Xiaoaiping injection combined with cisplatin and gemcitabine for non-small cell lung cancer:a meta-analysis
Yue Ji,Lin Wang,Changying Chen,Yihua Fan,Xuyan Wang,Hongtao Yang*
1The First Teaching hospital of Tianjin University of Traditional Chinese Medicine,300382,Tianjin,China.
2Chengdu University of Traditional Chinese Medicine,Chengdu 611137,China.
Introduction
Lung cancer is the most common malignant tumor with the highest morbidity and mortality in China,among which non-small cell lung cancer (NSCLC)accounts for 80%~85%.70%~80%of NSCLC patients are already in advanced stage when diagnosed, losing the opportunity of radical surgery.Therefore,palliative chemotherapy is the predominant treatment for advanced NSCLC [1-3]. Combination chemotherapy based on platinum is still the main first-line treatment for advanced NSCLC according to the clinical practice guidelines for NSCLC formulated by the national comprehensive cancer network (NCCN). The regimen of GP, as one of the standard first-line chemotherapy,has superior efficacy than other combination with the response rate 30% for advanced NSCLC patients[4].However, GP is associated with serious toxic effects,such as granulocytopenia and gastrointestinal reactions,which affect the patient's performance status significantly, even resulting in the suspension of treatment process[1]. Many studies have shown that XAPI, as the marsdenia tenacissima extract, has antitumor effect through inhibiting the proliferation of tumor cells, promoting the release of hormone and cytokines associated with tumor growth, inducing angiogenesis and apoptosis.Meanwhile,XAPI also has the function of antiasthma, depressurization,bacteriostasis, immune regulation and liver protection[6]. Presently, there were many clinical trials concerning about the efficacy of XAPI combined with GP for NSCLC patients. But it is still controversial on the efficacy of XAPI due to the difference of dosage,frequency and course of XAPI application in clinical trials. Therefore, the authors performed the present meta-analysis to assess the effectiveness of XAPI plus GP for advanced NSCLC so as to provide guidance for clinical application.
1 Methods
1.1 Types of studies
Randomized controlled trials (RCTs), whether adopting blind methods or allocation concealment,with the language of Chinese and English.
1.2 Types of participants
All the involved participants were diagnosed as advanced NSCLC with clinical stage of Ⅲb- Ⅳ(followed as the TNM classification formulated by WTO) according to the pathological, cytological, and radiological features. In addition, the Karnofsky performance score (KPS) was above 50 in all patients and the survival time is expected to be more than 3 months. The patients had no contraindications of chemotherapy or TCM injection, and no seriousliver and kidney dysfunction.
1.3 Types of interventions
Patients in the experimental group were given XAPI combined with GP; Patients in the controlled group were given GP alone.There is no limitation in the drug dosage and frequency.
1.4 Exclusion criteria
(1) The interventions contain other TCM thera pies. (2) Studies with no outcome measures;(3) Repeated published data; (4) RCTs for whi ch full-text versions were unavailable.
1.5 Types of outcome measures
Primary outcome was the clinical total effective rate. According to the therapeutic effect criterion of the World Health Organization for solid tumors[7],the clinical total effective rate was calculated by the following formula: (number of complete response patients + number of partial response patients)/total number of patients * 100%.Efficacy assessment follows RECIST standard.Complete response:complete disappearance of the tumor in response to treatment. Partial response:The shrinkage of tumor size ≥50%. Stable disease(SD): Tumor sizes do not appear to change.Progressive disease: tumor size increase and the disease is progressing or worsening. Secondary outcomes were performance status and adverse drug reaction (ADR). Performance status was assessed by the KPS.KPS increase ≥10 points was considered improvement of performance status.KPS decrease ≥10 points was considered lower performance status. KPS increase or decrease <10 points was considered stable performance status.The ADR assessment met the common toxicity criteria of chemotherapy drugs drafted by WHO.Assessed ADRs include leukocyte level decrease,hemoglobin level decrease, platelet decrease,gastrointestinal adverse reactions, phlebitis, liver function impairment,kidney function impairment.
1.6 Searching strategies
The retrieval was performed in the following databases from their inception to December,2018:Cochrane Library,Pubmed,Embase,CNKI,CBM,VIP and the Wanfang Database. Retrieval terms include “Xiaoaiping Injection”, “Gemcitabine,Chemotherapy”, “Non-small cell lung cancer”,“lung cancer”, “lung neoplasm”, “NSCLC”.References of important articles retrieved were manually searched.
1.7 Literature Selection and Data Extraction
Relevant literatures from all databases were collected. Endnote software was utilized to filter the duplication. Two reviewers independently examined the title and abstract of the articles to make the preliminary screening to exclude the studies that do not meet criteria. They continued to review the main text.When there existed disagreement, they discussed with a 3rd independent reviewer to resolve it.The following information was collected in this meta-analysis:literature resources,the first author’s name,publication year, baseline data of patients, intervention, course of treatment,and the data of outcome.
1.8 The assessment of methodological bias risk
The methodological bias risk of all studies was evaluated by using the Cochrane evaluation handbook of RCTs(5.1.0).The bias risk indices were the random sequence generation, the allocation concealment, the blinding of participants, data integrity, the selective reporting and the other bias.Each item was judged on three levels such as“Yes”for a low risk of bias, “No”for a high risk of bias and“Unclear”.
1.9 Statistical analysis
The meta-analysis was performed using Review Manager (RevMan) 5.3. . All data were expressed as odds ratio (OR) and 95% confidence intervals (CI),and P < 0.05 was considered to be statistically significant.The chi-square test was applied to evaluate heterogeneity among studies, and I2was used to show the magnitude of this heterogeneity.Results of P ≥0.1 and I2≤ 50% suggested a lack of significant heterogeneity; the fixed-effect model was used accordingly. For cases with P < 0.1 and I2> 50%, we adopted a random-effect model,and subgroup analysis was presented to explore the sources of heterogeneity.Funnel plots were used to reveal the potential publication bias when studies were ten or more.
2 Results
2.1 Search results
Initially,112 potentially relevant articles were retrieved.Endnote software was used to exclude 48 repeated articles.Then the titles and abstracts were read to reject the 45 irrelevant studies.Finally,7 articles[8-14]were included for this meta-analysis after reviewing full texts to exclude the studies with experimental group using other interventions, case reports, non-RCTs.The flow diagram in Figure 1 illustrates the selection process.
2.2 Characteristics of the included trials
This meta-analysis involved 7 articles and the experimental groups and control groups involved 270 and 264 cases respectively. Four studies reported the stages of NSCLC patients [8, 10, 11, 14]. Four studies records the expected survival time of patients [8,10, 11, 14]. Four studies mentioned the KPS of patients [8, 10, 11, 14]. In one study[14], the patients in experimental group were given XAPI by intramuscular injection and the control group was given 0.9% normal saline as placebo. The dose of XAPI was 2-80ml / time. The treatment time per cycle was 8-15days. The detailed chemotherapy regimens and other information are described in Tables 1.
2.3 The risk of methodological bias
The random sequence was generated in only 3 trial[12-14]. None of the trials provided the detailed informations about the allocation concealment and blindingof the participants. No absence of data and no selective reporting existed(Figure 2,Figure 3).
3 Outcomes
3.1 The clinical response
In this analysis, we made heterogeneity test and found that there was no statistical heterogeneity among the 7 trials(P=0.49,I2=0%).Therefore,the data was calculated by using a e fixed-effects model. The result showed that there is no statistical significance in total effective rate[OR=1.39, 95%CI (0.97, 1.98), P = 0.07] between GP alone and XAPI combined with GP(Figure 4).
3.2 Performance status improvement
Five studies[9, 10, 12-14] reported KPS of patients had been included in the analysis,and the experimental groups and control groups involved 163 and 163 cases respectively. There was no statistical heterogeneity among the 5 trials( P =0.58, I2= 0% ) and the fixed-effects model was performed. The result showed that compared with chemotherapy alone, the result showed that XAPI plus chemotherapy increased the OR, and the difference was statistically significant[OR= 3.78,95% CI (2.24, 6.38), P < 0.0001], indicating that XAPI plus chemotherapy could improve the performance status of advanced NSCLC.(Figure 5)
3.3 Hemoglobin decrease
Four studies [8, 10, 11, 14] recorded the hemoglobin. The heterogeneity was small (P =0.41, I2= 0%); thus, the fixed effect model was implemented. Meta-analysis revealed that the statistically significant difference was detected between experimental group and control group[OR = 0.49, 95%CI (0.26, 0.92), P = 0.03](Figures 6).

Figure 1.Flowchart of literature search

Tables 1 The basic characteristics of the included studies

Note:G,Gemcitabine;C,Cisplatin;XAPI,Xiaoaiping injection.

Figures2 Risk of bias summary
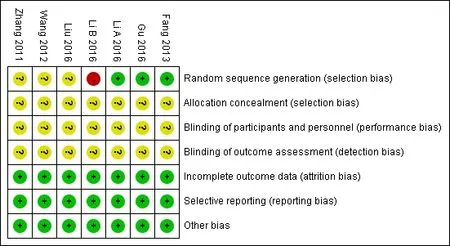
Figures3 Risk of bias summary

Figures 4 Forest plot of the clinical total effective rate.

Figures 5 Forestplot of theperformance status.
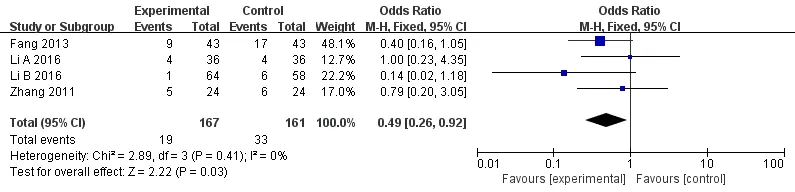
Figures 6(0.22,0.87),P=0.02](Figures 8).
3.4 Leukopenia
Four studies [8, 10, 11, 14] recorded the leukopenia.There was no statistical heterogeneity among the 4 trials(P=0.29,I2=20%)and the fixed-effects model was performed. Meta-analysis revealed that compared with the control group only using GP, XAPI with GP can reduce leukopenia. Besides, the statistically significant difference was detected between these two groups [OR = 0.40, 95% CI (0.21, 0.74), P = 0.004](Figures 7).
3.5 Platelet decrease
Four studies [8, 10, 11, 14] adopted the decrease rate of platelet as one index of side effects. There was no statistical heterogeneity (P = 0.92,I2= 0%) and the fixed effect model was implemented. Meta-analysis revealed that XAPI with GP can prevent platelet decreasing and the statistically significant difference was detected between two groups [OR = 0.43, 95%CI
3.6 Gastrointestinal reactions
Four studies [8, 10, 11, 13] reported gastrointestinal reactions in the two groups.Heterogeneity test showed that heterogeneity was high among the trails ( P = 0.07,I2= 58%);therefore, the random effect model was used.Meta-analysis indicated that compared with GP alone, there is no statistical significance in gastrointestinal reactions between the two groups[OR = 0.44, 95%CI (0.16, 1.23), P = 0.12](Figures 8).
3.7 Publication bias
The amount of studies involved was less than ten and the publication bias analysis was not performed.

Figures 7 Forest plot of the leukopenia rate

Figures 8 Forest plot of the platelet decrease rate

Figures 9 Forest plot of the gastrointestinal reactions rate
Discussion
In traditional Chinese medicine (TCM), lung cancer is associated with the concepts of lung accumulation,cough,hemoptysis and chest pain.The pathogenesis of lung cancer in TCM is normally identified as the deficiency of vital Qi,unbalance of Yin and Yang,and functional disturbances of the viscera, resulting in decreasing body's disease-resistant ability and invading pathogenic Qi. Then the pathogenic Qi is retained in the chest,leading to the stoppage of lung-qi and blood.As a result, fluid can not circulates properly and gathers into phlegm which will stagnate and congeal together,and form the tumor ultimately. In the past 10 years, studies about TCM in lung cancer have shown that TCM could relieve clinical symptoms, prolong survival time with tumor, improve the quality of life and combine with radiation and chemotherapy to increase efficiency and reduce toxicity. In the study performed by WU Tao [15], 80 cases of patients with advanced NSCLC were randomly divided into control group and observation group.The control group was treated with conventional chemotherapy treatment, the observation group combined with Yiqi Fuzheng herbs, 28 days for 1 courses of treatment, continuous observation of 2 courses. The results revealed that the observation group of chemotherapy in NSCLC patients showed obvious curative effect with significant difference compared with the control group (P <0.05). The quality of life in the observation group was significantly better than the control group(P<0.05), side effects were significantly less than the control group (P < 0.05), the immune indexes of blood lipid was significantly better than the control group. Xu Quansheng[16] observed the clinical efficacy of Yiqi Yangyi Sanjie therapy on advanced lung cancer and found that the shrinkage of the solid tumor and improvement of life quality in the treatment group were better than the control group with a significant difference. These findings indicate that the integrative medicine on advanced lung cancer showed better advantages than chemotherapy alone.According to the TCM theory, chemotherapy can damage the body's vital Qi, resulting in myelosuppression, immunodepression, gastrointestinal reaction, hepatic and renal dysfunction. Therefore,protecting and enhancing vital Qi is the fundamental principle of preventing toxic side effects of chemotherapy.
XAPI is the marsdenia tenacissima extracts and its constituents contain polysaccharides, saponins and alkaloids. Many studies have shown that XAPI has clinical effectiveness on gastrointestinal tumors and lung tumors,especially for patients with advanced lung cancer[17-20]. The anticancer mechanisms of XAPI may be blocking the tumor cell cycle and making cells arrest in M phase, disturbing the synthesis of nucleic acids and DNA. Meanwhile, due to the relieving asthma and anti - inflammation function of XAPI,it is also helpful to dyspnea,shortness of breath,wheezing and secondary infection caused by lung tumors[21]. Gu Ning[9]found that total effective rate of XAPI plus GP group was 82.5%,higher than that of control group (58.33%), and the difference was significant. Our research found that there is no statistical significance in total effective rate between GP alone and XAPI combined with GP, may be due to the reason that the amount of studies included was relatively small. In addition, Fang Huan showed that the statistical significance was also not found between GP alone and XAPI combined with GP in total effective rate. Hence, these results require confirmation with more clinical trails in the future.The present study also show that XAPI can prevent myelosuppression and improve the patient performance status, indicating that XAPI alleviates the toxic side effects of chemotherapy to some extent.Moreover, the synergistic relationship between XAPI and GP and the anti-tumor mechanism are worthy of further study.
There were some limitations in this study. Firstly,Chinese and English databases were retrieved, but not otherlanguage bases. These resulted in a source of selection bias. Secondly, the quality of the RCTs enrolled in this study was not high.Most RCTs did not refer to the specific random grouping method, and the allocation concealment were not clear as well. Due to the particularity of TCM injection, the blind methods were difficultly performed except the outcome observers. Besides, the application of blind methods should follow the internationally accepted scales and indicators and avoid the subjective outcome indicators.Thirdly, these patients involved were given different drug dosages and courses, which increased the heterogeneity between the groups.
Above all, due to the limitations of the current meta-analysis, the efficacy of XAPI plus GP need further study, and the strength of evidence needs to be promoted by more well-designed RCTs.
1. Wang XQ, Liu J, Lin HS, et al. A multicenterrandomized controlled open-label trial to assess the efficacy of compound kushen injection in combination with singleagent chemotherapy in treatment of elderly patients with advanced non-small cell lung cancer: study protocol for a randomized controlled trial.Trials,2016,17:124.
2. Gandara DR, Hammerman PS, Sos ML, et al.Squamous cell lung cancer: from tumor genomics to cancer therapeutics. Clin Cancer Res,2015,21:2236-2243.
3. McElnay P, Lim E. Adjuvant or neoadjuvantchemotherapy for NSCLC.Journal of Thoracic Disease, 2014, 6: S224-S227.
4. Paz-Ares LG, Biesma B, Heigener D, et al.Phase III,randomized,double-blind,placebocontrolled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer.J Clin Oncol, 2012, 30: 3084-3092.
5. Wang QM, Sun LM, Xu JF. Research on the antitumor mechanism of marsdenia tenacissima and its Xiaoaiping extractive.Clinical Misdiagnosis & Mistherapy, 2017,30:102-105.
6. Wang F, Fan QX, Wang HH, et al. Efficacy and safety of Xiaoaiping combined with chemotherapy in the treatment of advanced esophageal cancer.Zhonghua Zhong Liu Za Zhi,2017,39:453-457.
7. Keil S, Barabasch A, Dirrichs T, et al.Target lesion selection: an important factor causing variability of response classification in the response evaluation criteria for solid tumors 1.1 .Investigative radiology, 2014, 49: 509-517.
8. Fang H,Wang J,Pan CF.Xiaoaiping Injection Combined with Cisplatin and Gemcitabine for Non-Small Cell Lung Cancer: A Clinical Observation. Evaluation and Analysis of Drug-Use in Hospitals of China, 2013, 13:165-168.
9.Gu N, Li ZG. Clinical Observation of Xiaoaiping Injection Combined With Chemotherapy in Treatment With Advanced Non-small Cell Lung Cancer. China Health Standard Management.2016,7:120-121.
10. Li QL, Cheng B.A clinical research of Xiaoaiping Injection Combined with GP chemotherapy for Non-Small Cell Lung Cancer. Chinese Archives of Traditional Chinese Medicine, 2016, 4: 785-787.
11. Li XG, Jiang XJ. Xiaoaiping Injection Combined with GP chemotherapy for Non-Small Cell Lung Cancer: A Clinical Observation. Chinese Journal for Clinicians,2016,44:62-64.
12. Liu AF. The comprehend of Xiaoaiping Injection Combined with GP chemotherapy for advanced Non-Small Cell Lung Cancer in clinical practice.Journal of clinical medical literature, 2016, 3:4415-4416.
13. Wang YT. A clinical research of Xiaoaiping Injection Combined with GP chemotherapy for advanced Non-Small Cell Lung Cancer. Qingdao Medical Journal,2012,44:246-248.
14. Zhang FY, Li QW, Guan JZ. Clinical Observation of Xiaoaiping Injection Combined With Chemotherapy in Treatment With Advanced Nonsmall Cell Lung Cancer. Journal of Basic and Clinical Oncology,2011,24:415-417.
15. Wu T. Clincal study on the efficacy of synergistic and attenuated action of Yiqi Fuzheng herbs on chemotherapy in the patients with non-small cell lung cancer. World Journal of Integrated Traditional and Western Medicine,2018,13: 642-645.
16. Xu QS, Wu X. Clinical observation on treating advanced lung cancer by the Yiqi Yangyin Sanjie therapy. Clinical Journal of Chinese Medicine,2017,9:82-83.
17. Yao XY.Research progress on the mechanism of xiaoyuping injection in the treatment of advanced malignant tumors. The Asia-pacific Traditional Medicine,2014,10:41-42.
18. Xiao N,Zhou XM.Meta-analysis on treatment of non-small cell lung cancer with Xiaoaiping injection in combination with platinum-contained first-line chemotherapy. Modern Journal of Integrated Traditional Chinese and Western Medicine,2013,22:3669-3674.
19. Wang MX, Lou DH. Meta-analysis on treatment of advanced non-small cell lung cancer with Xiaoaiping injection. Jiangsu Medical Journal,2013,39:1287-1290.
20. Tao XX, Yu H, Bai JL. Meta-analysis and Therapeutic Effect of Xiaoaiping Injection Combined with Chemotherapy for NSCLC.Herald of Medicine,2014,33:48-53.
21. Liu JR,An YH, Yang WB, et al. Clinical Study on Xiaoaiping Injection Combined with GP Regimen in the Treatment of Advanced Lung Squamous Cell Carcinoma. Chinese Medical Innovations,2016,13:97-101.
 Clinical Research Communications2019年1期
Clinical Research Communications2019年1期
- Clinical Research Communications的其它文章
- 18F-FDG PET/CT for Detecting Vulnerable Carotid Plaque:A Systematic Review of Literatures
- A meta-analysis of randomized controlled trials of combined treatment with DGSN and western medicine on diabetic peripheral neuropathy
- A case report of acupuncture of dysphagia caused by herpes zoster virus infection
- Clinical observation on TCM fumigation and washing for treatment of diabetic peripheral neuropathy by self-made water-washing footbath
