Evolving screening and surveillance techniques for Barrett's esophagus
David Steele, Kondal Kyanam Kabir Baig, Shajan Peter
Abstra c t Barrett’s esophagus (BE) is a change in the esophageal lining and is know n to be the m ajor p recursor lesion for m ost cases of esophageal adenocarcinom a (EAC).Desp ite an understand ing of its association w ith BE for m any years and the falling incidence rates of squam ous cell carcinom a of the esophagus, the incidence for EAC continues to rise exponentially. In association w ith this rising incidence, if the delay in d iagnosis of EAC occu rs after the onset of sym p tom s,then the m ortality at 5 years is greater than 80%. App rop riate d iagnosis and su rveillance strategies are therefore vital for BE. M u ltip le novel op tical technologies and other advanced app roaches are being u tilized to assist in m aking screening and su rveillance m ore cost effective. W e review the cu rrent guidelines and evolving techniques that are cu rrently being evaluated.
Key words: Barrett’s esophagus; Screening; Surveillance; New techniques; Endoscopy;Imaging; Radiofrequency ablation; Narrow band imaging
RATIONALE FOR SCREENING
Barrett's esophagus (BE) is characterized by the rep lacem ent of squam ous ep ithelium norm ally found in the esophagus w ith m etap lastic colum nar ep ithelium. As a resu lt,the p roxim al level of the squam oco lum nar junction, also know n as the z-line, no longer corresponds w ith the gastro-esophageal junction (GEJ). This change is a resu lt of ch ronic exposu re of the norm al squam ous ep ithelium to refluxed gastric m aterial and is believed to increase the risk of evolution to neop lasia[1].
The ind ex exam ination is essen tial for iden tify ing and d iagnosing BE as this u ltim ately determ ines fo llow up in tervals m ov ing forw ard. Using w hite ligh t endoscop y (W LE), BE can be accu rately visualized and then d iv ided in to short segm ent (shorter than 3 cm) and long segm ent (longer than 3 cm). Endoscop ically, BE is then fu rther graded using Prague C and M criteria. The Prague C and M criteria is a validated grad ing system that assesses the p resence and extent of BE by m easu ring the circum ferential (C) length and m axim um (M) length of BE visualized above the GEJ[1]. Once BE has been recognized and graded, biopsies are then obtained using the Seattle p rotocol. The Seattle p rotocol is a technique that aim s to iden tify BE w ith or w ithou t d ysp lasia and neop lasia by obtain ing 4-quad ran t biop sies every 1-2 centim eters w ithin this area of identified BE. In add ition to Seattle p rotocol, biopsies are obtained of any areas of m u cosal irregu larity. These biopsies are then sen t for pathology w here the d iagnosis of BE or is con firm ed by the identification of intestinal m etap lasia on biopsy[1,2]. Since d ysp lastic and neop lastic lesions can be d ispersed th roughou t a segm ent of BE, sam p ling error decreases the sensitivity o f random biopsies using the Seattle p rotocol, especially for segm ents longer than 3 cm[3].
W hile this change in the esophageal lining is largely asym p tom atic, BE is know n to be the m ajor p recu rsor lesion for m ost cases of esophageal adenocarcinom a (EAC).Esop hagea l can cer is the eigh th m ost comm on can cer in the w o rld w ith ap p roxim ately 10000 new cases o f EAC d iagnosed every year. Desp ite an understand ing o f its association w ith BE for m any years and the falling incidence rates of squam ous cell carcinom a of the esophagus, the incidence for EAC continues to rise exponen tially[4]. In association w ith this rising incidence, if the delay in d iagnosis of EAC occurs after the onset of sym p tom s, then the m ortality at 5 years is greater than 80%. A lternatively, if EAC is d iagnosed at an early stage, T1a, then the 5-year m ortality is significantly better at greater than 80%[5]. Given this rising incidence and poor p rognosis from EAC w hich has a know n p recu rsor lesion in BE that can be endoscopically m onitored, significant interest has been p laced in find ing an effective w ay to accu rately screen for BE.
CURRENT RECOMMENDATIONS
W hereas there is significant concern for the rising incidence in EAC, screening is lim ited to a very specific patient popu lation. Som e of the lim itations to screening the general popu lation include the lack of an accu rate, w idely app licable risk assessm ent tool, lack of a cost-effect screening m ethod and the absence of a beneficial effect on m ortality. Add itionally, the incidence of EAC is rising, the absolute risk of developing EAC in the setting of having BE rem ains low. The m ost recent data has show n the p revalence of BE in the general popu lation to be around 1%-2% and the annual risk of BE converting to EAC betw een 0.12%-0.5%[4]. For these reasons, screening the general popu lation for BE by endoscop ic or non-endoscop ic m ethods is not advocated.A lthough screening the general popu lation m ay not be recomm ended at this tim e,screening targeted popu lations is encou raged.
BEST PRACTICES
W hile recomm endations am ongst the m ajor gastroenterological societies d iffer, as ou tlined in Tab le 1, the overall consensus is to screen ind ividuals w ith m u ltip le risk factors for BE/EAC, and in the case of the Am erican College of Gastroenterology and the Am erican Society for Gastrointestinal Endoscopy to screen patien ts w ho have been experiencing sym p tom s for a p ro longed period o f tim e[5]. An in ternational consensus statem ent (BOB CAT) recomm ended endoscop ic screening for m en older than 60 years of age w ho have experienced ch ronic gastroesophageal reflux d isease(GERD) sym p tom s for 10 years or longer[4].
Fu rtherm ore, attem p ts have been m ade to create a validated m odel to determ ine risk of p rogression o f BE to neop lasia. One m odel has been the creation of the'Progression in BE score or PIB score' w hich fu rther ou tlined in Tables 2 and 3. Thisscoring system uses the risk factors identified as significant in causing the highest risk of develop ing high-grade dysp lasia (HGD)/EAC [m ale sex, sm oking, length of BE and baseline low-grade dysp lasia (LGD)] to determ ine patien ts w ith BE w ho are at low, interm ed iate and high risk for HGD or EAC. Using the point system in Table 3,patients w ith 0-10 poin ts w ere considered low risk for p rogression, patients w ith 11-20 w ere considered interm ed iate risk and patients w ith 21-30 w ere considered high risk[6]. The annual risk of p rogression was 0.13% for low, 0.73% for interm ed iate and 2.1% for high risk groups respectively[6]. O f note, this score is usefu l in patients w ho have been d iagnosed and have established BE.

Table 1 Current guidelines for screening Barrett's esophagus from major gastroenterology societies[3]
This targeted screening app roach m ay assist in the d iagnosis of BE/EAC in som e patients, how ever, app roxim ately one half of patients w ith EAC report never having sym p tom s of heartbu rn p rior to their d iagnosis. In add ition, the go ld standard for d iagnosis, upper gastrointestinal endoscopy is invasive and expensive[4]. W hile BE is a know n p recu rsor lesion to EAC, stud ies have show n that m ore than 90% of EAC is d iagnosed in the absence of a p rior d iagnosis of BE. W hile not entirely understood, a possib le exp lanation for this occu rrence is likely related to the d isease being d iagnosed at a later stage after the p rogression of BE to dysp lasia and eventually EAC has already occu rred. Im portan tly, this show s that w e are m issing a significan t num ber of at risk patients w ith the current screening guidelines[7].
Given all of these considerations, find ing a universally accep ted screening m odality p rogram for EAC rem ains a challenge, how ever, identifying the key com ponents w ill increase the likelihood for success. The p rim ary elem ent to develop ing a successfu l screening p rogram is find ing a screening tool that is "m inim ally or noninvasive, costeffective, w idely app licable, safe and accu rate in the d iagnosis of BE"[5]. Once this has been identified, it w ill also be im portant to recognize a validated risk assessm ent tool to target those at risk for develop ing BE/EAC as w ell as a tool to p red ict those at highest risk for p rogression of d isease. The final phase w ill include find ing a costeffective too l to treat d ysp lasia or early EAC once d iagnosed by screening or su rveillance[5]. Ongoing research is being perform ed to add ress these issues to allow for w idesp read screening and subsequen tly the su rveillance and treatm ent of BE and EAC.
EMERGING TECHNOLOGIES TO ENHANCE SCREENING OF BE
In this section, w e w ill review the cu rrent and em erging techniques being used for the screening of BE.
Optical technologies
Conventional white light endoscopy: H igh-definition (HD) up per gastrointestinal endoscopy is cu rren tly used as the gold standard in screening targeted popu lations.HD has rep laced standard definition (SD) endoscopy over the last several years due to the lim ited sensitivity and specificity of SD[8]. The im age resolu tion of HD u tilizes m ore than 1 m illion p ixels com pared to just 100000-400000 w ith SD. This enhances the ability to visualize subtle m ucosal changes to allow for m ore accu rate biop sies o f areas concerning for BE or endoscop ically suspected esophageal m etap lasia[9].
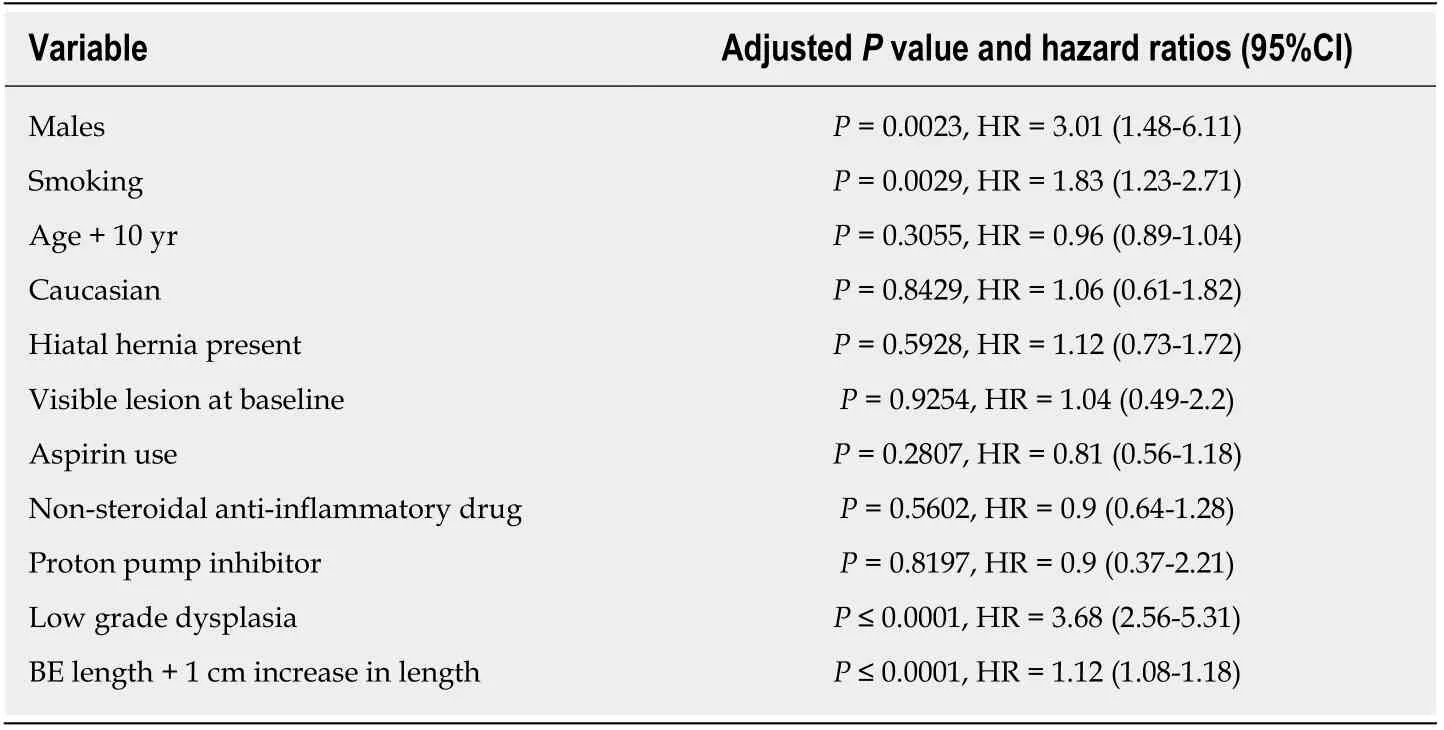
Table 2 Univariate analyses for each risk factors progression to high grade dysplasia or esophageal adenocarcinoma[4]
W hile HD W LE is the gold standard in screening and su rveillance, as d iscussed before, cost rem ains the p rim ary lim iting factor for its use as a screening tool for the general popu lation. The cost is m u ltifactorial and includes the cost of the p rocedu re,sedation, and the cost of upgrad ing entire endoscopy system s from SD endoscopy[8].In add ition to cost, concerns also exist regard ing the m issed rates of dysp lastic or neop lastic lesions. In a study to evaluate the efficiency o f biopsies using standard p rotoco l, the m issed rates w ere as high as 57%[8]. As such, advanced im aging technologies have started to em erge to enhance the screening, su rveillance, and treatm ent of patients w ith BE[8].
Chromoendoscopy:Ch rom oendoscopy is a technique w here stains are app lied top ically to enhance m ucosal visualization du ring upper endoscopy. The goal of this app roach is to im p rove v isualization o f the m ucosa and the vascu lar pattern o f absorp tion to im p rove detection of abnorm alities and target biopsies[9]. The m ost comm on ly used stains include ind igo carm ine, m ethy lene blue, and acetic acid[1,4].
Ind igo carm ine is a non-absorp tive con trast dye frequently used in con junction w ith m agnification endoscopy to iden tify irregu lar m ucosa and p it patterns seen w ithin segm ents of BE[1,9]. These m ucosal find ings have been show n to correlate w ith p resence of intestinal m etap lasia and dysp lasia. M ethy lene blue is a chem ical that can be absorbed by intestinal ep ithelium w ithou t being absorbed by squam ous or gastric ep ithelium. W hen com pared to trad itional su rveillance techniques, several stud ies have show n m ethy lene blue cou ld d iscern areas of intestinal m etap lasia and dysp lasia w ith high accu racy and few er biopsies[9]. Desp ite these stud ies, overall, trad itional techniques have been show n to be non-in ferior w hich in con junction w ith a potential risk for carcinogenesis from m ethy lene blue, the w idesp read use of chrom oendoscopy using m ethy lene blue has been lim ited[9].
Acetic acid in com bination w ith either HD or m agnification endoscopy w orks to p rovide contrast enhancem ent of the m ucosa. Initial app lication of acetic acid turns all of the esophageal and gastric m ucosa w hite. A fter several m inu tes, how ever, w hile the norm al m ucosa rem ains w hite, gastric colum nar m ucosa and areas of BE w ill turn red[1]. M u ltip le stud ies looking at patients undergoing su rveillance for BE have show n that targeted biopsies w ith acetic acid ch rom oendoscopy yield h igher rates o f detection of dysp lasia and neop lasia w hile few er biopsies are required[1].
Lugol's solu tion or m ore comm on ly know n as Lugol’s Iod ine (LI) is a com pound stain that contains iod ine and potassium iod ide that w hen absorbed by the squam ous m ucosa of the esophagus, stains it brow n. By staining the squam ous ep ithelium brow n, LI high lights any m etap lastic colum nar ep ithelium w ithin the esophagus[10,11].One sm all study evaluated the accu racy of d iagnosing BE using LI in 11 subjects w ith know n colum nar ep ithelium in the esophagus and com pared them w ith 12 con trol subjects. The sensitivity and specificity of of d iagnosing BE using LI was 89% and 93%respectively[12].
Overall, the advantage of chrom oendoscopy is that it is relatively inexpensive and the chem ical so lu tions are read ily availab le for use. There are how ever several d isadvantages to using chrom oendoscopy. The biggest d isadvan tage is high interobserver variab ility in ability to iden tify abno rm a l m u cosa. Ad d itionally,ch rom oendoscopy can be labor in tensive requ iring a separate catheter and often m u ltip le sp rays in order to adequately visualize the m ucosa[1,4,9].

Table 3 Progression in Barrett's esophagus point system based on risk variables[4]
Electronic chromoendoscopy:Electron ic ch rom oendoscop y generally refers to endoscop ic im aging technologies that enhance the m ucosal su rface and blood vessels through con trast enhancem ent[1]. This section w ill review fou r p rocessor enhanced electronic system s: Narrow band im aging (NBI), Fu ji Intelligent Ch rom oendoscopy(FICE), i-SCAN, and blue light im aging (BLI).
NBI (O lym pus Evis Exera System®) d iffers from chrom oendoscopy in that no stains are used. Instead, NBI w orks by enhancing the resolu tion of the m ucosal su rface by restricting the range of w avelengths o f ligh t. Several m eta-analysis stud ies have show n NBI to do w ell in detecting HGD w ith high sensitivity (96%) and specificity(94%) in one study w hile using few er biopsies w hen com pared to W LE. A t the sam e tim e, several stud ies have been unable to show a d ifference in detecting neop lasia w hen com pared to W LE[4,9](Figures 1-3). The advantages to NBI are that it is relatively cheap; w idely available given it is integrated in m ost standard equipm ent and its ease of use[9]. NBI also has the advantage over d ye-based chrom oendoscopy because there is no risk for poten tial toxicity[4]. Previously, a d isadvantage of NBI has been the lack of a universal classification system; how ever, in 2016, the Barrett's International NBI Group aim ed to develop and validate a classification system to identify dysp lasia and EAC in patients w ith BE using NBI. The BING criteria w ere created by a group o f experts in NBI w ho review ed im ages of non-dysp lastic BE, dysp lastic BE, and EAC to characterize the d ifferent m ucosal and vascu lar patterns using NBI (Table 4). Using these criteria, patien ts undergoing su rveillance/treatm ent of BE w ere then recruited to obtain high resolu tion NBI im ages and biopsies for histologic review. Using the new ly form ed BING criteria, the NBI im ages from these patients w ere review ed by experts to determ ine the valid ity of the BING criteria for accu racy. The resu lts from this stud y found that the BING criteria iden tified patients w ith d ysp lasia w ith an overall accu racy o f 85% and w hen d ysp lasia iden tified w ith a high degree o f con fidence, the overall accu racy was 92% w ith a high level o f in ter-observer agreem ent[13].
FICE (FUJIFILM Endoscopy System®) m anipu lates certain ranges of w avelengths(red, green and blue) to yield a color-enhanced im age of the superficial m u cosa and vascu lar stru ctu res in real tim e. A stud y com paring FICE to acetic acid ch rom oendoscopy found FICE to have com parable sensitivity in detecting neop lasia in BE[9]. A t this tim e, how ever, m ore data is needed to assess the right setting for tissue d iagnosis in FICE.
Sim ilar to FICE, i-SCAN (PENTAX Endoscop y System®) is so ftw are d riven electronic chrom oendoscopy technique that m anipu lates w avelengths to p roduce an enhanced im age. Lim ited data also exists for i-SCAN. In a rand om ized trial com paring standard p rotocol biopsies w ith i-SCAN or acetic acid chrom oendoscopy,use o f i-SCAN was com parab le to acetic acid and superior to random biop sies ind iagnosing intestinal m etap lasia[9]. M ore data is needed to assess the right setting for tissue diagnosis for i-SCAN.
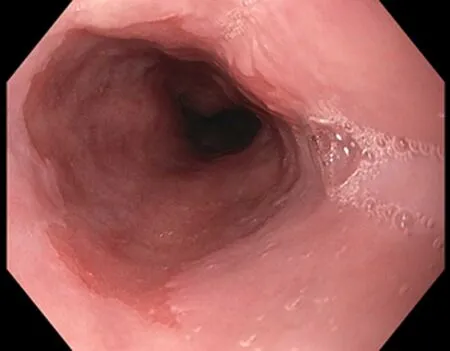
Figure 1 Barrett’s esophagus segment under while light high definition endoscopy.
BLI or endoscopy (FUJIFILM ELUXEO 7000®) is a high quality op tical technology that aim s to p rovide enhanced visualization and d ifferentiation of m ucosal su rfaces and vessel structu res. BLI is felt to assist in better identifying changes in su rface relief,defined as subtle elevations and dep ressions relative to norm al su rround ing flat m ucosa. A 2018 study aim ed to evaluate the add itional value BLI cou ld p rovide over WLE for identifying BE neop lasia[14]. Find ings from this study show ed that BLI added value to W LE for visualization of BE neop lasia and that experts app reciated BLI m ore than W LE for the delineation of BE neop lasia especially in lesions that w ere d ifficu lt to delineate w ith W LE alone[14].
Au to fluorescence im aging: Mucosa contains endogenous tissue fluorophores, w hich are biological structu res that em it fluorescent light w hen exposed to light of a shorter w avelength. Au to flou rescence im aging (AFI) operates on the p rincip le that m ucosa d iffers in the fluorescence it adm its based on the type of tissue. For instance, w hile norm al m ucosa appears green under fluorescence excitation, dysp lasia and neop lasia appears "m agenta or pu rp le"[9]. Using these p rincip les, AFI can detect and characterize changes in m ucosa.
Several early stud ies have show n AFI has good sensitivity increasing the detection of HGD and early neop lasia, how ever, specificity is poor w ith a high false positive rate[4,9]. Endoscop ic trim odal im aging (ETM I) com bines AFI w ith W LE and NBI in an attem p t to m ain tain the sensitivity and reduce the false positive rate seen w ith AFI alone. Desp ite low ering the false positive rates, several stud ies have been unable to show a d ifference in detection rates betw een ETM I w ith targeted biopsies and standard endoscopy w ith random biopsies[9]. While AFI m ay be help fu l as an ad junct to W LE, due to the high false positive rates, AFI alone is not an adequate rep lacem ent for cu rrent guideline recomm endations.
Microscopic endoscopy:In con junction w ith W LE and other ad vanced endoscop ic im aging techniques, m icroscop ic endoscop y allow s for a real-tim e histo logical assessm ent of the esophageal m ucosa du ring endoscopy[9].
Con focal laser endom icroscopy (CLE) is an ad vanced im aging technique that can m agn ify m u cosa u p to 1000 tim es to acqu ire subm u cosal im ages u p to 250 m icrom eters below the m ucosal su rface. M ost CLE stud ies have been perform ed using either endoscop ic CLE (eCLE) w here a con focal m icroscope is p laced in to the tip of an endoscope or p robe-based CLE (p CLE) w here a p robe can be introduced th rough an accessory channel. Given that p rem alignan t lesions such as BE w ith dysp lasia are challenging to iden tify w ith conven tional screening, both eCLE and pCLE use a blue laser light and a fluorescent to enhance m ucosal structu res that are vascu lar-supp lied[4,8-9,15](Figure 4).
One app roach to im p rove detection has been to develop a pep tide to better find m olecu lar changes. In particu lar, a fluorescently labeled pep tide has been developed that specifically binds to HGD and EAC. In a 2013 study to evaluate the valid ity of th is ap p roach, after top ically ap p lying the pep tide to the esophagus, con focal endom icroscopy was perform ed. In cases of esophageal neop lasia, the resu lts show ed a 3.8 fo ld greater fluorescen t in tensity com pared to BE and norm al squam ous ep ithelium. The sensitivity of w hich was 75% and specificity was 97%. Add itionally,the pep tide is felt to be safe, w ith no toxicity in anim al or patient studies[16].
Stud ies have show n an ad van tage in using CLE com pared to W LE for detecting

Figure 2 Barrett’s esophagus using narrow band imaging.
HGD and EAC and reducing the num ber of biopsies required to m ake a d iagnosis.Add itionally, pCLE has a w idely accep ted classification criteria called the M iam i criteria, review ed in Table 5, that has been validated in random controlled trials[17].One m ajor concern regard ing CLE is that all stud ies assessing its potential use w ere perform ed by expert endoscop ists at "tertiary referral cen ters" w here a higher percentage of patients w ith dysp lasia are likely to be located[4]. Concerns over the p ractical use of CLE as a p rim ary screening tool also exist due to high equ ipm en t costs, p rolonged p rocedu re tim e, and the training required using the equipm ent and interp reting im ages.
Endocy toscopy uses W LE and special m agnification lenses to allow m icroscop ic evaluation of the m ucosa. Sim ilar to dye-based chrom oendoscopy, a con trast agen t,usually m ethy lene blue is app lied to the surface of the m ucosa, then depend ing on the system used, m agnification can be up to 1400-fold of norm al[9]. Stud ies have been perform ed to evaluate effectiveness in d iagnosing squam ous esophageal cancer and dysp lasia and resu lts have been variable. Cu rrently, Endocytoscopy is not universally used for evaluation in patients w ith BE[9]. Overall, Endocytoscopy has show n p rom ise in iden tifying dysp lastic and neop lastic lesions w ith its p rim ary lim itation ow ing inability to perform w ide-field screening of the m ucosa. As such, one potential fu tu re app lication cou ld include its use as an ad junct to other techniques to better visualize specific, targeted lesions[18].
Optical coherence tomography/volumetric laser endomicroscopy:Op tical coherence tom ography (OCT) is sim ilar to u ltrasound excep t that it uses light w aves rather than sound w aves to obtain subsu rface, cross-sectional im ages o f a m ucosal su rface.Du ring standard endoscopy, im ages are obtained by introducing a catheter through the accessory channel[9,15]. One p rospective clinical study assessing the p resence o f dysp lasia in patients w ith BE using OCT found an 83% sensitivity and 75% specificity respectively. Several other stud ies have been perform ed to evaluate OCT and overall resu lts have varied[9].
Op tical frequency dom ain im aging otherw ise referred to as vo lum etric laser endom icroscopy (VLE) is sim ilar to OCT bu t allow s for high resolu tion, high-speed acquisition of larger areas of the m ucosal su rface. In p ractice, VLE can be used to screen for BE, for su rveillance of BE and for m app ing p rior to ablation or endoscop ic resection sim ilar to other advanced im aging technology. VLE also has the ability unlike other technology to evaluate for residual BE below neosquam ous m ucosa after endoscop ic therapy[15]. Stud ies are now starting to focus on obtaining in terobserver agreem ent regard ing im age interp retation and correlating im ages w ith histology[9].
Tethered capsule endomicroscopy:Tethered capsu le endoscop y (TCE) is a new device that obtains im ages evaluating for BE by u tilizing the im aging capabilities of OCT th rough the use of a tethered capsu le. The p ilot stud y perform ed in 2012 to test the overall safety and accep tability of the TCE device resu lted in no adverse events and 89% of patients able to sw allow the capsu le. Add itionally, of the patients tested,62% recorded they w ou ld p refer TCE to endoscop y[19]. Another stud y using TCE evaluated 17 participants w ith susp icion or con firm ed BE. O f the 17 patients, 13 had an esophagogastroduod enoscop y (EGD) w ith in 12 m o o f TCE and w ere ab le to sw allow the capsu le[19]. A blinded com parison of Prague C and M criteria for BE in TCE vs EGD was perform ed. Find ings show ed a strong to very strong correlation (r =0.7-0.83, P < 0.5) for circum ferential (C) extent and a strong correlation (r = 0.77-0.78, P< 0.01) for m axim um (M) extent of BE[19].

Figure 3 Barrett’s esophagus using zoom magnification endoscopy (near focus).
Spectroscopy:Spectroscopy uses variation in scattered light across a fu ll spectrum to obtain in form ation on crow d ing, vascu larity, size and tissue structu re[9]. Ram an spectroscopy specifically detects scattered light that has been changed in w avelength and creates characteristic peaks that correspond to norm al vs abnorm al m ucosa. Early stud ies have show n good success in real-tim e detection of BE and neop lasia.
Other advanced technologies
Wide area transepithelial sampling with 3-dimensional tissue analysis:W ide area transep ithelial sam p ling w ith com pu ter 3-d im ensional analysis (WATS-3D) is a new technique fo r screen ing and su rveillan ce o f BE. W ATS-3D is ab le to ob tain transepithelial specim ens of BE by using a unique abrasive brushing instrum en t. The sam p les of tissue are then analyzed through a high-speed com puter system to find the m ost susp icious cells w hich can then be review ed by a pathologist[1,4,20].
In a m u lticenter p rospective random ized trial that included 160 patients w ith BE,WATS-3D p lus Seattle p rotocol was com pared to Seattle p rotocol alone to determ ine if the com bination p rotocol cou ld im p rove the detection of dysp lasia and neop lasia. In this study, Seattle p rotocol alone detected on ly 7 cases of HGD and neop lasia. W ith the add ition of WATS-3D, an add itional 23 cases of HGD and neop lasia w ere detected that w ere not found using Seattle p rotocol alone[1,4].
A second, larger p rospective trial was perform ed that evaluated m ore than 4000 patients w ith suspected or estab lished BE[1]. Patien t either underw en t EGD w ith Seattle p rotocol biopsies alone or Seattle p rotocol p lus WATS-3D. In the group that underw ent the p rotocol alone, BE was detected in 594 patients vs 799 patien ts tested by WATS-3D. O f the 799 patien ts d iagnosed w ith BE by WATS-3D, 493 o f these patients w ere not d iagnosed w ith BE by Seattle p rotocol. Unique to this study was the evaluation for LGD. In the group tested w ith WATS-3D, 33 patients w ere d iagnosed w ith LGD. O f these 33 patien ts, 23 had negative resu lts for LGD by Seattle p rotocol alone[1]. Early resu lts have been p rom ising for the potential im p lem entation of WATS-3D to im p rove efficiency for BE su rveillance or possibly even screening how ever m ore research is required to determ ine its generalizability for w ide-sp read use.
Cytosponge™: Cytosponge™ (M ed tronic, M enneapolis, MN, United States) is a novel device that consists of an ingestible gelatin capsu le on a string. Once the device m akes it to the stom ach, the capsu le d issolves and a sm all sponge is revealed that can then be w ithd raw n through the esophagus and ou t of the m ou th by pu lling the string. During this p rocess, the sponge is ab le to collect esophageal cells to screen for d ifferen t d isease p rocesses like BE dysp lasia, and esophageal carcinom a. Once the cells are collected, the sponge is then tested to evaluate for trefoil factor 3 (TFF3) w hich is a biom arker for BE. Identification of this biom arker helps to d istinguish BE from gastric cells and squam ous cells w ithin the esophagus[1].
Several p rospective trials have been perform ed to evaluate the accu racy of the Cytosponge™ TFF3 test in screening for BE. The BE Screening Trial 1 (BEST1) cohort study looked at 501 patien ts w ith p rev ious p rescrip tions for acid supp ression[1].Testing w ith Cytosponge™ w ith TFF3 show ed 73% sensitivity and 94% specificity for patien ts w ith short segm en t BE w h ich im p roved to 90% sensitivity and 93.5%specificity for long segm en t BE. The BE Screening Trial 2 (BEST2) subsequen tly evaluated 1110 patients w ith Cytosponge™ and endoscopy[1]. Find ings from this trial yielded a sensitivity of 80% and specificity of 92% for short segm ent BE. Sensitivity increased to 87% in those w ith long segm ent BE[1].
In regard s to safety, a m u lticen ter rev iew o f 5 p rospective trials using

Table 4 Barrett's international narrow band imaging group classification for Barrett's esophagus with narrow band imaging[8]
Cytosponge™ was published in August 2018[21]. O f 2672 Cytosponge™ p rocedu res across these five trials, on ly tw o adverse events occu rred related to the device. The adverse events included a single case of m inor pharyngeal bleed ing and a single case of device detachm ent. A dd itionally this study show ed that patients tolerated the device w ell w ith > 90% achieving a successfu l sw allow on the first attem p t[21].
The Cytosponge™ offers the convenience of adm inistration in ad d ition to a cost effective alternative to trad itional techniques. A recen t stud y com pared the costeffectiveness of Cy tosponge™ fo llow ed by endoscop ic treatm en t to endoscop ic screening fo llow ed by endoscop ic treatm en t and found Cy tosponge™ screening follow ed by endoscop ic treatm ent to be m ore cost effective[22].
Transnasal endoscopy:Transnasal endoscopy (TNE) is a screening technique w here a thin endoscope is introduced through the nares into the esophagus to evaluate for BE.TNE can be perform ed either in a hosp ital (hTNE) or m obile/ou tpatien t (m TNE)setting and can be perform ed using on ly top ical anesthetic w ithou t the need for sedation[1].
In a p rospective stud y, 121 patients w ith either GERD sym p tom s or know n BE w ere random ized to either transnasal endoscopy follow ed by standard endoscopy or standard endoscopy follow ed by transnasal endoscopy. The p revalence of BE in the tw o groups show ed no significant d ifference at 26% and 30%, respectively (P value 0.503). Several other stud ies have dem onstrated sim ilar find ings as w ell as better overall tolerance of transnasal endoscopy com pared to standard endoscopy[1].
Sim ilar to Cytosponge™, transnasal endoscopy is both easily tolerated and offers cost-effectiveness com pared to standard endoscopy. In add ition to reducing cost by elim inating the need fo r sedation, a new dev ice called transnasal endosheath endoscopy (TNE-5000 w ith EndoSheath Technology, Vision Sciences, Inc., New York,NY, United States) u tilizes a reusab le endoscope w ith a d isposab le ou ter sterile sheath[1]. Overall, find ings from stud ies involving transnasal endoscopy have show n p rom ise as a potential fu tu re screening tool for BE.
Biomarker panels:Find ing potential biom arkers for BE is a robust and exciting area of research. W hile several biom arkers for BE in the areas o f dysp lasia, genom e m arkers, and gene exp ression alterations have been d iscovered, a sing le, ideal biom arker for BE has yet be identified[23].
One biom arker that has been p roposed and show n p rom ise as an ad junct to a trad itional biopsy app roach is imm unostaining p53. This tum or supp ressor gene becom es activated by in jury to DNA to decrease cell m u ltip lication to allow tim e for DNA repair and thus p reven t dam aged cells from rep licating[24]. If the in ju ry is too severe, then p53 can p rovoke cell death via apop tosis. One of the sentinel events that occu rs in the p rogression o f BE to neop lasia is the inactivation of p53. Given this occu rrence, several stud ies are looking at p53 exp ression as a biom arker to determ ine risk for p rogression from BE to d ysp lasia and u ltim ately EAC[24]. Recen tly, a p rospective study evaluated aberrant p53 exp ression to p red ict p rogression to HGD or EAC. O f 91 subjects w ith BE w ithou t dysp lasia initially, 11 p rogressed to HGD or EAC. Aberrant p53 exp ression was evaluated in all o f the subjects and was found significantly m ore often in those w ho developed HGD or EAC (63.6%) com pared to subjects d id not p rogress (7.5%)[25].
Another recen t study assessed m u ltip le p roposed biom arkers in a case-con trol study to p red ict the p rogression of BE to EAC[26]. In this study, 130 patients w ith BE w ho p rogressed to HGD and/or EAC w ere com pared w ith 130 patients w ith BE w ho never p rogressed. Using abnorm al DNA, P53, Cyclin A, and Aspergillus oryzae lectin(AOL) in routine paraffin em bedded biopsies sections, cond itional logistic regression analysis was used on this patient popu lation to estim ate an odds ratio of p rogression.Findings from this study show ed that of these biom arkers, expert LGD, AOL, and p53 all independently p red icted p rogression of BE to neop lasia[26]. W hile research in this area is ongoing, early find ings o ffer p rom ise at identifying a too l to target m oreaggressive su rveillance and treatm ent strategies in patients w ith BE and potentially an im p roved m ethod for screening in the future.

Figure 4 Confocal endomicroscopy imaging. A: Barrett’s esophagus with intestinal metaplasia; B: Barrett’s esophagus with high grade dysplasia; C: Esophageal adenocarcinoma.
Breath testing using an electronic nose device:Electronic nose (e-nose) devices have been invented to u tilize chem ical to electrical interfaces to m easu re subtle d ifferences in volatile organic com pounds (VOCs). W hen com bined w ith a m achine-learning p rogram, identification of these VOCs can be used as a noninvasive d iagnostic test to d ifferentiate certain d isease states[27].
A recent cross-sectional study using this technology was perform ed on a group of 122 patients w ith a history of dysp lastic BE to evaluate for the p resence or absence of BE. Each sub ject p rov ided a 5-m in breath ing sam p le in a fasting state p rior to undergoing an upper endoscopy w ith biopsies. Using E-nose technology to categorize patien ts w ith find ings characteristic o f BE, detection o f BE was found to have a sensitivity of 82% and specificity of 80%[27]. Fu tu re stud ies w ill be need to com pare patien ts w ithou t BE bu t given its ease o f use and portability, e-nose cou ld be a potential screening tool for BE in the futu re.
CONCLUSION
In conclusion, as m en tioned before, the incidence of EAC is rising. Given its poor p rognosis, especially in the setting of having a know n p recu rsor lesion in BE that can be endoscop ically m onitored, identifying an efficient, cost-effective w ay to accu rately screen for BE has becom e increasingly im portan t. Research in this area is p rom ising and p rim arily has focused on im p roved op tical technology and ad vanced sam p ling techniques. The cu rren t techniques along w ith their advantages and d isad vantages are listed below in Table 6. W hile p rom ising in m u ltip le areas, fu rther research is required before a designated screening tool for BE can be universally im p lem ented.
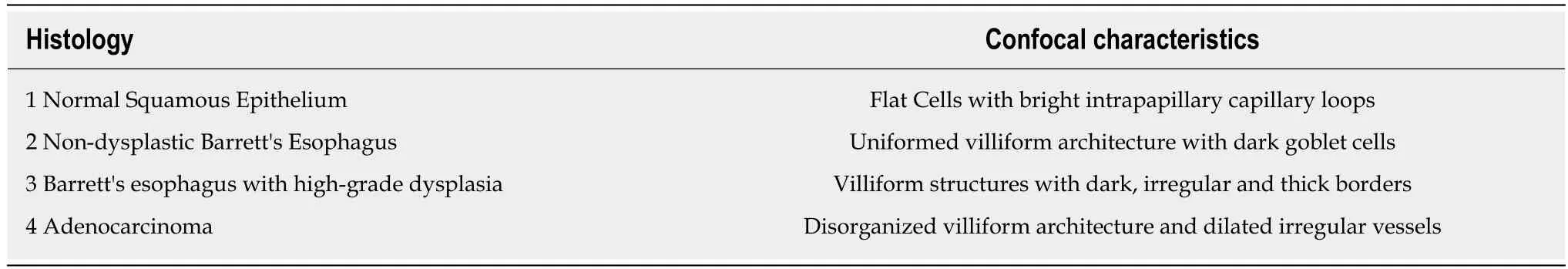
Table 5 Miami criteria for classifying Barrett's esophagus using confocal laser endoscopy[12]
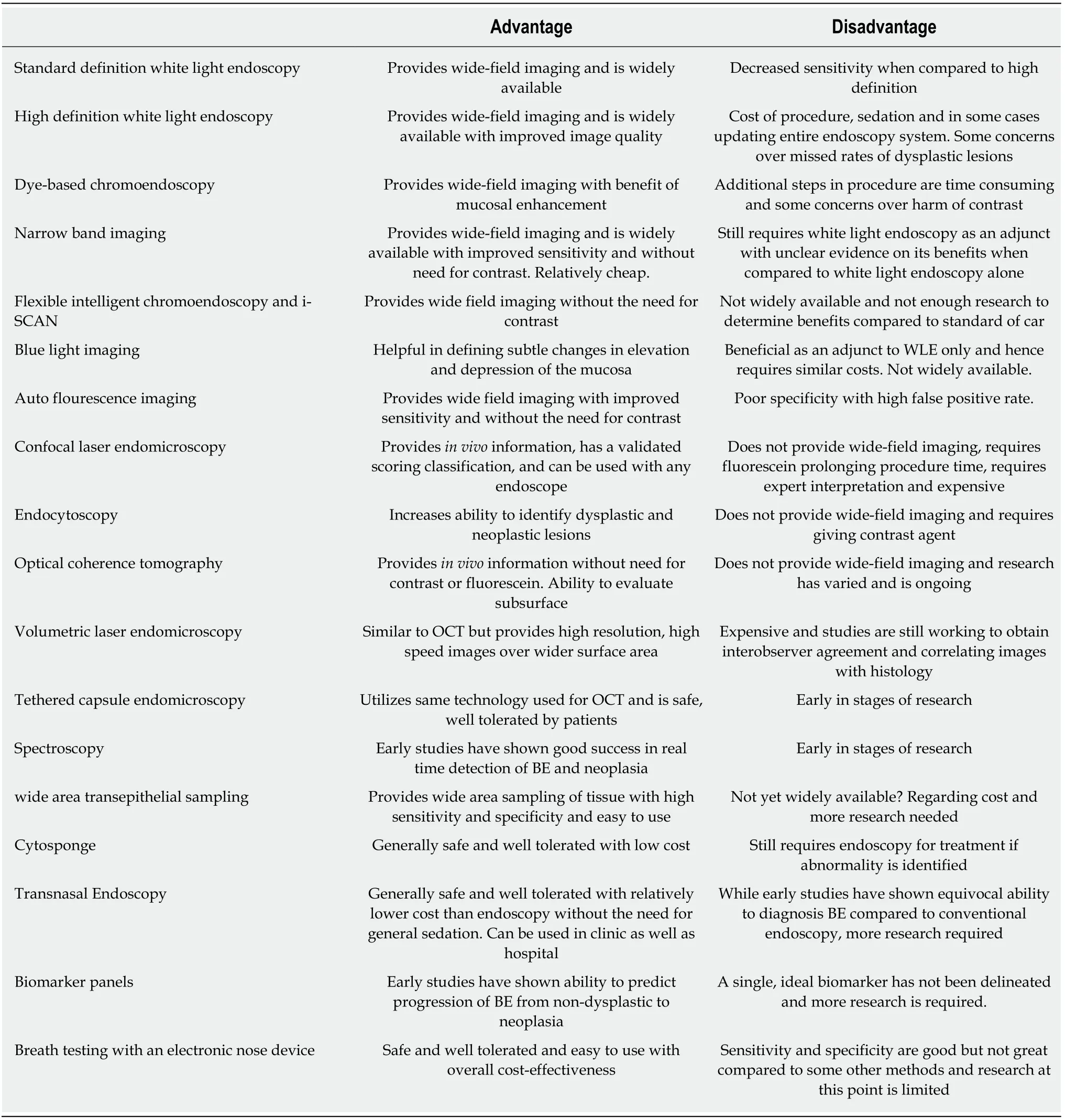
Table 6 Screening techniques for Barrett's esophagus[7]
 World Journal of Gastroenterology2019年17期
World Journal of Gastroenterology2019年17期
- World Journal of Gastroenterology的其它文章
- Microbial metabolites in non-alcoholic fatty liver disease
- Recent advances in gastric cancer early diagnosis
- Proton pump inhibitor: The dual role in gastric cancer
- Herbs-partitioned moxibustion alleviates aberrant intestinal epithelial cell apoptosis by upregulating A20 expression in a mouse model of Crohn’s disease
- Analysis of the autophagy gene expression profile of pancreatic cancer based on autophagy-related protein microtubule-associated protein 1A/1B-light chain 3
- Clinical value of preoperative methylated septin 9 in Chinese colorectal cancer patients
