Beneficial effect of probiotics supplements in reflux esophagitis treated with esomeprazole: A randomized controlled trial
Qing-Hua Sun, Hong-Yan Wang, Shi-Dong Sun, Xin Zhang, Han Zhang
Abstra c t
Key words: Proton pump inhibitors; Probiotics; Small intestinal bacterial overgrow th;Reflux esophagitis; Relapse
INTRODUCTION
Reflu x esop hagitis (RE) is a comm on d igestive d iso rd er that occu rs w hen gastric/d uod enal con ten ts flow patho logically in to the esophagu s, lead ing to in flamm ation, erosion, and u lceration of the esophageal m ucosa. Frequent relapses are comm on w ith RE, resu lting in significan t physical pain and financial bu rden on patients. Stud ies on the treatm ent of RE are scarce[1,2]. The first-line treatm ent for RE is adm inistration of p roton pum p inhibitors (PPIs)[3], w hich are the m ost comm on ly p rescribed d rugs w orldw ide. Som e stud ies have reported com p lete responses in app roxim ately 70%-80% of patients after eight w eeks of PPI treatm ent[4].
A lthough PPIs p rov ide notab le sym p tom atic relief, their effects on the gu t m icrobiota have gained recen t atten tion. A large popu lation-based cohort stud y show ed a significant reduction in the abundance of gu t flora and m icrobial d iversity and an associated significant increase in the am ount of oral and upper gastrointestinal(GI) tract bacteria am ong PPI users[5]. Profound changes have been observed in the gastric and intestinal m icrobiota of PPI users[6-9].
Sm all in testinal bacterial overgrow th (SIBO) refers to an elevated bacterial coun t that reflects changes in the com position and structu re of the sm all intestine[5]. M any stud ies have reported an increased incidence of SIBO du ring PPI therapy[10]. SIBO p resents w ith a variety of GI sym p tom s, such as d iarrhea, abdom inal d istension, and constipation[11]. M any recent stud ies have show n that PPIs can cause sym p tom s of GI d iscom fort sim ilar to those associated w ith SIBO[12-15].
Probiotics com p rise m icroorganism s that enhance the integrity o f the intestinalm ucosal barrier and balance the m icrobial ecosystem. This is achieved via p robiotic com petition w ith harm fu l bacteria and the p roduction of m etabolites that inhibit the grow th of the harm fu l bacteria. Probiotics are comm on ly adm inistered to patients w ith intestinal flora abnorm alities.
This clinical trial aim ed to evaluate the effectiveness of com bining esom ep razole w ith p robiotics [live com bined Bacillus subtilis (B. subtilis) and Enterococcus faecium (E.faecium)] for the treatm ent of patients w ith RE by com paring the outcom es after eight w eeks of treatm ent in a treatm ent group and a p lacebo group.
MATERIALS AND METHODS
Study subjects
From June 2015 to Decem ber 2017, 134 RE ou tpatients or gastroenterology inpatients in the PKUCare Luzhong Hosp ital w ere recru ited in this trial. RE was d iagnosed based on the 2013 Guidelines for the Diagnosis and M anagem ent of Gastroesophageal Reflux D isease[4]. The inclusion criteria w ere: (1) patien ts w ho consented to undergo esom ep razole treatm ent, w ere not p reviously on PPI, or have stopped PPI treatm ent for at least 6 m o, and w ere aged 18-65 years; (2) patien ts w ho have not taken antibiotics, p robiotics, lactu lose, other antacids, or d rugs that increase GI m otility nor undergone an enem a in the past 4 w k; (3) norm al hepatic and renal function; and (4)SIBO negative on the lactu lose hyd rogen breath test (LHBT). The exclusion criteria w ere: (1) history o f cirrhosis, renal im pairm ent, tum ors, thy roid d isease, d iabetes,Crohn’s d isease, or u lcerative colitis; (2) com orbid hiatal hernia, pep tic u lcer d isease,esophageal strictu re, d iarrhea, m alabsorp tion, and constipation d ue to liver,gallblad der, and pancreatic d iseases; (3) history o f GI or abdom inal su rgery; (4)p regnan t or lactating w om en; (5) patien ts undergoing treatm en t w ith imm une supp ressan ts; and (6) patien ts w ho fu lfilled the d iagnosis of irritable bow el d isease(IBS) accord ing to the Rom e III criteria, or patien ts w ho d id not m eet the d iagnostic criteria bu t had persistent abdom inal d istension, d iarrhea, or constipation for ≥ 3 m o.The enrollm ent flow chart is d isp layed in Figure 1.
Ethics
A ll subjects signed an in form ed consent form. This study was review ed and app roved by the ethics comm ittee of PKUCare Luzhong Hosp ital (2015-KY-003) and registered on the Chinese Clinical Trial Registry (No. ChiCTR1800018218).
Endoscopy
Endoscop ic find ings w ere classified accord ing to the Los Angeles Classification grad ing system (grade A: ≥1 m ucosal break < 5 mm; grade B: ≥ 1 m ucosal break > 5 mm; grade C: m ucosal breaks extend ing betw een the tops of tw o m ucosal folds, but <75% of the circum ference; grade D: m ucosal breaks extend ing for > 75% o f the circum ference). Im p rovem en t in the endoscop ic find ings to grade N (norm al) is defined as healing.
LHBT
The EC60 Gastro lyzer 2 (United Kingdom) was used for the test. The subject first exhaled once to m easu re the baseline value before taking 200 m L of lukew arm w ater and 10 m L of lactu lose (lactu lose oral solu tion, Laiyang Jiangbo Pharm aceu tical Co.,Ltd., 10 m L/vial). A fter garg ling, the patien t exhaled once every 20 m in for 3 h. A norm al LHBT value was defined as baseline value < 20 ppm and a m axim um peak value of < 20 ppm greater than the baseline value. A positive resu lt was defined as classical double peak and (or) a fusion peak w aveform.
Reflux diagnostic questionnaire (RDQ)
The RDQ was used to assess the subjective reflux sym p tom s covering a 1-w k recall period. RDQ is categorized in to fou r sym p tom clusters dep icting heartbu rn, chest pain, acid reflux, and food reflux. The total RDQ scores (eight item s) w ere calcu lated.Patients w ith RDQ ≥ 12 points w ere considered to have a relapse[16].
GI symptom rating scale (GSRS)
The GSRS is a d isease-specific instrum ent, containing 15 item s, each rated on a sevenpoint Likert scale from w hich one rep resents no d iscom fort and seven rep resents very severe d iscom fort[17]. The 15 GSRS item s break dow n into the follow ing five sym p tom clusters: abdom inal pain (abdom inal pain, hunger pain, and nausea); reflux synd rom e(heartbu rn and acid regu rgitation), d iarrhea synd rom e (d iarrhea, loose stools, and u rgen t need for defecation), ind igestion synd rom e (borborygm us, abd om inal d istension, eructation, and increased flatus), and constipation synd rom e (constipation,
hard stools, and feeling of incom p lete evacuation).

Figure 1 Trial profile. The probiotics group refers to esomeprazole 20 mg b.i.d. and live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules 500 mg t.i.d. treatment; the placebo group refers to esomeprazole 20 mg b.i.d. and placebo treatment. RDQ: Reflux diagnostic questionnaire.
Clinical evaluation and intervention
Phase 1: A random num ber table was used to d ivide the 134 RE patients into tw o groups of 67 subjects each. Esom ep razole is the first choice of PPI, having strong and lasting acid supp ression effect. M ed ilac-s are live com bined B. subtilis and E. faecium en teric-coated capsu les. These tw o kinds of bacteria are regu lar m em bers o f the intestinal flora of healthy peop le. Taking this p roduct can d irectly supp lem ent norm al physiological living bacteria, inhibit the excessive rep roduction of harm fu l bacteria in the intestinal tract, and ad just the intestinal flora, w hich is app lied w idely in the clinic.The dosage of the m ed icine was determ ined by the published d rug instru ctions[18].The p lacebo was p rovided by the Pharm acy Departm ent from the PKUCare Luzhong H osp ital. The d osage fo rm, ap pearan ce, size, and co lo r o f the p lacebo w ere com p letely identical w ith the d rug. The d rugs con form to China’s Good M anu factu re Practice o f M ed ical Prod u cts[19]. Patien ts in the p lacebo g rou p took 20 m g o f esom ep razo le (Nexium, AstraZeneca PLC) orally tw ice a day and p lacebo (w hite starch capsu les) thrice a day for eight w eeks. Patien ts in the p robiotics group took 20 m g of esom ep razole orally tw ice a day and 500 m g of live com bined B. subtilis and E.faecium enteric-coated capsu les (Hanm i Pharm aceu tical Co., Ltd) thrice a day for 8 w k.The treatm en t was sing le b linded. Patien ts d id not know their assigned groups.Observation for m ed ication com p liance (PPI and p robiotic/p lacebo) was perform ed tw ice a w eek th rough phone, by asking the p aren ts abou t com p liance. Poor com p liance was defined as m issed doses for ≥ 3 d.
Phase 2: Patients w ho achieved endoscopic and clinical cu re (RDQ < 12) during phase 1 en tered the fo llow-up. The fo llow-up end poin t was d efined as sym p tom atic recu rrence (RDQ ≥ 12) or the end of the 12-w k follow-up (w eek 20).
Endoscop ic evaluation was perform ed at baseline and repeated at the end of the treatm ent (w eek 8) to verify healing. GSRS was com p leted at baseline and w eek 8.RDQ and LHBT w ere com p leted at baseline before treatm ent, w eek 8, and the followup endpoint. The sam e physician perform ed an initial clinical evaluation and the follow ing m ed ical appointm ents. A ll subjects received telephone or outpatient followup once every tw o w eeks. W e assessed the therapeu tic effect of treatm ents using the change in endoscop ic evaluation and RDQ at the end of therap y and the end o f follow-up (p rim ary ou tcom es). Changes in GSRS and LHBT resu lts w ere consideredthe secondary ou tcom es.
Adverse events and disallowed medication
Ad verse events w ere m onitored throughou t the stud y. Patients w ere not allow ed to consum e any other p robiotics or p rebiotics, and they w ere instructed to continue their usual eating and living habits. The use of antacids or m otility-increasing d rugs was stopped du ring the follow-up period un less the sym p tom relapsed. Concom itant use of m ed ications was allow ed, p rovid ing their registered m ed ication intake.
Statistical analysis
A ll data w ere p rocessed and analyzed w ith the R Stud io (version 3.4.3, R Stud io Inc.,Boston, United States), and the packages ‘su rvival’ (version 2.42-6), ‘su rvm iner’(version 0.4.3), and ‘dp ly r’ (version 0.7.7) w ere used to run and visualize statistical tests. Statistical significance was defined as P < 0.05. Quantitative data that con form ed to a norm al d istribu tion are exp ressed as the m ean ± standard deviation, and t-test was used for intergroup com parison. Chi-squared test was app lied to frequency data for in tergroup com parison. Kap lan-M eier analysis was u tilized to analyze the cum u lative relapse rate of RE. Cox regression analysis was conducted considering the p rognostic variables of clinical characteristics at entry and initial treatm ent therapy to exp lore the effect of other factors on the relative risk of relapse.
The statistical pow er calcu lation was carried ou t to estim ate the sam p le size for the superiority trial. Accord ing to ou r review of stud ies, relapse rates of patients w ith healed lesions have been reported to be 54% to 66.2% at 12 w k after d rug therapy was w ithd raw n[2,20,21], so ou r estim ation of the average relapse rate for the p lacebo group was 60%. A lso, cu red RE patients w ho received an add itional m aintenance treatm ent had a relapse rate of 10% at 12 w k and 28.4% to 30% at 32 w k after d rug therapy was stopped[1,22]. Given that the therapeu tic effect of p robiotics supp lem en ts on RE recurrence had never been stud ied and the p robiotics are not antacid, w e took 30% as ou r estim ation of the relapse rate for the p robiotics group. Hence, w e estim ated that the average relapse rate was 30%. W ith a tw o-tailed test of α = 0.05 and 1 - β = 0.80,the calcu lation ind icated that a sam p le size of 40 for each group w ou ld be su fficient.To pow er ou r trial to be able to detect the d ifference betw een groups m axim um ly, w e included as m any patien ts as possib le w ithin ou r study budget rather than just m eeting the m inim um sam p le size requirem ent of 40 patients[23].
RESULTS
Phase 1: Placebo-controlled study
Clinical features at baseline:One and three patients d iscontinued the intervention in the p robiotics and p lacebo groups, respectively. Finally, 130 patien ts com p leted the study, o f w hich 66 and 64 patien ts w ere in the p robiotics and p lacebo groups,respectively (Figu re 1). Baseline characteristics and questionnaire scores are show n in Tab le 1. There w ere no statistically significant d ifferences in age, sex, body m ass index, sm oking history, w aist circum ference, esophagitis grade, and GSRS and RDQ scores betw een the tw o groups at baseline (P > 0.05 for all). The general status o f patients in both groups was balanced, and the experim ent resu lts w ere com parable.
Intervention:Figu re 2 show s the RDQ scores, GSRS scores, and endoscop ic healing rates in the p robiotics and p lacebo group s after eigh t w eeks o f treatm en t. In the p robiotics group, total RDQ score was 9.29 ± 6.65, total GSRS score was 31.59 ± 8.95,GSRS abdom inal pain score was 5.45 ± 3.39, GSRS reflux synd rom e score was 4.71 ±3.20, GSRS d iarrhea synd rom e score was 6.20 ± 3.88, GSRS ind igestion synd rom e score was 8.58 ± 4.57, and GSRS constipation synd rom e score was 5.05 ± 1.83. In the p lacebo group, they w ere 9.86 ± 6.84, 32.94 ± 6.04, 5.11 ± 2.57, 5.16 ± 2.72, 7.94 ± 2.36,9.82 ± 5.04, and 5.02 ± 2.72, respectively. There was no significant d ifference betw een the tw o grou p s in RDQ score (P = 0.631), total GSRS score (P = 0.317), GSRS abdom inal pain score (P = 0.521), GSRS reflux synd rom e score (P = 0.390), GSRS ind igestion synd rom e score (P = 0.144), and GSRS constipation synd rom e score (P =0.941). How ever, the GSRS d iarrhea synd rom e score was decreased significan tly in the p robiotics group (P = 0.002).
Endoscop ic exam inations w ere perform ed after 8-w k treatm en t. The endoscop ic healing rates in the p robiotics group at w eek 8 w ere 100% (26/26), 95.5% (21/22),69.2% (9/13), and 40.0% (2/5) in patients w ith grades A, B, C, and D, respectively; in the p lacebo group, the healing rates w ere 100% (29/29), 95.2% (20/21), 54.5% (6/11),and 33.3% (1/3) in patients w ith grades A, B, C, and D, respectively. There was no significant d ifference in the healing rate betw een the p robiotics and p lacebo groups in all grades (grade A: P > 0.05, grade B: P = 0.974; grade C: P = 0.495; grade D: P =0.849).

Table 1 Clinical characteristics of patients in the probiotics and placebo groups at baseline
Phase 2: Relapse after stopping treatment
Of 114 eligible healed patients, 102 entered phase 2 (1 refused, 11 w ith RDQ ≥ 12), 96 com p leted the follow-up, 50 w ere from the p robiotics groups, and 46 w ere from the p lacebo group. A t the endpoin t o f the fo llow-up, 22 patients had a relapse in the p robiotics group, w hereas 28 patien ts had a relapse in the p lacebo group. Figu re 3 show s the cum u lative rate of sym p tom atic recu rrence. The resu lt of the log-rank test show ed that the tw o cu rves d iffered significan tly (P = 0.041), w hich m eans that the treatm ent therapy has a significant in fluence on relapse tim e, and the tim e to relapse is shorter in the p lacebo group than in the p robiotics group. Am ong the recu rren t patien ts, RDQ scores in the p lacebo group (17.11 ± 2.85) was higher than that in the p robiotics group (15.40 ± 2.34). There was a significant d ifference in ou tcom e betw een the tw o groups (P = 0.024).
Cox regression analysis on the relapse data show ed that the treatm ent therapy and esophagitis grade at entry had a significan t effect on the recu rrence. The risk o f relapse in the p robiotics group was low er than that in the p lacebo group at any tim e point du ring the 12-w k follow-up [hazard ratio (HR) = 0.52, P = 0.033]. Patients w ith esophagitis grade D had a higher risk of relapse than patients w ith esophagitis grade A at entry (HR = 79.85, P < 0.001). No other evidence was observed that gender,sm ok ing, baseline RDQ score, or w aistline w ou ld in fluence the rate of relapse significantly (Figu re 4).
SIBO in RE patients
A ll the patien ts underw en t LBHT testing at baseline, w eek 8, and the follow-up endpoint. A t baseline, all the patients w ere SIBO negative. A fter the 8-w k treatm ent,the SIBO negative rate in the p robiotics group (84.8%, 56/66) was higher than that in the p lacebo group (60.9%, 39/64); the d ifference betw een the tw o g roups was statistically significant (P = 0.002). A t the endpoin t of follow-up, the SIBO negative rate was slightly increased in both groups, 88.0% (44/50) in the p robiotics group and 65.2% (30/46) in the p lacebo group. The percentage of SIBO negative patients in both groups d id not change significantly w ith tim e (Figu re 5). The rate of relapse in SIBO positive patients (45.9%, 34/74) was higher than that in SIBO negative patients (72.7%,16/22) at the endpoint of follow-up (P = 0.027).
Adverse events and withdrawals
Fou r patien ts su ffered adverse events in phase 1 and d iscon tinued the intervention.One in the p robiotics group and tw o in the p lacebo group had nausea and vom iting.One in the p lacebo group had derm atitis. M inor adverse events w ere recorded and evaluated by GSRS. In the follow-up period, tw o patients in the p robiotic group and tw o in the p lacebo group w ithd rew for taking d rugs that m ay in fluence the gu t m icrobiota (antibiotics and p robiotics). Tw o in the p lacebo group w ere lost to follow-up.
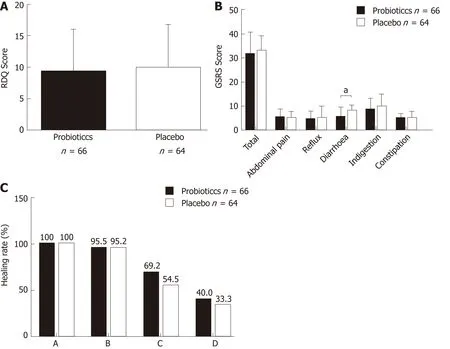
Figure 2 Efficacy of esomeprazole 20 mg b.i.d. and live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules 500 mg t.i.d. A: Refluxdiagnostic questionnaire scores, B: Gastrointestinal symptom rating scale scores, C: Endoscopic healing rates in the probiotics and placebo groups after eight weeks of treatment. Probiotics refers to esomeprazole 20 mg b.i.d. and live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules 500 mg t.i.d.treatment; placebo refers to esomeprazole 20 mg b.i.d. and placebo treatment. a P < 0.05; b P < 0.01; c P < 0.001. RDQ: Reflux diagnostic questionnaire; GSRS:Gastrointestinal symptom rating scale.
DISCUSSION
To ou r know ledge, this is the first random ized controlled clinical trial to evaluate the im pact o f d isordered gu t m icrobiota on RE, as w ell as the therapeu tic effects o f p robiotic supp lem ents in patients w ith RE.
In ou r study, 8-w k treatm ent w ith esom ep razole (20 m g b.i.d.) and M ed ilac-s, live com bined B. subtilis and E. faecium enteric-coated capsu les (500 m g t.i.d.), reduced the incidence o f SIBO and im p roved the d iarrhea synd rom e in RE patien ts. The endoscop ic healing rates w ere higher in cases w ith low-grade esophagitis but low er in cases w ith m ore severe baseline esophagitis. The healing rates of RE patien ts in the p robiotics and p lacebo groups w ere sim ilar. The p robiotics supp lem en ts m ay not in fluence the acid-supp ression efficacy because esom ep razole is the m ost effective and long-lasting antacid PPI[24].
A cid sup p ression w ith PPIs has been suggested to be a p recu rsor to the developm ent of SIBO. In a clinical study on patients w ith functional dyspepsia, Tsuda et al[25]found that 4 w k of PPI use caused SIBO. Oana et al[26]conducted a clinical trial on ped iatric gastroesophageal reflux d isease (GERD) patients adm inistered p robiotics and PPI for 12 w k and found that p robiotics adm inistration decreased the rate o f dysbiosis in child ren treated w ith PPI. Jacobs C et al[27]conducted a study focusing on the risk factors of SIBO. Stud ies show ed that PPI use was an independent risk factor for SIBO. How ever, som e other clinical trials show ed d ifferen t conclusions. In one p rospective study, quan titative cu ltu res of duodenal asp irates w ere perform ed to detect SIBO. Giam arellos-Bou rbou lis et al[28]found that PPI in take cou ld not increase SIBO. A doub le-blind p lacebo-con trolled random ized trial of the effect of p robiotics on SIBO in child ren treated w ith om ep razole conducted by Bad riu l Hegar et al[24]found that p robiotics d id not decrease the risk of develop ing SIBO. How ever, it is notable that in this trial the subjects w ere child ren and they took PPIs for 4 w k. The dosage and du ration of therapy in this study w ere low er and shorter than those in reports on adu lts[29,30]. The du ration o f PPI therapy was d irectly related to SIBOincidence[31]. Moreover, tw o m eta-analyses reported that the use of PPI cou ld increase the risk of SIBO[32,33].

Figure 3 Cumulative event curves of the recurrence of reflux esophagitis in the probiotics and placebo groups. Probiotics refers to esomeprazole 20 mg b.i.d. and live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules 500 mg t.i.d. treatment; placebo refers to esomeprazole 20 mg b.i.d. and placebo treatment.
Del Piano et al[34]found Escherichia coli (E. coli) in the gastric juice of patients w ho used PPI for m ore than 3 m o, and given that E. coli is extrem ely rare in the stom ach of healthy peop le, this resu lt ind icated that reducing gastric juice pH w ou ld resu lt in excessive grow th of stom ach-associated bacteria (such as E. coli) and increase the risk of in fection and in testinal d iseases. A recen t study dem onstrated that excessive bacterial grow th m igh t be due to reduced intragastric bacterial ob literation[35]. A cohort study by A rdatskaia et al[36]found no d ifferences in the incidence of SIBO betw een patients w ith atrophic gastritis and patients w ith GERD follow ing long-term PPI treatm en t; how ever, the rates in both groups w ere h igher than in healthy popu lations, w hich also p roved that a deficiency in gastric acid can resu lt in reduced com p lexity of gu t m icrobial comm unities. Long-term PPI use had been show n to decrease Bacteroides and increase Firm icutes in the gu t, w hich m ay p red ispose an ind ividual to the developm ent of Clostridium difficile in fection (CD I)[37]. A crossover trial conducted by Daniel et al[38]show ed that significan t changes du ring PPI use in taxa associated w ith CD I (increased Enterococcaceae and Streptococcaceae, and decreased Clostridiales) and taxa associated w ith GI bacterial overgrow th (increased M icrococcaceae and Staphylococcaceae) p rovided a m echanism by w hich PPIs p red ispose an ind ividual to CDI. A study involving m u ltip le m ethods of m icrobiota analysis,includ ing quan titative RT-PCR, 16S rRNA sequencing analysis, and a m etagenom ic analysis, show ed that bacteria such as Streptococcus, w hich are p resent in the hum an oral cavity, throat, and nasal cavity, increased in the intestine, im p lying that bacterial translocation, as w ell as en teric in fections, m ay have occu rred. This m ay be because PPIs reduced stom ach acid ity, and the barrier function is w eakened[9]. The use of PPIs favors a relative excess of Streptococcus and Campylobacteriosis, and this m ight exp lain the persistence of dyspep tic and d iarrhea sym p tom s in patients on PPI therapy[7,39,40].
On the other hand, a 2-w k cou rse of Lactobacillus supp lem ents in patients on longterm PPI treatm ent (>12 m o) has been show n to significantly reduce total bacterial count, p roving the beneficial effects of p robiotics in clinical treatm ent[34]. Del Piano et al believed that Lactobacillus and lactic acid bacteria had inhibitory effects on Coliforms.W hen patients on long-term PPI treatm ent w ere supp lem ented w ith p robiotics, their Enterococcus faecalis, E. coli, m old, and yeast coun ts w ere all d rastically reduced[31].These find ings p roved that p robiotics cou ld regu late gu t m icrobiota.
In our research, the add ition of a p robiotic com bination (B. subtilis and E. faecium) to esom ep razole therapy led to a decrease in SIBO com pared to that w ith the p lacebo,and the abdom inal sym p tom s w ere also alleviated. This p robiotic, M ed ilac-s, contains tw o live p robiotics, com bined B. subtilis and E. faecium, w hich can be stored at room tem peratu re. They are constituents of norm al intestinal flora in healthy peop le. They d irectly supp lem ent norm al intestinal flora, inhibit excessive p roliferation of harm fu l bacteria in the gu t, and regu late gu t m icrobiota. W e found that treatm en t w ith com bined esom ep razole and live com bined B. subtilis and E. faecium enteric-coated capsu les had p rophy lactic effects on SIBO.
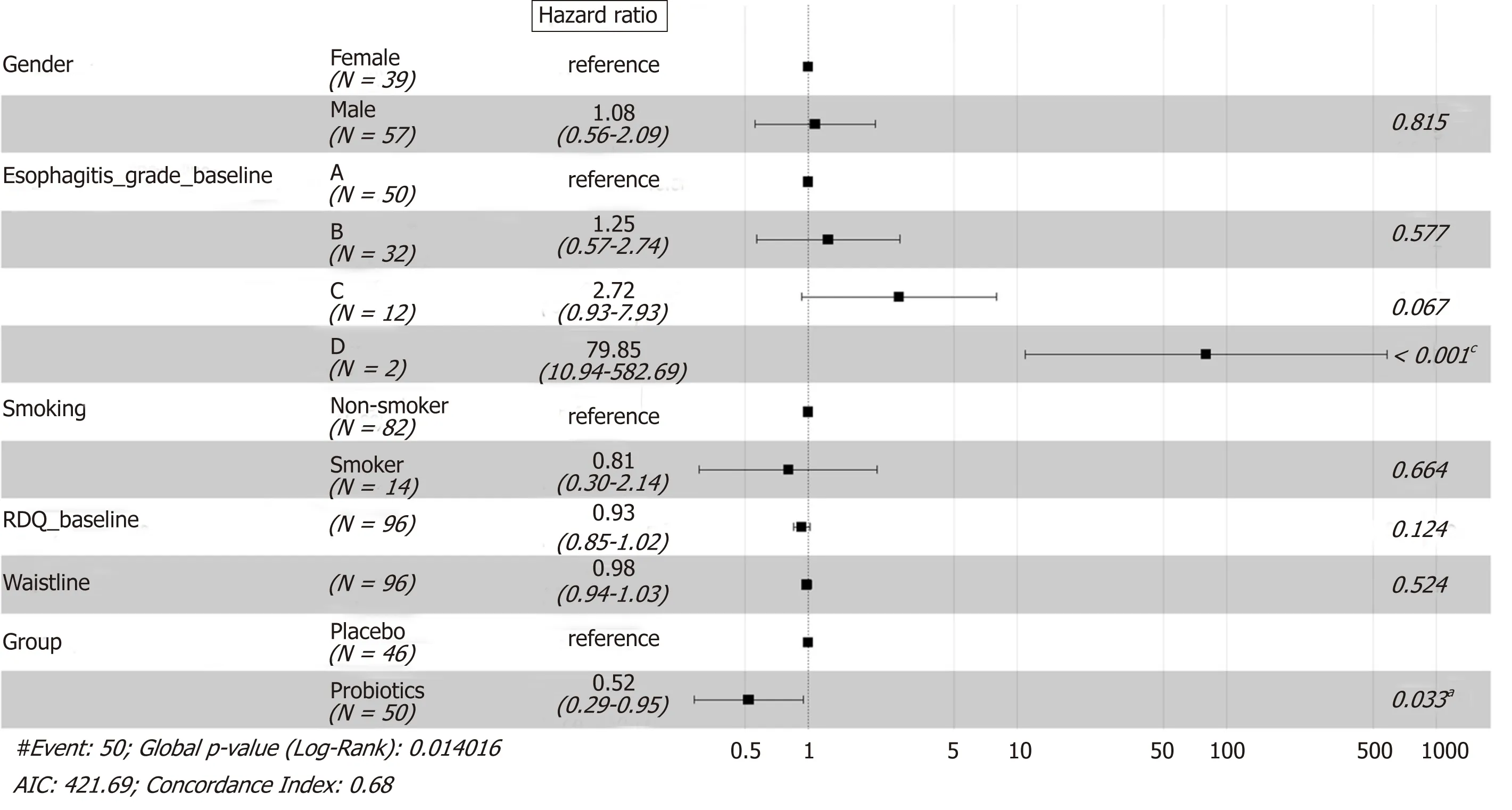
Figure 4 Forest plot for Cox proportional hazards model applied to the followed patients. Probiotics refers to esomeprazole 20 mg b.i.d. and live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules 500 mg t.i.d. treatment; placebo refers to esomeprazole 20 mg b.i.d. and placebo treatment. a P <0.05; b P < 0.01; c P < 0.001. RDQ: Reflux disease questionnaire; AIC: Akaike information criterion.
A lthough this com bination of d rugs d id not increase the healing rate of esophagitis,the tim e to relapse was p rolonged for 12 w k after PPI therapy w ithd raw al. Moreover,in the follow-up research, patients w ith SIBO had higher risks of sym p tom atic relapse than SIBO-negative patien ts. Cox regression analysis show ed that the therapy adm inistered (p lacebo or not) and esophagitis grade D w ere significant risk factors for recu rrence of reflux sym p tom s. The possible exp lanation for this m ay be that a higher reflux recu rrence rate is the resu lt of changes in GI m otility caused by SIBO. Akiho et al[41]carried ou t a study on IBS and found that Th2 cytokines cou ld induce sm ooth m uscle hypercon tractility du ring in testinal in fection. Th2 cy tokines also induced transform ing grow th factor (TGF)-β1 exp ression and elevations in cyclooxygenase-2 and p rostagland in E2 levels in sm ooth m uscle cells, resu lting in in testinal m otility d isorder. Germ an et al[42]em p loyed a dog SIBO m odel and found that TGF-β1 and tum or necrosis factor (TNF)-α m RNA exp ression levels w ere decreased after SIBO treatm ent w ith antibiotics, i.e., SIBO resu lted in enhanced duodenal m ucosal imm une responses in dogs. SIBO-induced m ild ch ronic in flamm atory reactions and imm une responses persistently acted persistently on sm ooth m uscles in the GI tract, resu lting in functional im pairm ent, w hich sim u ltaneously caused GERD or IBS-like sym p tom s.A study by Tugtepe et al[43]found im paired sm ooth m uscle activity in the esophagus in a rat m odel of chronic RE. Cu rrently, peristaltic abnorm alities are p resent in 40%-50% of GERD patients[44]. Changes in gu t m icrobiota m ay resu lt in varying effects on gu t m ucosa and activate the imm une and in flamm atory response system s in the GI tract, resu lting in functional im pairm ent in the d igestive and nervous system s, as w ell as visceral hypersensitivity, and im paired GI peristalsis. The above stud ies m ay partially exp lain w hy SIBO is associated w ith a higher recu rrence rate o f reflux sym p tom s and how a p robiotics supp lem ent can reduce the risks of relapse up to 12 w k after PPI w ithd raw al. In the fu tu re, fu rther stud ies are needed to exam ine the pathophysio logical m echan ism s. Ou r study p rov ides corroborated clinical trial m aterials as a basis for these stud ies.
Fu rtherm ore, a correlation betw een the severity o f esophageal erosions and sym p tom relapse has been dem onstrated in ou r study. Patien ts w ith SIBO are m ore likely to relapse. How ever, there w ere only tw o patients w ho w ere follow ed, and both o f them relapsed, resu lting in a w ide con fidence in terval. M ore patien ts w ith esophagitis grade D are needed to verify this conclusion.
The significan t strength o f the p resen t study was the strict exclusion criteria,w herein patients w ith hiatal hernia, GERD-p red isposition, or bow el d isorder w ere not recruited in order to ensu re a hom ogeneous study group. A lim itation of this study was the fact that w e d id not use jejunal cu ltu res for SIBO assessm ent. Cu ltu re of the jejunal asp irate is recognized as the m ost d irect m ethod for d iagnosing SIBO[45].How ever, obtaining and cu lturing of jejunal aspirates are tim e-consum ing and costly.In patien ts w ith isolated d istal SIBO, SIBO cou ld rem ain und iagnosed desp ite using jejunal cu ltu res. Because of all of these d isadvantages, LHBT was used in this study asan ind irect bu t reliable alternative test to assess SIBO. Another lim itation is that this was a sing le-center stud y w ith a lim ited sam p le size. Furtherm ore, the d ietary habits of the included patients m ay affect the m orbid ity of RE and SIBO, and the effects of on ly B. subtilis and E. faecium p robiotics on gut m icrobiota w ere studied. Furtherm ore,w e d id not perform endoscopy on asym p tom atic patients after p rim ary healing was achieved, and as a resu lt, w e w ere not ab le to detect asym p tom atic relap ses o f esophagitis erosions. Therefore, the actual rate of m u cosal relap se cou ld not be determ ined in ou r study.

Figure 5 Proportion of patients without small intestinal bacterial overgrowth at the beginning and endpoint of follow-up. Probiotics refers to esomeprazole 20 mg b.i.d. and live combined Bacillus subtilis and Enterococcus faecium enteric-coated capsules 500 mg t.i.d. treatment; placebo refers to esomeprazole 20 mg b.i.d. and placebo treatment. a P < 0.05; b P < 0.01; c P < 0.001. SIBO: Small intestinal bacterial overgrowth.
The com bined adm in istration o f p robio tics (B. subtilis and E. faecium) and esom ep razole cou ld reduce the incidence of SIBO and im p rove abdom inal sym p tom s in patients w ith RE. It m ay also p rolong the tim e to relapse, show ing the poten tial of p robiotics (B. subtilis and E. faecium) for the treatm ent and m anagem ent of RE.
ARTICLE HIGHLIGHTS
Research background
Profound changes have been observed in the gastric and in testinal m icrobiota o f p roton pum p inh ibitor users. Probio tics are comm on ly adm in istered to patien ts w ith in testinal flo ra abnorm alities. N o p rio r stud ies have been conducted to evaluate the therapeu tic effects o f p robiotics [Bacillus subtilis (B. subtilis) and Enterococcus faecium (E. faecium)] on patien ts w ith reflux esophagitis (RE).
Research motivation
W e conducted a random ized con tro lled clinical trial to evaluate the im pact o f d isordered gu t m icrobiota on RE as w ell as the therapeu tic effect of p robiotics supp lem en ts on patien ts w ith RE.
Research objectives
This clinical trial aim ed to study the RE patien ts treated w ith the com bination of p robiotic (B.subtilis and E. faecium) and esom ep razole.
Research methods
This study included 134 patients w ith RE w ho m et the criteria. In phase 1, patients w ere d ivided into tw o groups. The p robiotics group was given esom ep razole and live com bined B. subtilis and E. faecium en teric-coated capsu les for eigh t w eeks, and the p lacebo g roup was given esom ep razole and p lacebo for eight w eeks. Endoscopic evaluation, gastrointestinal sym p tom rating scale (GSRS), reflux d iagnostic questionnaire (RDQ), and lactu lose hyd rogen breath test(LHBT) w ere perform ed at the end of the treatm ent. In phase 2, patien ts w ho achieved endoscop ic and clinical cure (RDQ < 12) entered the follow-up. RDQ and LHBT w ere com p leted at the follow-up endpoint.
Research results
A fter eight-w eek treatm ent, the GSRS d iarrhea synd rom e score was decreased significantly in the p robiotics group, and the sm all intestinal bacterial overgrow th (SIBO) negative rate in the p robiotics group was significantly higher than that in the p lacebo group. Fu rtherm ore, the therapy had a significan t in fluence on relapse tim e, and the risk of relapse in the p robiotics group was low er than that in the p lacebo group at any tim e point during the 12-w k follow-up(hazard ratio = 0.52). How ever, on ly B. subtilis and E. faecium as p robiotics w ere studied on gut m icrobiota in our study. More kinds of p robiotics shou ld be stud ied.
Research conclusions
The com bined adm inistration of p robiotics (B. subtilis and E. faecium) and esom ep razole cou ld reduce the incidence of SIBO and im p rove abdom inal sym p tom s in patients w ith RE. It m ay also p rolong the tim e to relapse, show ing the potential of p robiotics (B. subtilis and E. faecium) for the treatm ent and m anagem ent of RE.
Research perspectives
The lim itation of this study is the fact that w e d id not use jejunal cu ltu res for SIBO assessm ent and d id not perform endoscopy on asym p tom atic patients after p rim ary healing was achieved.Add itional random ized controlled trials are needed to study m ore p robiotics and d ifferent dosages, and p rolong the follow-up tim e to evaluate the long-term effect.
 World Journal of Gastroenterology2019年17期
World Journal of Gastroenterology2019年17期
- World Journal of Gastroenterology的其它文章
- Microbial metabolites in non-alcoholic fatty liver disease
- Recent advances in gastric cancer early diagnosis
- Evolving screening and surveillance techniques for Barrett's esophagus
- Proton pump inhibitor: The dual role in gastric cancer
- Herbs-partitioned moxibustion alleviates aberrant intestinal epithelial cell apoptosis by upregulating A20 expression in a mouse model of Crohn’s disease
- Analysis of the autophagy gene expression profile of pancreatic cancer based on autophagy-related protein microtubule-associated protein 1A/1B-light chain 3
