Annual advances of traditional medicine toxicity in 2018
Shu-Li Man,Gn-Bi Wang,Chang-Xiao Liu,Wn-Yuan Gao
aState Key Laboratory of Food Nutrition and Safety,Key Laboratory of Industrial Microbiology,Ministry of Education,Tianjin Key Laboratory of Industry Microbiology,China International Science and Technology Cooperation Base of Food Nutrition/Safety and Medicinal Chemistry,College of Biotechnology,Tianjin University of Science & Technology,Tianjin,300457,China.bTianjin Key Laboratory for Modern Drug Delivery and High Efficiency,School of Pharmaceutical Science and Technology,Tianjin University,Tianjin,300072,China.cTasly Academy,Tasly Holding Group Co.,Ltd.,No.2 Pujihe East Road,Tasly TCM Garden,Beichen District,Tianjin 300410,China.dState Key Laboratory of Core Technology in Innovative Chinese Medicine,Tasly Holding Group Co.,Ltd.,No.2 Pujihe East Road,Tasly TCM Garden,Beichen District,Tianjin 300410,China.eThe State Key Laboratories of Pharmacodynamics and Pharmacokinetics,Tianjin 300193,China.
1They are regarded as co-first authors and contributed equally to this work.
Abstract
Keywords:Traditional medicine,Natural product,Herb,Toxicity,Toxic target organs,Risk assessment,Safety evaluation
Background
During 2018,there were a number of papers referred to the toxicity advance of traditional medicine (TM)such asBergenia ciliate[1],Rhododendron Molle(Ericaceae) [2],Arecae semenaqueous extract [3],Tithonia diversifolia (Hemsl.) A.Gray[4],Lambda-cyhalothrin[5],traditional Chinese patent medicine for hyperthyroid heart disease [6],phototoxicity of TM [7] and so forth.Meanwhile,multiple theories have been applied.For example,effect-toxicity-chemical study was used in the toxicity of radixWikstroemia indica[8].Toxicokinetic-toxicity correlation was applied for better understanding the toxic mechanisms ofRadix Aconiti Lateralis Preparata[9],while pharmacokinetic study for the health risk assessment of arsenic in realgar and Chinese medicine compound preparation Niuhuang Jiedu tablets (which is a widely used traditional Chinese medicine containing realgar) [10].For China had a long history and widely uses of TM in clinic,the number of its toxicology paper from Chinese researchers was the largest in the world.Therefore,China promoted a rapid upsurge in this field.Statistical analysis of annual publications of toxicological studies on TM by relative percentages on different countries was showed in Figure 1A.China,India [11-16],USA [17-19] and Morocco [20,21] were ranked from the first to fourth important countries researching the toxicology of TM.Furthermore,establishment of toxicology database of TM is very important for their rational application.
Organ toxicity
Liver was regarded as the top one toxic target organ of TM
As we known,drug-induced hepatotoxicity is one of the main causes inducing drug non-approval and drug withdrawal by Food and Drug Administration.During 2018,a large amount of researches have been reported to focus on liver toxicity.For example,Cortex Dictamniextracts displayed potential hepatotoxicity in mice which was associated with cell apoptosis[22]and metabolic activation primarily by CYP (cytochrome P450) 3A4 [23].CYP3A induction and glutathione depletion were involved in hepatotoxicity induced by emodin [24].Pyrrolizidine alkaloids as phytotoxins identified in over 6000 plant species worldwide,likeethnomedicine Arnebia benthamii[25],whose hepatotoxicity was induced by pyrrole-protein adducts[26].Polygonum multiflorum Thunband its processed products have been used in China for centuries due to their multiple beneficial effects to human body.In 2018,researchers focoused on its liver injuries associated with potential toxic ingredients,metabolite identification,metabolomics studies and exogenous contaminant determination [27].Dioscorea bulbifera rhizomecausing liver injury also mainly linked to its metabolites such as amino acid,lipid,purine,pyrimidine,bile acid,gut microflora,and energy metabolisms [28].Bavachinin as a natural product derived from the fruit of the traditional Chinese herbFructus Psoraleaeinduced liver oxidative damage via reactive oxygen species and the JNK/p38 signaling pathways [29].Interestingly,a paper reported that Kansui fry-baked with vinegar displayed protective effect in hepatic and gastrointestinal damage model,but caused the same damage to the normal animals in 2018,which was consistent with the traditional Chinese medicine application principle that traditional Chinese medicine shows different toxicity to patients and normal people[30].
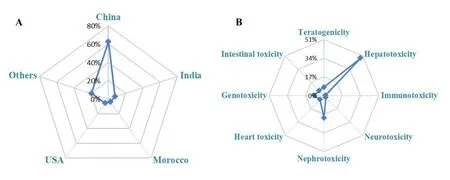
Figure1 Statistical analysis of annual publications on toxicological studies on traditional medicine by relative percentages on different countries and toxic target organs
Kidney was considered as the second toxic target organ of TM
In the year of 2018,researchers identified a new compound inEuphorbia pekinensisand described that it could disturb metabolic pathways of purine,amino acid,phospholipids and sphingolipids inducing nephrotoxicity in rats [31].Aristolochic acids (AAs)induced nephrotoxicity through the proapoptosis and oxidative stress of renal tubular cells,and inhibition of aquaporins.Furthermore,it promoted renal fibrosis by activating TGF-β-Smad signaling and the migration of macrophages,induced carcinogenesis based on the formation of covalent adducts with DNA and so forth[32].Previous study discovered that the carcinogenic mechanism of AAs involved in their promotion of A to T mutations in the TP53 tumour suppressor gene(Figure 2) [33].Gene mutations caused by different factors are different;the mutational signatures are different [34].The construction of mutational signatures database of TM will greatly deepen our understanding and monitoring of teratogenic and carcinogenic toxicity of TM.Cinnabar and realgar induced hepatorenal injury through the oxidative stress and inflammatory damage in mice [35].Terminalia chebulafruits rich in hydrolysable tannins were used in the treatment of various chronic ailments.Following their 28 days repeated dose oral administration,they caused mild disturbances in liver and kidney function which was indicated by reduced body weight,food and water intake,increase of serum urea,glucose and aspartate aminotransferase levels and a mild granulomatous inflammation in the liver[36].
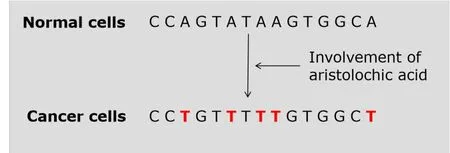
Figure2 Examples of gene mutations induced by aristolochic acid
Other toxic target organs of TM
As 2018 reported,the intestinal toxicity of Kansui was highly correlated with its regulation of Lactobacillus and Blautia genera.Its fry-baked with vinegar significantly relieved side effects of Kansui.Meanwhile,Kansui fry-baked with vinegar increased the production of short-chain fatty acids,which were regulated by gut microbiota [37].It was used as an example for explaining the reason that process should be applied in reducing toxicity of TM.
In 2018,a review listed several herbs such asAngelica sinensis,Tripterygium wilfordiiHook F,Lycopodium serratum,Erycibe henryi prain,Withania somnifera,Caulophyllum thalictroides,Pausinystalia johimbe,AconitumandEphedraspecies associated with heart related problems.Paper emphasized that phase IV post marketing surveillance should be used to diminish adverse events [18].Semen Strychniinducing neurotoxicity could be characterized by metabonomics approach [38].Psammosilene tunicoidescaused damages to heart,lung and kidney in rats [39].Alkenylbenzenes in botanical containing products induced genotoxic and carcinogen [40].These extracts should be used with caution.Taken together,statistical analysis of annual publication referred to different toxic target organs induced by TM was summarized in Figure 1B.
Current advances
Zebrafish embryoes were popular for evaluating the safety of TM
Right now,the safety evaluation has been applied in cellular,organ & individual levels.Rodents were regarded as the common individual models to analyze the safety of TM or natural products.For example,the acute and chronic toxicity of aqueous extract of the seeds ofCalycotome villosa(Poiret) Link (subsp.intermedia) were evaluated in rodents [21],and embryo-fetal development toxicity study with dimethylaminoethyl ginkgolide B in rats and rabbits[41].Meanwhile,a zebrafish model was increasingly considered to be a reliable model for the evaluation of embryo toxicity and a rapid,medium throughput,cost-effective method scince 2018.It was used in the toxicity evaluation ofSutherlandia frutescens(L.)R.Br.[42],Trapa natansleaf extracts [43],motherwort essential oil [44],detoxifiedRadix Aconiti Lateralis Preparata(lateral root of Aconitum carmichaeli) [45],saponin-rich plant extracts [46] and so forth.AlthoughCaenorhabditis elegans[47]andDrosophila[48]were popular in the safety evaluation of various chemical compounds recently,there were no research on that in TM.In the future,the application of these models can be focused on.
New toxicology study technology
Recently,multiple theories have been applied.For example,researchers set up an organ-on-a-Chip,a GelMA-based 3D culture platform,to mimic the microenvironment and basic functions of the kidney screening main compounds associated with nephrotoxicity in spearmint (Mentha spicata L.) [49].Meanwhile,effect-toxicity-chemical study was used in the toxicity of RadixWikstroemia indica[8].Toxicokinetic-toxicity correlation was applied for better understanding the toxic mechanisms ofRadix Aconiti Lateralis Preparata[9],while pharmacokinetic study for the health risk assessment of arsenic in realgar and Chinese medicine compound preparation Niuhuang Jiedu tablets [10].The changes in gut microbiota were also used to explain the reason why processing technology should be applied in reducing toxicity of TM[37].
Other hot issues in 2018
Adverse reaction of TM injection was always the key point for preclinical study and clinical utility.In addition,the safety assessment ofAconitum,Tripterygium,Strychnine,etc.was still hot issue.AlthoughRadix Aconiti Lateralis Preparatahas promising therapeutic effects,its toxicities were frequently observed.Aconitumalkaloids were responsible for its pharmacological activity and toxicity [50].In 2018,researchers focused on the toxicokinetics-toxicity relationship of its main diester-diterpenoid alkaloids [9],thein vivoacute toxicity of detoxifiedRadix Aconiti Lateralis Preparata[45],heart toxicity related toRadix Aconiti Lateralis Preparata[18],and safety of its stems and leaves [51].Tripterygium Wilfordii Hook Fcould be used on psoriasis vulgaris.Recently,pharmacokinetic,toxicological characteristics [52] and randomized controlled trials [53] ofTripterigiumglycosides and their derivatives have been reviewed in 2018.
Meanwhile,detoxification was also a research hotspot in 2018.It’s reported that ginger extract could ameliorate the toxicity ofLambda-cyhalothrinon the rat thyroid through the inhibition of oxidative stress[5].Total glucosides ofPaeoniae Radix Albaprotected againstSemen Strychni-induced neurotoxicity in rats through suppressing oxidative stress and reducing the absorption of toxic components[54].Chinese medicine compound preparation Angong-Niuhuang Pill displayed hepatorenal protective effects against cinnabar and realgar induced oxidative stress and inflammatory damage in mice[35].
Conclusion
Taken together,the annual researches showed that the methods of effect-toxicity-chemical study,toxicokinetics,gut microbiota,metabolism and organ-on-a-Chip were used in toxicology research since 2018.Besides rodents,zebrafish embryoes have been regarded as common models to evaluate the safety of TM.2018 toxicology research indicated that liver and kidney were the mainly toxic target organs of TM.Their toxic mechanisms included cell apoptosis,metabolic disorder,oxidative stress,inflammatory damage,liver and renal fibrosis and even inducing carcinogenesis.In addition,the safety assessment ofAconitum,Tripterygium,Strychnine,and detoxification methods were still hot issue.Therefore,the herbs mentioned in this paper should be used with caution.Combination of TM,processing drugs,quality control and dose control should be used in the prevention of TM toxicology in the future.
 Traditional Medicine Research2019年3期
Traditional Medicine Research2019年3期
- Traditional Medicine Research的其它文章
- Unveiling the book of Persian medicine-the official document of Persian medicine in Iran and delivering it to the WHO representative
- Leech therapy indications:a scoping review
- The potential role of grape (Vitis vinifera L.) in prevention of threatened abortion via immunomodulatory and anti-inflammatory abilities:a hypothesis
- Digestion process and causes of indigestion based on Avicenna's view and modern medicine
- Ethnobotanical and traditional uses,phytochemical constituents and biological activities of Eryngium species growing in Iran
- Application of herbal rectal suppositories beyond intestinal disorders in Persian medicine
