RP-HPLC法测定可溶性CD95-Fc融合蛋白中异天冬氨酸的含量
于雷 陶磊 毕华 李响 饶春明
[摘要] 目的 测定可溶性CD95-Fc融合蛋白中异天冬氨酸(IsoAsp)含量。 方法 采用ISOQUANT?誖异天冬氨酸检测试剂盒结合反相高效液相色谱法测定样品中IsoAsp含量,采用Phenomenex Synergi 4 μm Hydro RP-80 C18色谱柱(150 mm × 3 mm,4 μm),以7.9 mmol/L磷酸二氢钾-1.1 mmol/L磷酸氢二钾-10%甲醇为流动相A,以甲醇为流动相B,梯度洗脱,流速为0.43 mL/min。 结果 对照品和3批原液中IsoAsp含量相近(0.30~0.32 mol/mol sCD95-Fc),3批成品中IsoAsp含量明显增高(0.51~0.53 mol/mol sCD95-Fc);25℃和37℃处理24 h后成品中IsoAsp含量无明显变化,60℃处理后明显增加。 结论 该法适用于可溶性CD95-Fc融合蛋白中IsoAsp含量检测,高温处理可加快样品中IsoAsp生成。
[关键词] 可溶性CD95-Fc;异天冬氨酸;脱酰胺修饰;反相高效液相色谱
[中图分类号] R914 [文献标识码] A [文章编号] 1673-7210(2019)02(c)-0109-04
[Abstract] Objective To determine the content of isoaspartate (IsoAsp) in soluble CD95-Fc fusion protein. Methods IsoAsp content in samples was determined by ISOQUANT Isoaspartic Acid Detection Kit combined with reversed-phase high performance liquid chromatography. Phenomenex Synergi 4 μm Hydro RP-80 C18 column (150 mm × 3 mm, 4 μm) was used. Potassium dihydrogen phosphate-1.1 mmol/L potassium dihydrogen phosphate-10% methanol was used as mobile phase A, methanol as mobile phase B, gradient elution was performed at a flow rate of 0.43 mL/min. Results The content of IsoAsp in reference material was similar to that in three batches of raw liquor (0.30-0.32 mol/mol sCD95-Fc). The content of IsoAsp in three batches of finished products increased significantly (0.51-0.53 mol/mol sCD95-Fc). The content of IsoAsp in finished products did not change significantly after 24 hours of treatment at 25℃ and 37℃, but increased significantly after treatment at 60℃. Conclusion This method is suitable for the determination of IsoAsp in soluble CD95-Fc fusion protein. High temperature treatment can accelerate the formation of IsoAsp in samples.
[Key words] Soluble CD95-Fc; IsoAsp; Deamidization; Reversed-phase high performance liquid chromatography
重組蛋白类制品的化学稳定性对其安全性和有效性至关重要。在生产和储存过程中,某些热点氨基酸残基易受到不同类型的化学修饰,包括脱酰胺、异构化、氧化等[1]。其中,天冬酰胺(Asn)脱酰胺和天冬氨酸(Asp)异构化是比较常见的化学修饰,两者均会形成异天冬氨酸(IsoAsp),可能影响蛋白的体内生物学活性和体外稳定性。研究[2]表明,生产和储存过程可能会对蛋白中IsoAsp的含量造成影响。因此,IsoAsp的检测对重组蛋白类药物极为重要,其能够作为监测药物活性功能改变的重要指标,在药物质量控制、制剂配方优化、储存条件筛选等方面发挥重要作用[3-4]。目前已有大量研究[6-11]开发了针对重组蛋白的IsoAsp分析技术,尤其是单抗类制品,主要分析技术包括等电聚焦(IEF)、离子交换色谱(IEX)、疏水作用色谱(HIC)、亲水作用色谱(HILIC)[5]、胰蛋白酶肽图等。IsoAsp和Asp的分子量、电荷均相同,两者很难区分,往往需要先将大的蛋白质消化成小片段,经不同方式分离后使用质谱检测器进行鉴定[9,11-12]。这些方法通常耗时、低效,且在样品处理过程可能产生新的IsoAsp,从而造成结果不准确[10]。Promega公司推出了一款ISOQUANT?誖试剂盒,其基本原理是利用蛋白L-异天门冬氨酰甲基转移酶(PIMT)催化S-腺苷-L-蛋氨酸(S-adenosyl-L-methionine,SAM)上的甲基转移到样品中的IsoAsp羧基上,同时SAM转换成S-腺苷同型半胱氨酸(S-adenosyl homocysteine,SAH),SAH的摩尔含量与IsoAsp相同,SAH采用反相高效液相色谱法(RP-HPLC)分离定量[4,10]。该方法快速、准确,不需要质谱检测器,更适用于常规检测。
可溶性CD95-Fc(soluble CD95-Fc,sCD95-Fc)融合蛋白是一种用于治疗多形性成胶质细胞瘤(glioblastoma multiforme,GBM)和骨髓增生异常综合征(myel-odysplasticsyndrome,MDS)的创新生物技术药物[13-14]。它是由CD95胞外结构域和IgG1抗体Fc段组成的全人源融合蛋白,可以与肿瘤细胞表面的CD95L结合,从而抑制多个信号途径,阻断肿瘤细胞生长[15-17]。sCD95-Fc可显著延长复发性胶质母细胞瘤患者生存期[18-19]。建立和完善sCD95-Fc的质量控制体系是保障其安全性和有效性的重要手段。sCD95-Fc蛋白中的Asn位点均有不同程度的脱酰胺修饰,因此对样品中IsoAsp的含量进行监测是非常必要的。本研究采用ISOQUANT?誖试剂盒结合RP-HPLC法测定sCD95-Fc对照品、原液和成品中IsoAsp含量,并考察不同温度热处理后成品中IsoAsp含量变化。
1 仪器与试药
1.1 仪器
超高效液相色谱系统配PDA检测器(Waters公司)、H2O3金属浴(金银杏生物科技公司)、Phenomenex Synergi 4 μm Hydro RP-80 C18色谱柱(150 mm×3 mm,4 μm)(Phenomenex公司)。
1.2 试药
ISOQUANT?誖异天冬氨酸检测试剂盒(MA1010)购自Promega公司;磷酸二氢钾和磷酸氢二钾均为国产分析纯试剂;HPLC级甲醇购自Fisher Scientific公司。sCD9-Fc融合蛋白对照品、3批原液和3批成品为中国食品药品检定研究院留存样品。
2 方法与结果
2.1 方法
2.1.1 sCD9-Fc样品前处理 取出ISOQUANT?誖异天冬氨酸检测试剂盒、sCD95-Fc对照品和样品,解冻后于室温平衡30 min。分别用Q水稀释sCD9-Fc对照品和样品至2.0 mg/mL。取对照品、样品和Q水(空白对照)各80 μL,加入主反应混合物(Q水∶5×反应缓冲液∶SAM∶PIMT=16∶40∶4∶40,现用现配)80 μL,混匀后于27℃的加热块中放置45 min。加反应终止液32 μL,混匀后12 000 r/min离心1 min,离心半径3 cm,取上清上机测定。
2.1.2 异天冬氨酸-人促睡眠肽样品制备 异天冬氨酸-人促睡眠肽(IsoAsp-DSIP)是具有9个氨基酸的活性肽,其氨基酸序列为Trp-Ala-Gly-Gly-IsoAsp-Ala-Ser-Gly-Glu[20]。其IsoAsp摩尔浓度与蛋白摩尔浓度相同。取8 μL IsoAsp-DSIP母液(101.97 μmol/L)加入93.97 μL水,即为8.0 μmol/L。取80 μL参照“1.2.1”处理。
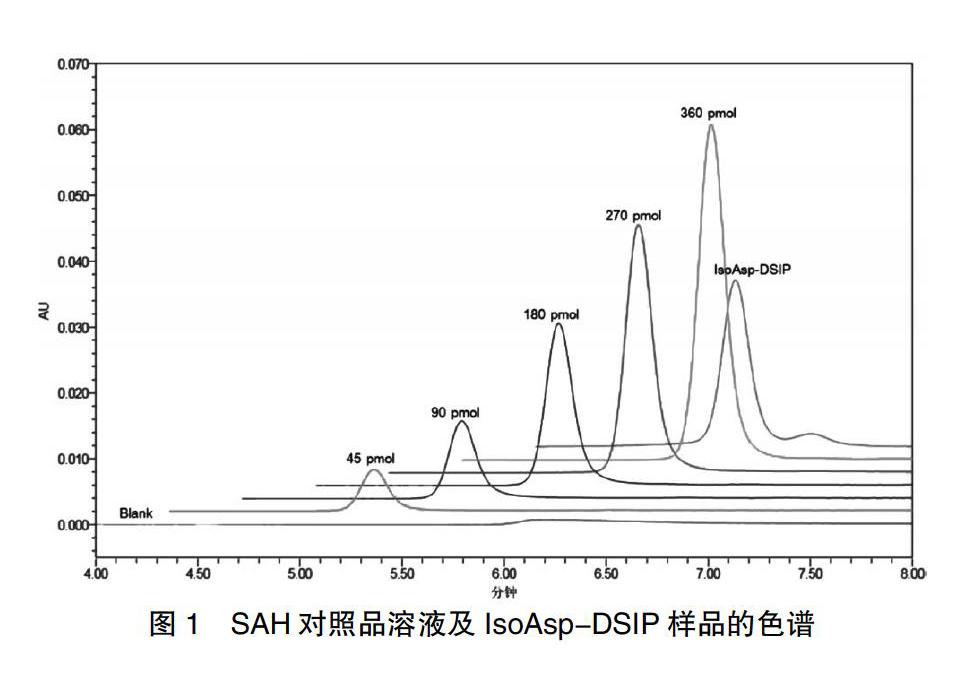
2.1.3 SAH对照品溶液制备 分别采用Q水稀释SAH对照品母液(15.00 μmol/L),制备5个不同浓度点SAH对照品溶液,使50 μL进样量中分别含有360、270、180、90、45 pmol SAH。
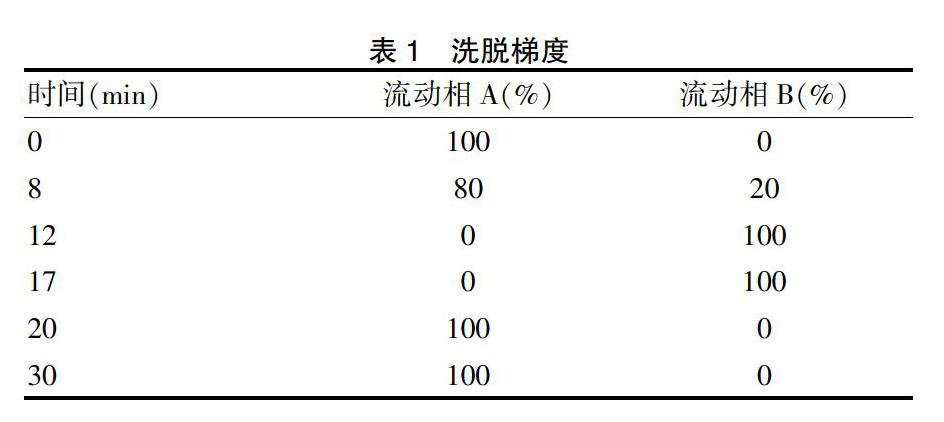
2.1.4 色谱条件 流动相A:7.9 mmol/L磷酸二氫钾-1.1 mmol/L磷酸氢二钾-10%甲醇;流动相B:甲醇。进样器温度:(5±3)℃;柱温:(25±3)℃;泵流速:0.43 mL/min;最大柱背压:5000 psi;PDA检测器:210~400 nm,3D,1.2分辨率;进样量:50 μL;梯度洗脱。洗脱梯度见表1。
2.2 结果
2.2.1 SAH最大紫外吸收波长测定 根据SAH对照品的3D色谱图,5.203 min处该组分最大紫外吸收波长为259.3 nm,同时260 nm色谱图的最大吸收峰也处于5.206 min。因此,本研究中所有的数据分析均基于260 nm色谱图结果。
2.2.2 系统适用性 序列前、中、后各进2针270 pmol SAH对照品,以在试验的不同阶段考察系统的适用性。6次进样的SAH峰面积RSD仅为0.36%,理论塔板数均>6000,拖尾因子均<1.5。
2.2.3 SAH标准曲线 分别取360、270、180、90、45 pmol SAH对照品溶液,注入色谱仪,按照“2.1.4”项下检测,记录色谱峰。以3次进样的峰面积均值对SAH含量进行线性回归,得到SAH标准曲线y = 1391.2x- 4272.8,R2 = 0.9998。360、270、180、90、45 pmol SAH对照品溶液及IsoAsp-DSIP样品的色谱图。
2.2.4 IsoAsp-DSIP回收率 理论IsoAsp含量为8 μmol/L(pmol/μL),进样体积为50 μL,进样量为8×80/192×50=166.7 pmol。将3次进样的峰面积均值代入标准曲线,计算实测SAH含量均值为169.5 pmol,回收率为101.7%。IsoAsp-DSIP样品的色谱图。
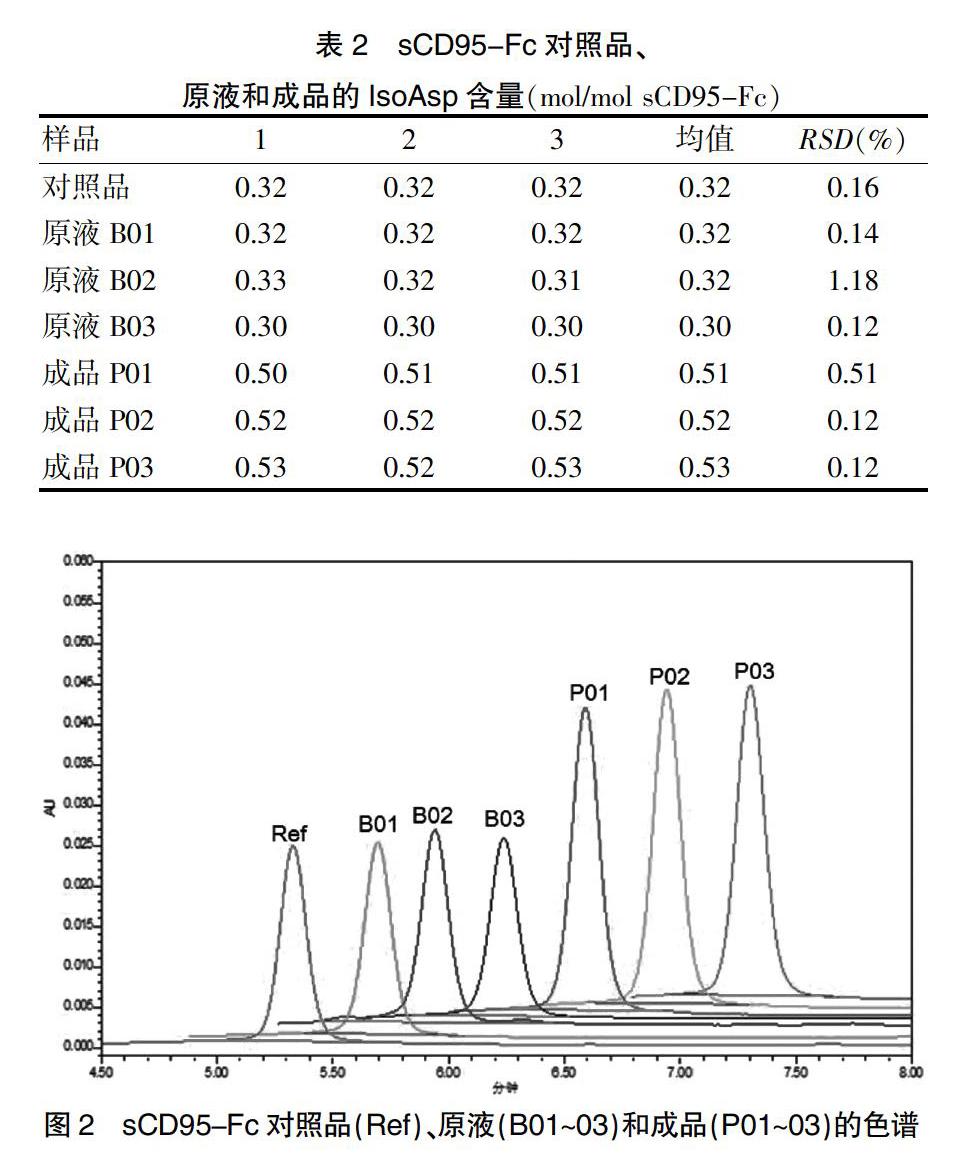
2.2.5 sCD95-Fc样品中IsoAsp含量 将sCD95-Fc对照品、原液和成品的峰面积均值代入标准曲线,计算进样SAH含量。sCD95-Fc蛋白的理论分子量为87 kD,2.0 mg/mL的摩尔浓度为23.0 μmol/L(pmol/μL)。进样sCD95-Fc蛋白含量为23.0×80/192×50=479.2 pmol。3批原液与参考品相近,而3批成品的IsoAsp含量明显增加。结果见表2。
2.2.6 热处理后sCD95-Fc成品中IsoAsp含量变化 本研究采用热加速实验,进一步考察储存过程对sCD95-Fc成品中IsoAsp含量的影响。将同一支样品分装至4个离心管中,分别于4℃、25℃、37℃和60℃放置24 h,同法测定IsoAsp含量。25℃、37℃下放置的样品与4℃下放置的样品比较,其IsoAsp含量无明显变化,而60℃处理后,其IsoAsp含量明显增加。不同温度处理后IsoAsp含量见表3,sCD95-Fc成品P01的色谱图。
3 讨论
本研究采用ISOQUANT?誖试剂盒结合RP-HPLC法测定sCD95-Fc对照品、原液和成品中的IsoAsp含量。对照品和原液的IsoAsp含量为0.30~0.32 mol/mol sCD95-Fc。sCD95-Fc对照品和原液中,除了N-糖基化位点的Asn之外,其他几个位点的Asn均有不同程度的脱酰胺修饰,其中第6位修饰率为14%左右,第312位为12%左右,两者理论上贡献IsoAsp含量为0.28 mol/mol sCD95-Fc,再加上另外几个Asn位点的潜在修饰(2%左右)以及Asp异构化形成的IsoAsp(极少量),IsoAsp总含量为30%左右。
3批成品间和3批原液间IsoAsp含量均相似,提示该产品不同批次的生产工艺较稳定。原液和对照品的IsoAsp含量相近,而成品中IsoAsp含量比原液增加了70%。分析原因可能有以下几点:①制剂缓冲液成分、稳定剂能可能引起Asn脱酰胺或Asp异构化;②制剂pH与原液不同;③储存温度,原液和对照品储存于-70℃,而成品储存于4℃,温度升高可能会引起IsoAsp生成速率加快。为进一步考察储存温度对IsoAsp含量的影响,将sCD95-Fc成品分别于4℃、25℃、37℃和60℃放置24 h后测定其IsoAsp含量。结果显示,尽管25℃和37℃下放置的样品与4℃下放置的样品比较,其IsoAsp含量无明显变化,但是60℃处理后,其IsoAsp含量明显增加,提示高温加速了IsoAsp的生成。综上所述,储存条件对IsoAsp生产速率影响显著,IsoAsp含量的检测对指导制剂配方优化和储存条件的筛选具有重要意义。此外,IsoAsp含量的监测也可成为考察生产工艺及储存过程稳定性的重要方式。
[参考文献]
[1] Jia L,Sun Y. Protein asparagine deamidation prediction based on structures with machine learning methods [J]. PLoS One,2017,12(7):e181 347.
[2] Diepold K,Bomans K,Wiedmann M,et al. Simultaneous assessment of Asp isomerization and Asn deamidation in recombinant antibodies by LC-MS following incubation at elevated temperatures [J]. PLoS One,2012,7(1):e30 295.
[3] BierczyńskaKrzysik A,?覵opaciuk M,Pawlakmorka R,et al. Investigation of asparagine deamidation in a SOD1-based biosynthetic human insulin precursor by MALDI-TOF mass spectrometry [J]. Acta Biochim Pol,2014,61(2):349-357.
[4] 畢华,韩春梅,丁有学,等.重组人血管内皮生长因子抑制剂中异天门冬氨酸含量检测[J].药物分析杂志,2015, 35(5):879-883.
[5] Badgett MJ,Boyes B,Orlando R. The Separation and Quantitation of Peptides with and without Oxidation of Methionine and Deamidation of Asparagine Using Hydrophilic Interaction Liquid Chromatography with Mass Spectrometry (HILIC-MS) [J]. J Am Soc Mass Spectrom,2017,28(5):818-826.
[6] Hsiao K,Alves J,Patel R,et al. A High-Throughput Bioluminescent Assay to Monitor the Deamidation of Asparagine and Isomerization of Aspartate Residues in Therapeutic Proteins and Antibodies [J]. J Pharm Sci,2017, 106(6):1528-1537.
[7] Fukuda M,Takao T. Quantitative analysis of deamidation and isomerization in beta2-microglobulin by 18O labeling [J]. Anal Chem,2012,84(23):10 388-10 394.
[8] Liu H,Wang F,Xu W,et al. Quantitation of asparagine deamidation by isotope labeling and liquid chromatography coupled with mass spectrometry analysis [J]. Anal Biochem,2013,432(1):16-22.
[9] Liu M,Cheetham J,Cauchon N,et al. Protein isoaspartate methyltransferase-mediated 18O-labeling of isoaspartic acid for mass spectrometry analysis [J]. Anal Chem,2012, 84(2):1056-1062.
[10] Puri A,Quan Y,Narang AS,et al. A Fluorescence-Based High-Throughput Coupled Enzymatic Assay for Quantitation of Isoaspartate in Proteins and Peptides [J]. AAPS Pharmscitech,2017,18(3):803-808.
[11] Mukherjee R,Adhikary L,Khedkar A,et al. Probing deamidation in therapeutic immunoglobulin gamma (IgG1) by ′bottom-up′ mass spectrometry with electron transfer dissociation [J]. Rapid Commun Mass Spectrom,2010,24(7):879-884.
[12] Mehl JT,Sleczka BG,Ciccimaro EF,et al. Quantification of in vivo site-specific Asp isomerization and Asn deamidation of mAbs in animal serum using IP-LC-MS [J]. Bioanalysis,2016,8(15):1611-1622.
[13] Dong S,Tan L,Chen G,et al. CD95-CD95L interaction mediates the growth control of MHV68 immortalized B cells by cytotoxic T cells [J]. Virol Sin,2017,32(3):257-259.
[14] Merz C,Strecker A,Sykora J,et al. Neutralization of the CD95 ligand by APG101 inhibits invasion of glioma cells in vitro [J]. Anticancer Drugs,2015,26(7):716-727.
[15] Hartmann N,Messmann JJ,Leithauser F,et al. Recombinant CD95-Fc (APG101) prevents graft-versus-host disease in mice without disabling antitumor cytotoxicity and T-cell functions [J]. Blood,2013,121(3):556-565.
[16] Tan L,Zhang C,Dematos J,et al. CD95 Signaling Inhibits B Cell Receptor-Mediated Gammaherpesvirus Replication in Apoptosis-Resistant B Lymphoma Cells [J]. J Virol,2016,90(21):9782-9796.
[17] Wick W,Fricke H,Junge K,et al. A phase Ⅱ,randomized,study of weekly APG101+reirradiation versus reirradiation in progressive glioblastoma [J]. Clin Cancer Res,2014,20(24):6304-6313.
[18] Blaes J,Thome CM,Pfenning PN,et al. Inhibition of CD95/CD95L (FAS/FASLG) Signaling with APG101 Prevents Invasion and Enhances Radiation Therapy for Glioblasto-ma [J]. Mol Cancer Res,2018,16(5):767-776.
[19] Tuettenberg J,Seiz M,Debatin KM,et al. Pharmacokinetics,pharmacodynamics,safety and tolerability of APG101, a CD95-Fc fusion protein,in healthy volunteers and two glioma patients [J]. Int Immunopharmacol,2012,13(1):93-100.
[20] Zhang XG,Wang WN,Zhang CS,et al. Expression and Purification of Delta Sleep-Inducing Peptide Fused with Protein Transduction Domain and Human Serum Albumin in Pichia pastoris [J]. Protein Pept Lett,2017,24(7):668-675.
(收稿日期:2018-03-23 本文編辑:王 蕾)

