 min. The actual decrease in absorption induced by the test compound was calculated by subtracting that of the control. The antioxidant activity of each test sample was expressed as an IC50value, i.e. the concentration in mg/ml that inhibits DPPH absorption by 50% and was calculated from the concentration-effect linear regression curve. BHT was used for positive control. The DPPH radical scavenging activity of each sample was calculated as the percentage inhibition.
% Inhibition of DPPH radical activity = [(A0− A1)/A0] × 100%
Where: A0is the absorbance of the DPPH itself; A1is the absorbance of sample and the positive control.
2.6. Determination of total phenolic contents (TPC)
The contents of total phenolic in basil samples extracts and oils were determined spectrophotometrically according to the Folin-Ciocalteau method of Kang et al. [17] with slight modification.The diluted extract and oil (1.25 and 5 mg/mL methanol), respectively were used in the analysis. A 0.2 mL aliquot of the diluted extract was mixed with 2.5 mL of 10% Folin-Ciocalteu’s reagent in water. The mixture was covered and incubated for 2 mints in dark place then 2 mL of 7.5% Na2CO3dissolved in water was added. The mixture was incubated for 1 h at room temperature. The absorbance was measured at 765 nm against blank. The blank had the same constituents except that the extract was replaced by distilled water.Pyrocatechol was used as standard for preparing the calibration curve. The total phenolic content was expressed as mg pyrocatechol equivalents (PE) per g of extract.
2.7. Statistical analysis
Data were analyzed statistically using Statistix Version 8.1 software. Differences between means were determined using the least significant difference test at P < 0.05. The data are presented as mean ± SD.
3. Results and discussion
3.1. Chemical composition of essential oils
The chemical compositions of the investigated essential oils of tested basil from three locations; Assiut, Minia and BeniSuef of Egypt are shown in Tables 1–3, respectively. GC–MS analysis of Assiut basil essential oil identified 33 constituents, representing 93.75% of the total oil. The major constituents of the essential oil were linalool (31.65%), estragole (17.37%), methyl cinnamate (15.14%) bicyclosesquiphellandrene (6.01%), eucalyptol (4.04%), α-bergamotene (3.94%), eugenol (3.59%), γ-cadinene(2.42%) and germacrene D (1.56%).31 constituents were identified from Minia basil essential oil, representing 93.20% of total oil.Linalool (28.18%), estragole (16.97%), methyl cinnamate (13.39%),eugenol (7.33%), bicyclosesquiphellandrene (6.83%), eucalyptol(4.73%), α-bergamotene (4.20%), γ-cadinene (2.64%) and germacrene D (2.41%) were the major constituents of the essential oil tested. Whereas, 34 constituents, representing 92.27% of total oil were identified for BeniSuef essential oil by GC–MS analysis and the major constituents were, linalool (27.64%),estragole (15.96%), methyl cinnamate (10.48%), bicyclosesquiphellandrene (7.01%), eucalyptol (5.48%), α-bergamotene (4.52%),γ-cadinene (3.26%), eugenol (2.78%), and germacrene D (2.37%).As a result, the composition of three basil essential oils was varied.

Table 2 Minia basil essential oil composition (%), obtained by GC–MS.

Table 3 BeniSuef basil essential oil composition (%), obtained by GC–MS.
The variation in chemical compositions of essential oils may be probably due to environmental conditions. Linalool and methyl chavicol and methyl cinnamate as the major oil constituents of essential oils. Our results in agreement to the previous results reported by Simon et al. [6] and Chenni et al. [18], linalool and methyl chavicol as the major oil compounds of essential oil from Egypt. However, Ismail [19] reported linalool, 1,8-cineole, eugenol,and methyl cinnamate as the major components of Egyptian basil essential oil.
A high diversity in the constituents of basil essential oil with different chemotypes from many regions of the world have been previously investigated; As reported by Nurzy´nska-Wierdak et al.[20], linalool, geraniol, and 1,8-cineole are the major compounds in basil leaf essential oil of Poland. These results are consistent with those obtained in previous works in Oman, Hanif et al [11] and Brazil, Oliveira et al. [21]. Marotti et al. [12] reported the presence of linalool, methyl chavicol, and eugenol as main components of Italian basil essential oil.
Klimankova et al. [22] reported linalool and eugenol are the major compounds of basil essential oil native to Czech Republic corroborating with Guinea, Keita et al. [23], as well as Reunion, Lucchesie et al. [24]. It was also reported that four major compounds characterize the basil essential oil of Austria: linalool, methyl chavicol, methyl cinnamate, and –cadinol, Politeo et al. [25]. These results are in agreement with those obtained in previous works in Bulgaria (Opalchenova et al. [26] and USA Lee et al. [27]. Linalool was reported as the main component in O. basilicum essential oil from Romania, Benedec et al [28].
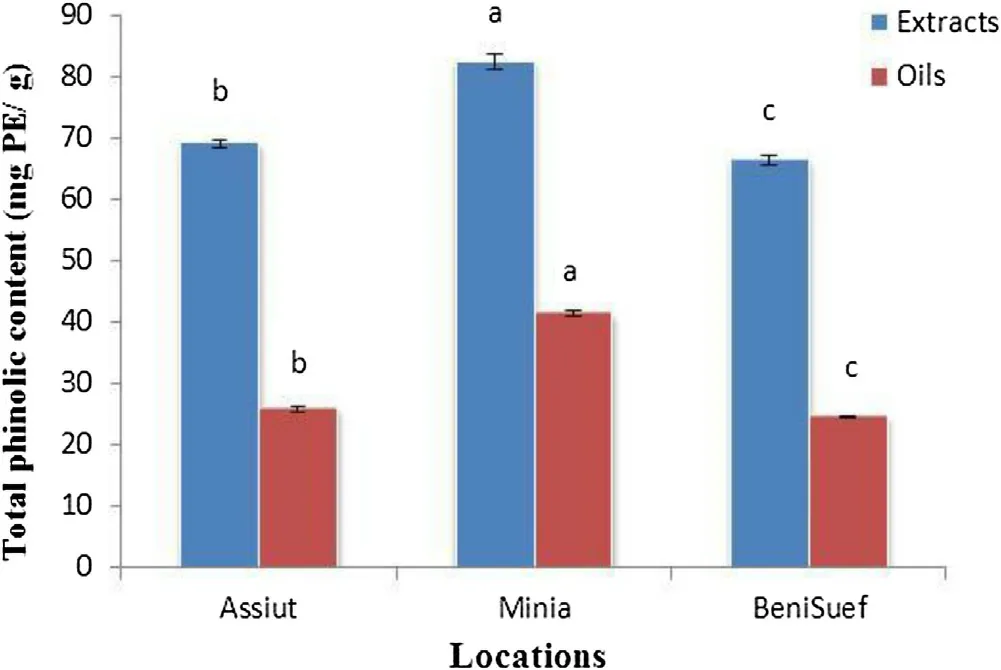
Fig.1. Total phenolic content in basil extracts and essential oils at different locations. Data are mean ± SD (n = 3). Columns in figure that are headed with the different letter are significantly different (P < 0.05) according to the least significant difference test.
The major compounds of essential oil extracted from Algeria leaves of basil, were linalool, linalyl acetate, elemol, and geranyl acetate Hadj-Khelifa et al. [29]. Regarding Pakistan basil, Hussain et al. [5] reported four major compounds were found: linalool,epi–cardinol, -bergamotene, and -cadinene. Concerning basil from Turkey, Chalchat et al. [30] cited three major compounds: methyl chavicol, limonene, and p-cymene. In addition, Iranian basil essential oil is rich in methyl chavicol, Shirazi et al. [31] as well as Thailand basil essential oil, Bunrathep et al. [32].
On the other hand, Purkayastha and Nath [33] reported that camphor, followed by limonene and –selinene were the major compounds in O. basilicum essential oil from Northeast India. However,methyl cinnamate was the first major compound from French Polynesia basil leaf essential oil, Adam et al. [34]. These variations in the constituents of basil essential oil between countries may be probably due to environmental conditions and genetic factors, different chemotypes, and the nutritional elements of the plants, as well as other factors that can influence the oil composition.
3.2. Total phenolic contents of essential oils and extracts
The total phenolic contents (TPC) for basil essential oils and extracts from different locations of Egypt are presented in Fig.1.The results indicated that the phenolic contents in different essential oils and extracts varied significantly. In among essential oils the highest TPC (41.3 mg PE/g) was obtained from Minia basil essential oil followed by 25.91and 24.61 mg PE/g for Assiut and BeniSuef basil essential oils, respectively. The TPC of extracts exhibited similar trends to TPC of essential oils. The highest TPC(82.45 mg PE/g) was obtained from Minia basil extract followed by 69.12 and 66.48 mg PE/g for Assiut and BeniSuef basil extracts,respectively. In general, basil extracts exhibited higher contents of phenolic than that of essential oils. This difference in the amount of TPC may be due to varied agroclimatic (climatical, seasonal, geographical) conditions of the locations.
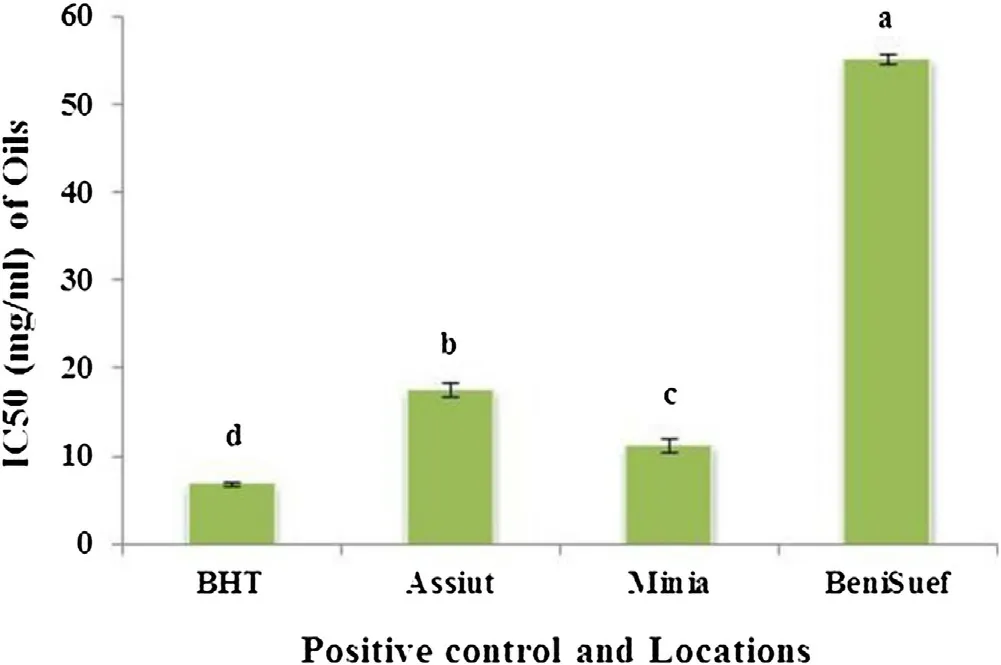
Fig.2. DPPH radical scavenging assay of basil essential oils at different locations.Data are mean ± SD (n = 3). Columns in figure that are headed with the different letter are significantly different (P < 0.05) according to the least significant difference test. BHT used as a positive control.

Table 4 The major constituents (%) in basil essential oils.
3.3. DPPH radical scavenging assay of essential oils and extracts
Free radical scavenging activities of the basil essential oils and extracts were measured by DPPH assay. Free radical scavenging capacity increased with increasing essential oil and extract concentrations. In Fig.2, basil essential oils from different locations exhibited good radical scavenging activity but lower than that of synthetic antioxidant BHT (IC50= 6.80 mg/mL). The highest radical scavenging activity was recorded by Minia essential oil with IC50value (11.23 mg/mL) followed by 17.52 and 55.15 mg/ml for Assiut and BeniSuef essential oils, respectively.
These variations were probably due to differences in environmental condition. Low correlation (R2= 0.441) was found between antioxidant activity data from DPPH assays (IC50) and total phenolic contents of basil essential oils from different locations (Fig.4). This result can be concluded that antioxidant activity of basil essential oils is not limited to phenolic. It may also come from the presence of other antioxidant secondary metabolites.
In this study the increment in radical scavenging activity of Minia essential oil was probably due to an increase of eugenol content which recorded higher concentration about 2 times and 3 times than that of Assiut and BeniSuef essential oils, respectively,as illustrated in Table 4. However eugenol was not the main component of oils. Our findings are in agreement with Politeo et al. [25]who clearly suggests that the antioxidant capacity (IC50= 1.4 g/L)of basil essential oil from Austria is due only or mainly to the presence of eugenol (5.9%) in its chemical composition and that other constituents do not have significant effect on eugenol capacity.
The same finding was reported by Pripdeevech et al. [35]who observed that the essential oil of O. basilicum from Thailand(linalool/eugenol chemotype) exhibited high scavenging ability of DPPH radicals (IC50= 26.53 g/mL). Also, Dabire et al. [36] reported that the decrease in the rate of eugenol in essential oil of O. basilicum causes a decrease of its antioxidant power. Lee et al. [27] also found that eugenol (0.896 mg/g) was the main contributor of the antioxidant activity of volatile extract of basil collected from USA than major components.

Fig.3. DPPH radical scavenging assay of basil extracts at different locations. Data are mean ± SD (n = 3). Columns in figure that are headed with the different letter are significantly different (P < 0.05) according to the least significant difference test.BHT used as a positive control.
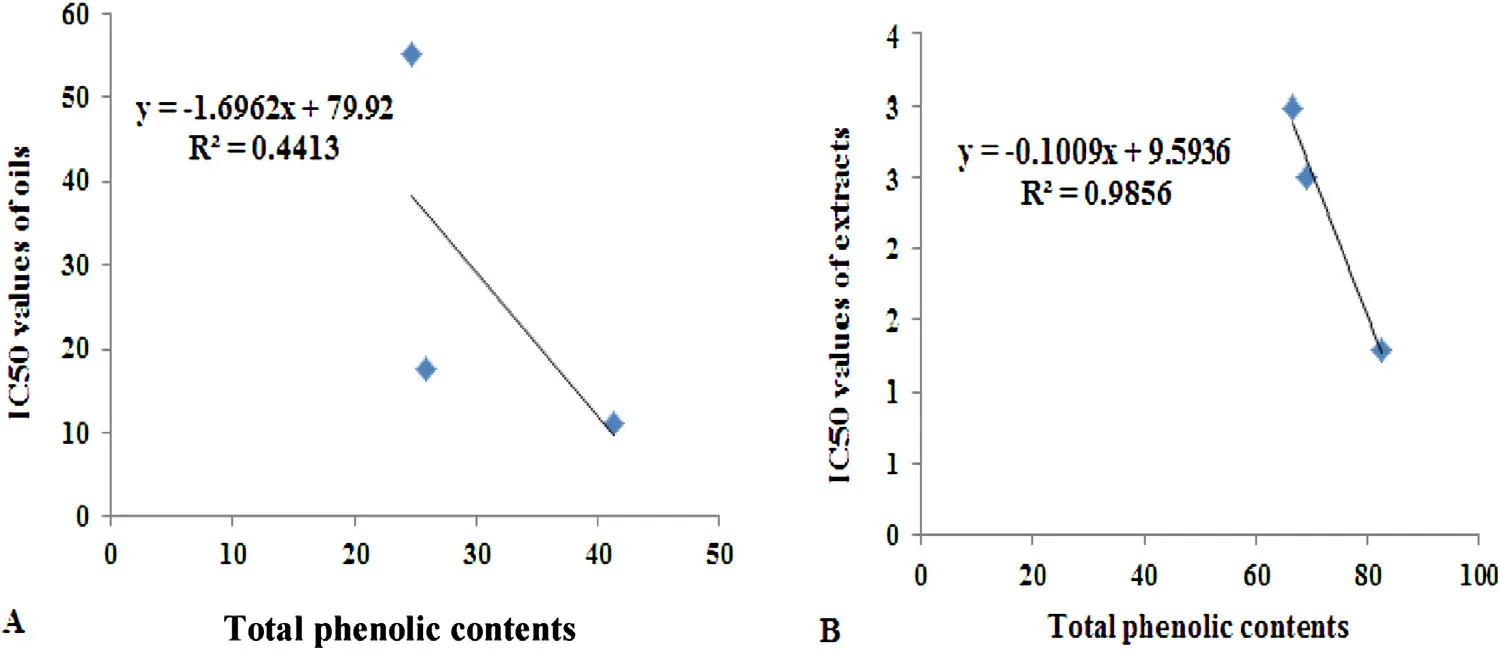
Fig.4. Linear correlation between the antioxidant activity data from DPPH assays (IC50) and the total phenolic content of basil from different locations.
The variation in the antioxidant activity of essential oil in different origins has been previously investigated by other researchers.Hadj-Khelifa et al. [29] who reported that O. basilicum essential oil from Algeria containing linalool as a major compound exhibited lower antioxidant effects (IC50= 83.4 mg/mL) than the vitamin E (IC50= 22.0 mg/mL). Hussain et al. [5] showed that sweet basil essential oil from Pakistan which is rich in linalool offered antioxidant activity comparable to synthetic antioxidant BHT. However,Bozin et al. [37] found that basil oil containing methyl chavicol(45.8%) and linalool (24.2%) as major components exhibited very strong radical scavenging activity, reducing the DPPH radical formation (IC50) in the range of 0.4 g/mL.
Quite a different situation was observed by Mahmoud et al. [38]who found that the main component methyl chavicol exhibit a moderate antioxidant activity (DPPH assay). Also, Dawidowicz et al.[39] which showed that the main component methyl chavicol, do not exhibit antioxidant properties.
Depending on our results and pervious researches we can conducted that the main component does not always determine the antioxidant activity of the examined essential oil. It is possible that components present at lower concentrations might be involved in some type of synergy with other active compounds.
On the other hand the ethanol extracts of basil from different locations exhibited stronger radical scavenging activity than that of synthetic antioxidant BHT (IC50= 6.80 mg/mL). As illustrated in Fig.3 Minia basil extract recorded the highest radical scavenging activity in among locations with IC50value (1.29 mg/mL) followed by 2.50 and 2.98 mg/ml for Assiut and BeniSuef extracts, respectively.
On contrary high correlation (R2= 0.985) between antioxidant activity data from DPPH assays (IC50) and total phenolic contents of basil extracts from different locations was observed(Fig.4). This result suggests that the antioxidant capacity of basil extracts results from the contribution of phenolic contents. Also indicates that the variation in radical scavenging activity was due to differences in phenolic content which recorded higher concentration in Minia basil extract than that of another two locations.
Our findings are in agreement with Javanmardi et al. [40] who reported that 71% of the antioxidant capacity of Iranian Ocimum accessions results from the contribution of phenolic compounds.In general, the free radical scavenging activity of ethanol extracts was superior to that of essential oils.
Phenolic compounds are very important plant constituents because of their scavenging ability on free radicals due to their hydroxyl groups. Therefore, the phenolic content of plants may contribute directly to their antioxidant action [41]. Several investigations of the antioxidant activity of plant extracts have confirmed a correlation between total phenolic content and antioxidant activity [42,43].
4. Conclusions
Our results showed that variations in chemical composition of essential oils of O. basilicum obtained from three locations; Assiut,Minia and BeniSuef of Egypt. The basil extracts contained appreciable levels of total phenolic contents and exhibited good DPPH radical scavenging capacity higher than that of essential oils. High correlation between antioxidant activity and total phenolic contents of basil extracts was observed. High variation in free radical scavenging activity of essential oils was found. On contrary, low correlation between antioxidant activity and total phenolic contents of basil essential oils from different locations. The results of the present investigation demonstrated significant variations in the antioxidant activities of sweet basil essential oils and extracts from Egypt and it should be addressed in future research.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgement
This work was supported by National cooperation project of Kaifeng City (1806004).
- 食品科学与人类健康(英文)的其它文章
- Risk assessment of chemical substances of safety concern generated in processed meats
- Seaweed nutraceuticals and their therapeutic role in disease prevention
- Occurrence, properties and biological significance of pyroglutamyl peptides derived from different food sources
- Antioxidant peptides encrypted in flaxseed proteome: An in silico assessment
- Hyperinsulinemia, cancer and maqui berry: The promise of nutritional supplementation
- Fermentation-enabled wellness foods: A fresh perspective

